Obesity is now reaching epidemic proportions in both developed and developing countries and is affecting not only adults but also children and adolescents. Over the last 20 years, obesity has become the most prevalent nutritional problem in the world, eclipsing undernutrition and infectious disease as the most significant contributor to ill health and mortality. It is a key risk factor for many chronic and noncommunicable diseases.
In Canada, the prevalence of overweight and obesity has increased over recent decades among both children and adults in all areas of the country. According to the most recent estimates from the 2004 Canadian Community Health Survey,1 59% of the adult population is overweight (i.e., body mass index [BMI] ≥ 25 kg/m2) and 1 in 4 (23%) is obese (i.e., BMI ≥ 30 kg/m2). The sheer numbers of people who are overweight and obese highlight a pressing public health problem that shows no signs of improving in the near future. What is more alarming is the problem of obesity among children and adolescents in Canada, which is advancing at an even more rapid pace than obesity among adults. In 2004, 1 in 4 (26%) Canadian children and adolescents aged 2–17 years was overweight. The obesity rate has increased dramatically in the last 15 years: from 2% to 10% among boys and from 2% to 9% among girls.1,2 This increase is cause for concern, since there is a tendency for obese children to remain obese as adults. Moreover, obesity-related health problems are now occurring at a much earlier age and continue to progress into adulthood. Given the recent temporal obesity trends among children and youth, the prevalence of obesity among adults will likely continue to increase as the current generation of children enters adulthood.
Obesity should no longer be viewed as a cosmetic or body-image issue. There is compelling evidence that overweight people are at increased risk of a variety of health problems, including type 2 diabetes, hypertension, dyslipidemia, coronary artery disease, stroke, osteoarthritis and certain forms of cancers. It has recently been estimated that about 1 in 10 premature deaths among Canadian adults 20–64 years of age is directly attributable to overweight and obesity. In addition to affecting personal health, the increased health risks translate into an increased burden on the health care system. The cost of obesity in Canada has been conservatively estimated to be $2 billion a year or 2.4% of total health care expenditures in 1997.3 Thus, the continuing epidemic of obesity in Canada is exacting a high toll on the health of the population.
The cause of obesity is complex and multifactorial. Within the context of environmental, social and genetic factors, at the simplest level obesity results from long-term positive energy balance — the interaction of energy intake and energy expenditure. The rapid increase in the prevalence of obesity over the past 20 years is a result of environmental and cultural influences rather than genetic factors. With progressive improvements in the standard of living in developed and developing countries, overnutrition and sedentary lifestyle have supplanted physical labour and regular physical activity, which has resulted in positive energy balance and overweight.
Considerable advances have been made in dietary, exercise, behavioural, pharmacologic and bariatric surgical approaches to successful long-term management of obesity. Lifestyle interventions remain the cornerstone of the treatment of obesity, but adherence is poor and long-term success is modest because of significant barriers both on the part of affected individuals and health care professionals responsible for the treatment. Pharmacotherapy and bariatric surgery are useful adjuncts for improving the health outcomes of overweight people, but, for a variety of reasons, these modalities of treatment are not widely adopted.
Despite steady progress in the management of obesity, its prevalence continues to rise. To date, population interventions have tended to focus on individual risk factors and have been largely ineffective. Hence, sweeping prevention and intervention strategies are required to slow, and hopefully reverse, the alarming increase in obesity prevalence in Canada and globally.
A number of clinical practice guidelines on the assessment and management of obesity have been published in the past. These have been largely based on consensus statements by expert panels. Moreover, most of these guidelines focus on individuals rather than on communities and the population as a whole. Recognizing these deficiencies, Obesity Canada — a not-for-profit organization founded in 1999 to improve the health of Canadians by decreasing the occurrence of obesity — convened a panel of experts to determine whether a comprehensive set of guidelines could be developed to address not only the management but also the prevention of obesity in both adults and children. Members of the Steering Committee and Expert Panel unanimously agreed on an evidence-based approach. Through the process of developing these guidelines, which began in the spring of 2004, members of the Steering Committee and Expert Panel identified major gaps in knowledge regarding obesity treatment and prevention. Considerable research is required in many areas to optimize management and to prevent the increasing prevalence of overweight and obesity in Canada. The authors' recommendations range from the need for enhanced surveillance and population-based data to new research on the biological, social, cultural and environmental determinants of obesity as well as research on effective treatment strategies, policies and interventions.
Because obesity is increasingly viewed as a societal issue, members of the Steering Committee and Expert Panel unanimously agreed to include chapters on the prevention of obesity in children and adults at the population level, as well as implications of the guidelines for health policy-makers and other interested parties.
The challenges of disseminating and implementing these guidelines were acknowledged by the Steering Committee, and a dissemination strategy has been developed to ensure that they are translated into clinical practice.
Publication of these guidelines is not the end of this process; it is only the beginning. Ongoing evaluation and revision of the various chapters and recommendations will be undertaken, as appropriate. It is hoped that, with continuing new knowledge from research, regular updating of these guidelines will accord greater certainty to many of the recommendations and expected outcomes.
Formulation of recommendations: an overview
We have attempted to use a rigorous, evidence-based approach to the development of the practice recommendations, while also acknowledging the breadth of topics to be assessed and the inherent limitations of the obesity literature on these topics.
In addition to making recommendations for treatment interventions, the most common application of clinical practice guidelines, we have also provided recommendations on interventions related to screening and prevention at the individual and population levels.
The recommendations are based on a prespecified process that was overseen by the Steering Committee. Specific chapters of the guidelines were delegated to a group of content experts within the Expert Panel, who performed a systematic literature review and were responsible for drafting the recommendations for each chapter. Recommendations were appraised by an independent Evidence-based Review Committee, members of which assessed whether the assigned level of evidence reflected the strength of the existing literature. The interactive process by which the recommendations were developed, reviewed and revised included 4 joint meetings of the Steering Committee and Expert Panel. The final draft of the guidelines was reviewed by the Steering Committee and by external stakeholders and experts, who included representatives from academia, industry and government and nongovernment officials.
The approach used to formulate the recommendations was based on the following conventions:
• A clear question or well-defined issue surrounding an obesity-related intervention was the starting point for review of the literature and formulation of recommendations.
• Each recommendation is evidence-based — arrived at through a systematic review of the literature — and reflects the consensus of the Steering Committee and relevant Expert Panel members.
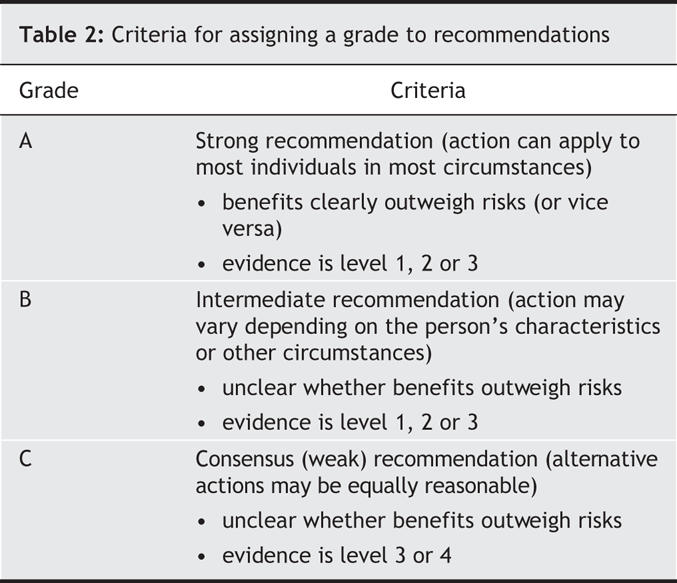
• Each recommendation includes a level of evidence (1 to 4 [Table 1]) and a grade (A, B or C [Table 2]).
Table 1
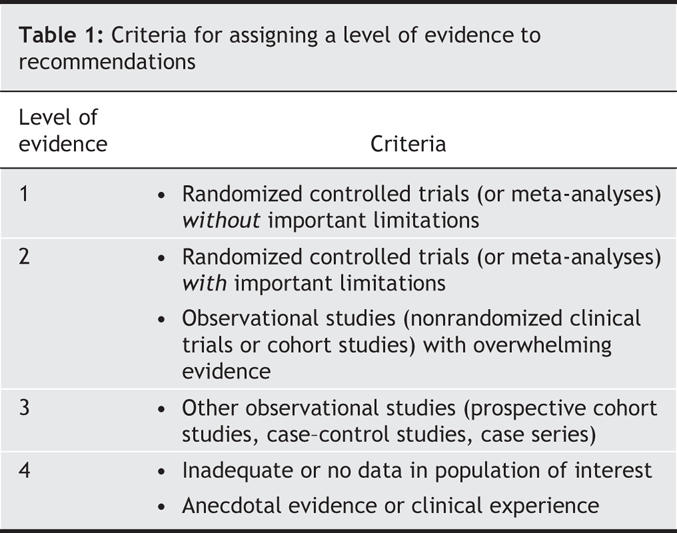
Table 2
• The level of evidence informs the reader about the strength of the evidence in favour of (or against) the intervention and is based on prespecified objective criteria.
• The grade informs the reader about whether an intervention should (or should not) be implemented and reflects both the level of evidence supporting the recommendation and a consideration, where applicable, of the harms and costs of the intervention and its importance and value to the individual or population.
• The level of evidence assigned to an intervention is not necessarily linked to a corresponding grade. However, a high grade is less likely in the setting of low-quality evidence.
• The wording “we recommend” is used to express a grade A recommendation. The wording “we suggest” is used to express a grade B recommendation. The wording used for grade C recommendations varies but reflects the uncertainty surrounding the benefits and risks of the intervention.
• A consensus recommendation, which is classified as grade C, is a statement that provides a reasonable approach or guideline for that domain of study. A consensus recommendation reflects insufficient evidence to inform clinical practice or anecdotal evidence only.
Research recommendations
Research recommendations were developed to identify gaps in existing knowledge and to establish priorities for future research. Research recommendations were formulated by content experts and were subjected to iterative reviews and modifications by the Steering Committee.
Objectives and content of the guidelines
These Canadian guidelines on the prevention and management of obesity were developed to address the need for evidence-based recommendations for the management of obesity at the individual and population levels, to identify gaps in knowledge and to establish an agenda for future research in this area.
Objectives
The specific objectives of the guidelines were:
• to establish a process for developing evidence-based guidelines for the screening, prevention and treatment of obesity in Canada
• to provide recommendations regarding application of the following interventions to individuals and populations:
- screening for obesity (using BMI and waist circumference)
- screening for obesity-related conditions (e.g., dyslipidemia, diabetes)
- screening for psychosocial disorders (e.g., mood disorders, eating disorders)
- prevention of obesity (individual and community-based interventions)
- dietary intervention
- physical exercise therapy
- cognitive-behaviour therapy
- pharmacotherapy
- bariatric surgery
- alternative or nontraditional therapy
- health care team support
• to disseminate material to a broad spectrum of health care providers
• to assist in public health policy development
• to identify gaps in knowledge and suggest a research agenda.
Population and special groups of interest
In developing these guidelines, we aimed to address a broad range of populations and patients, encompassing all age groups and subgroups (the latter classified according to comorbidity or ethnic background):
• children, adolescents and adults who are overweight or obese, or with an increased waist circumference
• population subgroups (e.g., people with type 2 diabetes, hypertension, dyslipidemia, severe obesity [BMI ≥ 40 kg/m2], and ethnic groups [e.g., Aboriginal people, Asian people]).
Outcomes of interest
The guidelines identify several key outcomes of interest. These were considered, whenever possible, in the formulation of recommendations:
• Anthropometric outcomes: body weight, BMI and waist circumference
• Biochemical or physical outcomes: fasting glucose, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, ratio of total cholesterol to HDL cholesterol, and systolic and diastolic blood pressure
• Clinical outcomes: cardiovascular disease and other morbidities, mortality and quality-of-life measures
• Psychosocial outcomes: depression, mood disorders and eating disorders
Using the guidelines: an illustrative case scenario
These guidelines are intended as a practical guide that can be used by health care professionals in everyday clinical practice. When using the guidelines, the reader can refer to the list of recommendations (listed at the end of this summary) to find key points rapidly. Alternatively, the reader can refer to individual chapters (available on the CMAJ Web site at www.cmaj.ca/cgi/content/full/176/8/S1/DC1) for a more in-depth review of specific issues. Each of the 26 chapters contains the recommendations listed at the end of this summary that pertain to a specific issue along with an outline of the background and rationale for those recommendations.
With either approach, we suggest that the reader review chapter 1, which provides a rationale for the grades and levels of evidence assigned to the recommendations. Because there is no standardized method for developing clinical practice guidelines, the reader may be aided by a prior understanding of the approach used by the committees. In brief, the level of evidence is an objective assessment that reflects the quality of evidence in published studies. The grade is a subjective assessment that considers both the level of evidence and other factors (e.g., harms and costs) relevant to an intervention when applied at the level of the individual or population.
The following case scenario illustrates how the guidelines may be used in the management of a typical adult patient assessed in clinical practice. Fig. 1 summarizes the assessment and stepwise management of the overweight or obese adult, which can be applied to the following case.
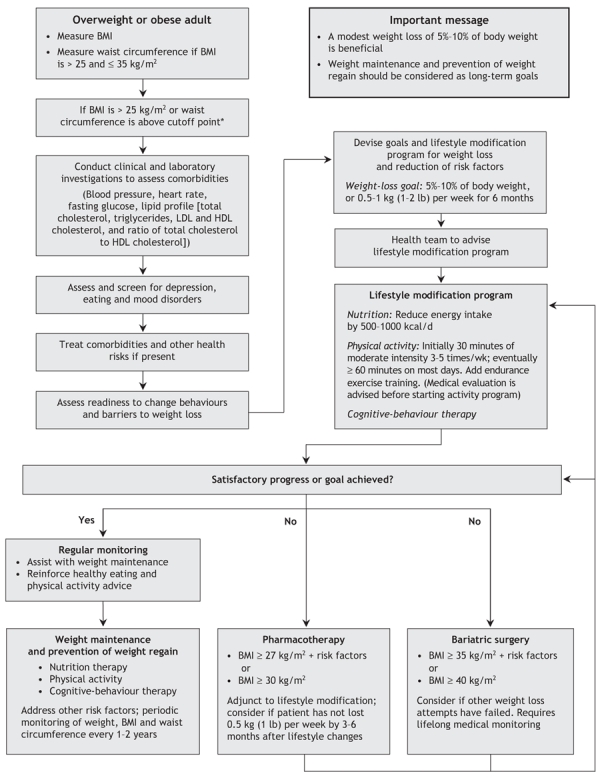
Fig. 1: Algorithm for the assessment and stepwise management of the overweight or obese adult. LDL = low-density lipoprotein, HDL = high-density lipoprotein. *Body mass index (BMI) and waist circumference cutoff points are different for some ethnic groups; see Table 3 for ethnic-specific waist circumference cutoff points.
Ms. A is a 46-year-old woman who is married with 2 children and is seen for an initial primary care assessment. She has experienced some “weariness and increased irritability” in recent months but otherwise has been in good health. Her menses are regular, and she does not have any climacteric symptoms. She is not taking any prescription medications but occasionally takes an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) for low back pain. She is a former smoker who works in a sedentary occupation and does not exercise regularly (because of lack of time). Her mother (age 72 years) has type 2 diabetes, and her father (age 75 years) has coronary artery disease.
On physical examination, Ms. A appears generally well. She weighs 89 kg and is 1.6 m tall. Blood pressure is 135/85 mm Hg, heart rate is 72 beats/min, chest is clear to auscultation and percussion, heart sounds are normal, no abdominal organomegaly is palpable, and there are no musculoskeletal or skin abnormalities. Her body habitus suggests a central (abdominal) pattern of obesity.
As the primary care physician, you are concerned that Ms.A is obese (with a central pattern of fat distribution) and is at risk of obesity-related diseases, in this case type 2 diabetes and hypertension. On further questioning, the patient states that she has poor eating habits and admits to frequent “snacking” on energy-dense, low-nutrient foods. She also has symptoms that suggest a depressive mood disorder, which is common in obese adults. She does not have any symptoms suggestive of obstructive sleep apnea, which is also associated with obesity.
Initial patient assessment and investigations
In chapters 3–8, issues relating to the initial assessment of overweight or obese patients are addressed. Based on a clinical assessment of Ms. A, supplemented by key points from relevant chapters, we recommend the following interventions.
Measurement of BMI and waist circumference
Rationale: Measuring BMI and waist circumference is an essential first step to determine the level and distribution of adiposity and is a grade A recommendation when screening for overweight and obesity in individuals. These measures are straightforward and easy to perform. The measurement of BMI (weight divided by height squared) and waist circumference can be used to help determine a patient's risk profile for cardiovascular disease and overall health risk; they also provide a reference point for monitoring BMI or waist circumference over time, especially if a weight management intervention is planned.
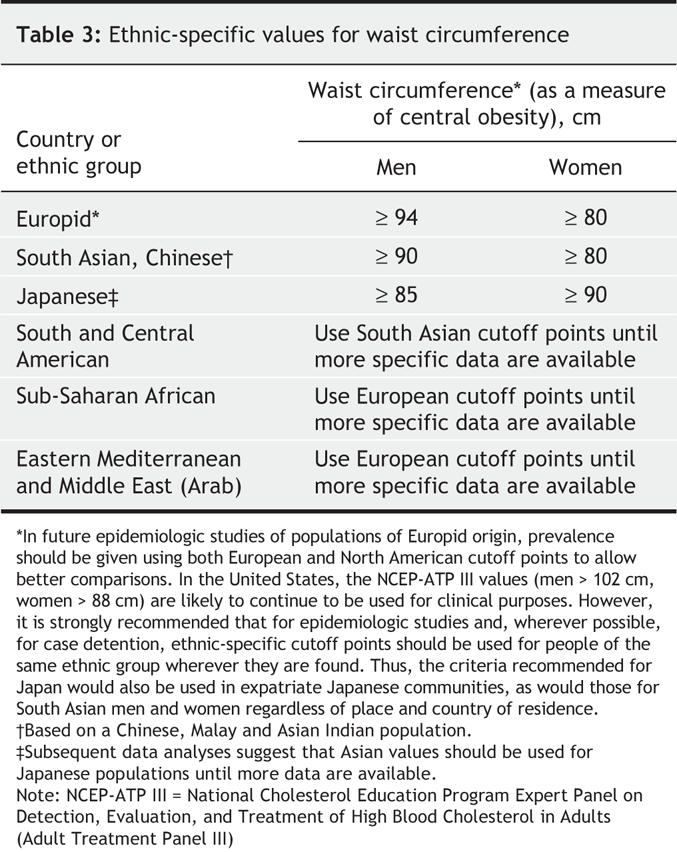
Ms. A's BMI is 34.8 kg/m2 (89 kg ÷ [1.6 m]2). Based on current guidelines for body weight classification (outlined in chapter 3), Ms. A is approximately at the cutoff point between class I obesity (BMI 30–34.9 kg/m2) and class II obesity (BMI 35–39.9 kg/m2). Her waist circumference, at 98 cm, is high for her sex (i.e., ≥ 80 cm), indicating a central (abdominal) pattern of obesity (see Table 3). The combination of these 2 measures of health risk puts this patient at “very high risk” of obesity-related diseases.
Table 3
Measurement of laboratory parameters
Laboratory parameters include fasting blood glucose level, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, ratio of total cholesterol to HDL cholesterol, liver enzyme levels and urinalysis.
Rationale: Fasting blood glucose level and lipid profile are important components in the assessment of an overweight or obese person, and this is a grade A recommendation (chapters 6 and 8) because patients with obesity (especially central obesity) are at high risk of pre-diabetes (impaired fasting glucose or impaired glucose tolerance, or both), type 2 diabetes and dyslipidemia. Early recognition of such disorders may permit dietary and lifestyle changes that will mitigate the risk of disease development and progression. Assessment of liver enzymes and urinalysis is a grade B recommendation (chapters 6 and 8) because obese patients may be at increased risk of liver disease (nonalcoholic steatohepatitis) and impaired renal function.
For Ms. A, laboratory testing reveals the following: fasting glucose level 6.4 mmol/L, total cholesterol 5.75 mmol/L, LDL cholesterol 3.59 mmol/L, HDL cholesterol 1.26 mmol/L, triglycerides 1.98 mmol/L, ratio of total:HDL cholesterol 4.56, normal liver enzyme levels and normal urinalysis results.
Assessment for depression and other mood disorders
Rationale: Major depression and other mood disorders are common in patients with obesity; they occur in 20%–60% of women aged 40 years or older with a BMI > 30 kg/m2, the group into which Ms. A falls. Furthermore, the presence of a mood disorder may adversely affect adherence to weight management interventions. Screening for such disorders in appropriate individuals is a grade B recommendation (see chapter 7). Treatment of a major depressive disorder should be undertaken in concert with any planned weight management intervention, because some pharmacologic weight-loss treatments must be avoided in patients who are taking antidepressant medications.
Ms. A is considered to have a mild depressive disorder that appears to be linked, in part, to concerns and anxiety about her body image. At this time, a serotonin-specific re-uptake inhibitor (SSRI) and psychotherapy are considered.
Developing the health care team for a weight management program
Based on the above clinical and laboratory findings, you conclude that Ms. A has impaired fasting glucose (and pre-diabetes), dyslipidemia and a mild depressive disorder. She is at risk of cardiovascular disease and increasing depressive symptoms. In addition, her blood pressure (135/85 mm Hg) requires monitoring because of the risk of hypertension.
Using chapter 9 as a guide, we recommend that Ms. A begin a weight management program. Before its initiation, discuss with her the need for a multidisciplinary health care team to help her carry out the program. The health care team will include a coordinating health professional (who may be a primary care physician, medical specialist or registered nurse), a dietary professional, an exercise professional and a clinical psychologist.
The coordinating health professional will engage the patient's family so that they are made to feel part of the health care team. The health professional will make specific recommendations to help the patient adhere to the weight management program and identify potential barriers to the changes in lifestyle that will become an integral part of the program. The health care professional will also discuss the need for reasonable weight management goals that are sustainable. As discussed in chapter 9, the use of a multidisciplinary, lifestyle-based intervention for the management of obese individuals is a grade A recommendation. Furthermore, discussion of the weight management program before its initiation among members of the health care team and the patient to establish management goals and review potential barriers to achieving these goals is a grade B recommendation.
Initiating a weight management program
Based on chapters 10–16 of the guidelines, we recommend that Ms. A undertake a weight management strategy focusing on dietary therapy and lifestyle interventions. To initiate and allow ongoing follow-up of these interventions, refer the patient to appropriate health professionals, who will help to initiate the interventions and monitor the patient's progress over time. Implicit in all weight management programs is long-term monitoring of the patient's status, similar to the long-term monitoring undertaken in patients with other chronic conditions, such as hypertension or diabetes. Follow-up visits are used to provide ongoing counselling about dietary and lifestyle management, education and, perhaps most important, ongoing support to the patient so that she maintains the dietary and lifestyle changes over the long-term.
Dietary and lifestyle interventions
Rationale: Dietary and lifestyle interventions aimed at decreasing energy intake and increasing energy expenditure through a balanced dietary and exercise program are an essential component of all weight management programs. In chapter 12, we provide a grade A recommendation for a healthy diet and regular physical activity as the first-line treatment option for overweight or obese adults to attain clinically important weight loss and reduce obesity-related symptoms. We also give a grade A to the recommendation for diet and exercise therapy in overweight or obese adults with risk factors for type 2 diabetes, as is the case with Ms. A, who has pre-diabetes (with impaired fasting glucose) and a family history of type 2 diabetes.
Assessment by a nutrition health professional
Rationale: To outline a dietary treatment plan and to provide adequate education requires counselling by a health professional with expertise in dietary management. Typically, many physicians do not have adequate time to devote to dietary management and may not have the expertise required to recommend a diet therapy plan. Using a qualified and experienced health professional (preferably a registered dietitian) for dietary counselling and to implement an optimal dietary plan for achieving and maintaining a healthy body weight is a grade B recommendation (chapter 11).
For Ms. A, the nutrition professional will focus on a dietary regimen tailored to the patient with impaired glucose tolerance or emerging type 2 diabetes.
Assessment by an exercise health professional
Rationale: Physical exercise is an integral component of a weight management program, especially for weight maintenance. As with dietary treatment, many physicians do not have the time or expertise to advise patients on an exercise program that is tailored to individual needs and capabilities. Advice from an exercise health professional is required. For a typical patient, physical activity (30 minutes a day of moderate intensity, increasing, when appropriate, to 60 minutes a day) as part of a weight management program is a grade B recommendation (chapter 13).
For Ms. A, an exercise program is problematic because of time constraints related to balancing work-related and family-related duties. An exercise professional will help develop a program that is workable within these confines.
Assessment by a clinical psychologist (or psychiatrist)
Rationale: In chapter 10, we give a grade A to the recommendation for 1 or more of behaviour modification, cognitive behavioural therapy, activity enhancement and dietary counselling as part of a comprehensive weight management intervention.
For Ms. A, who appears to have had mild depressive symptoms that may be related, in part, to body image perception and suboptimal eating habits, a psychologist (or psychiatrist) with expertise in the management of obese patients will aid in providing appropriate behaviour modification and, if required, psychotherapy and psychotropic medication.
Long-term monitoring of a patient in a weight management program
Many patients who initiate a weight management program may experience initial health benefits characterized by weight loss and improvements in physical measures (symptom reduction), laboratory measures (glucose and lipid levels) and psychologic measures (mood improvement). However, many patients also experience a relapse or worsening of these measures over the long term.
Although specific patient management issues are beyond the scope of these guidelines, we do provide an assessment of treatment options to be considered after the first-line treatment relating to dietary and lifestyle interventions. These treatment options, which are intended to supplement the first-line treatment, are discussed in chapters 14–16. Based on individual patient characteristics that reflect baseline health risks and benefits accrued from first-line weight management therapy, a patient may be eligible to receive pharmacologic therapy for obesity, bariatric surgery or alternative treatments, which include herbal and other dietary supplements.
RECOMMENDATIONS
SECTION ONE: CLINICAL
Epidemiology of obesity
1. Because of the health impact of the rising prevalence and incidence of overweight and obesity in Canada, we recommend implementing strategies directed at the prevention and treatment of overweight and obesity in children, adolescents and adults [grade A, level 3].
2. Because of the lack of adequate information on the prevalence of obesity and related risk factors in Canada, particularly among subgroups of the population, we recommend the creation of a national surveillance system that incorporates, at a minimum, measurements of height, weight and waist circumference [grade A, level3].
Classification of overweight and obesity in adults and children
3. We recommend measuring body mass index (BMI; weight in kilograms divided by height in metres squared) in all adults [grade A, level 34–6] and in all children and adolescents (aged 2 years and older). We recommend using the growth charts of the US Centers for Disease Control and Prevention for BMI to screen children and adolescents for overweight (≥ 85th to < 95th percentile) and obesity (≥ 95th percentile) [grade A, level 37].
4. We recommend measuring waist circumference in all adults to assess obesity-related health risks [grade A, level 35,6].
Assessment of overweight and obesity in adults and children
Assessment of readiness to change
5. We suggest that health care professionals assess readiness and barriers to change before an individual implements a healthy lifestyle plan for weight control or management [grade B, level 38,9].
Clinical and laboratory assessment of overweight and obese adults, adolescents and youth
6. We recommend that the clinical evaluation of overweight and obese adults4,10–12 and children13,14 include a history and a general physical examination to exclude secondary (endocrine or syndrome-related) causes of obesity and obesity-related health risks and complications [grade A, level 3].
7. We recommend measuring fasting plasma glucose level and determining lipid profile, including total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and ratio of total cholesterol to HDL cholesterol, as screening tests in overweight and obese adults [grade A, level 310,15], and we suggest that these screening tests be done in children aged 10 years and older [grade B, level 313,16]. We suggest repeating these tests at regular intervals as needed [grade C, level 4].
8. We suggest additional investigations, such as liver enzyme tests, urinalysis and sleep studies (when appropriate), to screen for and exclude other common obesity-related health problems [grade B, level 317–19].
9. We suggest that the health care professional screen the overweight or obese adult for eating disorders, depression and psychiatric disorders, as appropriate [grade B, level 320–31].
Management of obesity in adults and children
Role of health care team professionals in obesity counselling and management
10. We recommend a comprehensive healthy lifestyle intervention for overweight and obese people [grade A, level 1]. We suggest that members of the health care team discuss with those willing to participate in weight management programs appropriate education, support and therapy as adjuncts to lifestyle interventions [grade B, level 2].
11. Primary care health professionals are encouraged to work with other health care team members to develop a comprehensive weight management program for the overweight or obese person to promote and maintain weight loss [grade C, level 3].
12. Primary care health professionals are encouraged to create a nonjudgmental atmosphere when discussing weight management [grade C, level 4].
13. Health care professionals are encouraged to consider the barriers people might have concerning obesity and its management [grade C, level 4].
Lifestyle intervention
14. We recommend an energy-reduced diet and regular physical activity as the first treatment option for overweight and obese adults32,33 and children34–36 to achieve clinically important weight loss and reduce obesity-related symptoms [grade A, level 2].
15. In children, we recommend ongoing follow-up by health professionals for a minimum of 3 months [grade A, level234–37].
16. We recommend diet and exercise therapy for overweight and obese people with risk factors for type 2 diabetes [grade A, level 138–40] and cardiovascular disease [grade A, level 241,42].
17. We suggest that individuals willing to participate in weight management programs be provided with education and support in behaviour modification techniques as an adjunct to other interventions [grade B, level 243–45].
18. We recommend comprehensive lifestyle interventions (combining behaviour modification techniques, cognitive behavioural therapy, activity enhancement and dietary counselling) for all obese adults [grade A, level 139,46–48].
19. When treating obesity in children, we suggest using family-oriented behaviour therapy [grade B, level 149–53].
Dietary intervention
20. We suggest that the optimal dietary plan for achieving healthy body weight and dietary counselling for adults, adolescents and children be developed with a qualified and experienced health professional (preferably a registered dietitian) together with the individual and family to meet their needs [grade B, level 254–56].
21. We recommend that a nutritionally balanced diet (designed to reduce energy intake) be combined with other supportive interventions to achieve a healthy body weight in overweight and obese people of all ages and to ensure the maintenance of growth in adolescents and youth [grade C, level 4].
22. We suggest a high-protein or a low-fat diet (within acceptable macronutrient distribution ranges indicated in the Dietary Reference Intakes) as a reasonable short-term (6–12 months) treatment option for obese adults as part of a weight-loss program [grade B, level 257,58].
23. Meal replacements may be considered as a component of an energy-reduced diet for selected adults interested in commencing a dietary weight-loss program [grade C, level 259,60].
Physical activity
Adults
24. All those considering initiating a vigorous exercise program are encouraged to consult their physician or health care team professionals [grade C, level 4].
25. We suggest long-term, regular physical activity, which is associated with maintenance of body weight or a modest reduction in body weight for all overweight and obese people [grade B, level 261,62].
26. Physical activity and exercise should be sustainable and tailored to the individual. We recommend that the total duration be increased gradually to maximize the weight-loss benefits [grade A, level 263–65].
27. We suggest physical activity (30 minutes a day of moderate intensity, increasing, when appropriate, to 60 minutes a day) as part of an overall weight-loss program [grade B, level 262,63].
28. Endurance exercise training may reduce the risk of cardiovascular morbidity in healthy postmenopausal women, and we suggest its use for adults with an increased BMI [grade B, level 2].
Children and adolescents
29. We recommend that the primary care physician or health care team encourage children and adolescents to reduce sedentary pursuits and “screen time” (i.e., television, video games) [grade A, level 267–69].
30. We recommend that activity prescribed for children be fun and recreational, with lifestyle activities tailored to the relative strengths of the individual child and family [grade A, level 270]. Health professionals are encouraged to emphasize the short-term benefits of physical activity rather than the long-term health benefits to children [grade C, level 4].
Pharmacotherapy
Adults
31. We suggest the addition of a selected pharmacologic agent for appropriate overweight or obese adults, who are not attaining or who are unable to maintain clinically important weight loss with dietary and exercise therapy, to assist in reducing obesity-related symptoms [grade B, level 271–76].
32. We suggest the addition of a selected pharmacologic agent for overweight or obese adults with type 2 diabetes, impaired glucose tolerance or risk factors for type 2 diabetes, who are not attaining or who are unable to maintain clinically important weight loss with dietary and exercise therapy, to improve glycemic control and reduce their risk of type 2 diabetes [grade B, level 271,72,77–82].
Children and adolescents
33. We suggest that orlistat be considered to aid in weight reduction and weight maintenance when added to a regimen of lifestyle intervention among adolescents [grade B, level 1].
34. Because of lack of data for prepubertal children, the use of pharmacologic agents in this group should be considered only within the context of a supervised clinical trial [grade C, level 4].
Bariatric surgery
35. We suggest that adults with clinically severe obesity (BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 with severe comorbid disease) may be considered for bariatric surgery when lifestyle intervention is inadequate to achieve healthy weight goals [grade B, level 283].
36. We suggest that bariatric surgery in adolescents be limited to exceptional cases and performed only by experienced teams [grade C, level 4].
37. We suggest that a minimally invasive approach be considered for weight loss surgery when an appropriately trained surgical team and appropriate resources are available in the operating theatre [grade C, level 384].
Alternative therapies
38. There is insufficient evidence to recommend in favour of or against the use of herbal remedies, dietary supplements or homeopathy for weight management in the obese person [grade C, level 485–98].
Prevention of obesity in adults and children
39. The use of surveillance systems and measurement tools is encouraged to determine the effectiveness and efficacy of obesity prevention programs and interventions. The development of a comprehensive, coordinated and rigorous surveillance plan with strong links among program developers, advocates, policy-makers and other stakeholders is encouraged as a key component in obesity prevention [grade C, level 4].
40. Obesity prevention should take a multisector approach— similar to that used for tobacco control in Canada. Prevention efforts should invest in and target all age groups and span life from infancy to old age. Innovative ways to provide access and programs to less economically viable citizens should be developed [grade C, level4].
41. Programs that combine a low-fat or energy-reduced diet and endurance exercise have not been shown to be more effective than programs using either component alone for obesity prevention; both approaches should be considered [grade B, level 3].
42. We suggest that individual and small-group counselling for dietary interventions be considered for the prevention of obesity in adults [grade B, level 2]. Counselling by telephone, counselling by mail and financial incentives do not appear to be effective, and we do not encourage their use [grade C, level 3].
43. There is insufficient evidence to recommend in favour of or against broad community interventions aimed at cardiovascular disease risk reduction for the prevention of obesity [grade C, level 3].
44. Discussion of the prevention of childhood obesity with the pregnant mother is encouraged [grade C, level 4].
45. Exclusive breast-feeding of infants is encouraged until at least 6 months of age to prevent later obesity [grade C, level 4].
46. Discussion of limiting consumption of energy-dense snack foods high in sugar and fat during childhood and adolescence is encouraged [grade C, level 4].
47. We suggest limiting “screen time” (i.e., watching television, playing video or computer games) to no more than 2 hours a day to encourage more activity and less food consumption, and to limit exposure to food advertising [grade B, level 3].
48. The role of schools as pivotal settings for the promotion of healthy active living and school-based prevention programs to reduce the risk of childhood obesity is encouraged, as are interventions to increase daily physical activity through physical education class time and opportunities for active recreation [grade C, level 4].
49. The development of programs in multiple settings targeting behaviour change with parental and family involvement is encouraged [grade C, level 4].
SECTION TWO: RESEARCH, POLICY, EDUCATION
Research and policy
50. Normative data from representative samples of the Canadian population should be collected to allow the development of Canadian-specific growth curves for BMI and waist circumference. Research efforts should be directed at developing reference data that are based on health-related criteria or outcomes rather than being merely representative of the population [grade C, level 4].
51. Population-based research is needed to help establish ethnic-specific cutoff values for waist circumference, with optimal sensitivity and specificity for discriminating clinical events [grade C, level 4].
52. Future research should be directed at determining the clinical utility of waist circumference in the identification of health risk among children and youth, independently or in combination with BMI [grade C, level 4].
53. For population surveillance of overweight and obesity in children, we recommend that the BMI thresholds of the International Obesity Task Force be used to classify children and youth as overweight and obese. Where possible, we recommend that prevalence be presented using both the US Centers for Disease Control and Prevention thresholds and the International Obesity Task Force cutoff points to facilitate international comparisons [grade C, level 4].
54. Future research should be directed at understanding the impact of gender, biological maturation, nutrition, physical activity levels, sociocultural milieu, built environments, ethnic background, biological factors, psychological factors and genetics on obesity and obesity-related health risk in the context of the Canadian population [grade C, level 4].
55. The screening criteria for obesity-related health consequences should be assessed in the clinical setting for sensitivity, specificity and clinical value in improving the health of children with obesity [grade C, level 4].
56. Investigation of the prevalence of the health consequences of obesity in childhood should be undertaken in diverse populations and should include longitudinal studies to examine the prognosis of hyperinsulinemia, impaired glucose tolerance, impaired fasting glucose level and the clustering of cardiovascular risk factors [grade C, level 4].
57. Studies to determine optimal intervention strategies for children with established health consequences related to obesity are urgently required [grade C, level 4].
58. Randomized clinical trials are needed to examine the effect of the treatment of depression on outcomes of treatment for obesity. Further studies with improved diagnostic methods and prospective designs are also needed to delineate the association between obesity and major depressive disorder [grade C, level 4].
59. Research should be undertaken to develop and evaluate the organization of care for overweight and obese people and to determine the cost-effectiveness of these strategies [grade C, level 4].
60. Long-term, randomized controlled trials of nutritional therapy for obesity treatment in the pediatric population are urgently required. Such studies should consider different ethnic groups and ages, should be with and without energy restriction and should address dietary efficacy, acceptability, long-term compliance, impact of the diet on overweight status, and physical and psychological health risks [grade C, level 4].
61. Research should be undertaken to further validate models and tools for assessing intention to change as well as the effectiveness of interventions to improve readiness to change [grade C, level 4].
62. Research to find drugs that do not cause weight gain in treatment areas such as psychiatry is needed [grade C, level 4].
63. Funding of all types of research at all levels to address knowledge gaps and answer outstanding questions in the area of obesity is a high priority. Research is needed to develop, test and refine effective policies and interventions (best practices) in obesity prevention to enhance the evidence base for future public health interventions. Specific emphasis must be placed on translating research into policy, programs and practice [grade C, level 4].
Education
64. Undergraduate curricula and education for graduate health practitioners should be improved to fulfill knowledge, skills and attitude goals with respect to management and prevention of obesity [grade C, level 4].
65. Continuing education activities that provide physicians and health professionals with the skills they need to counsel people confidently in healthy weight management should be developed [grade C, level 4].
Implementation of the guidelines
66. Dissemination of the guidelines can be orchestrated by a central organization, but implementation should be carried out locally by individuals or local organizations [grade C, level 4].
67. The transfer of information into clinical practice should focus on establishing weight reduction and weight control as an important secondary prevention strategy for diabetes and cardiovascular disease [grade C, level 4].
68. More research is needed to improve understanding of the mechanisms of clinical practice guidelines implementation [grade C, level 499].
69. The guidelines should be disseminated in a simple, clear format that will be well received and accepted [grade C, level 4].
70. A network of local key opinion leaders should be developed as an important component of a successful dissemination and implementation strategy [grade C, level 4100].
71. A multifaceted global dissemination and implementation plan should involve a sequence of events, including publication in peer-reviewed and non-peer-reviewed journals [grade C, level 4101].
72. To ensure continual quality improvement, a committee should be created to measure outcomes, then monitor the effectiveness of the implementation program [grade C, level 4].
Supplementary Material
Acknowledgments
Financial assistance for the development of these guidelines was generously provided in the form of arm's-length grants-in-aid from Abbott Laboratories Ltd., AstraZeneca Canada Inc., GlaxoSmithKline Inc., Merck Frosst Canada Ltd., Pfizer Canada Inc., Hoffmann–La Roche Ltd., Johnson and Johnson Medical Products, sanofi-aventis Canada Inc. and Unilever Canada Inc. Sponsors were not involved in any aspect of the guidelines development, the literature interpretation, the decision to publish or any other aspect of publication of the guidelines. The funds were used for conference calls and for organization of and travel to meetings for the members of the Steering Committee and Expert Panel Committee. None of the members of these committees received any financial or in-kind remuneration for their contribution to this work.
Appendix 1.
Footnotes
This is a summary of the full document, which consists of this summary as well as 26 chapters on specific aspects of obesity prevention and management. The full document can be found at www.cmaj.ca/cgi/content/full/176/8/S1/DC1.
The recommendations were developed, reviewed and revised by the Expert Panel and the Steering Committee. The final draft of the guidelines was reviewed by the Steering Committee and by external stakeholders and experts, who included representatives from academia, industry and government and nongovernment officials.
Contributors: David Lau and James Douketis were the principal coauthors of the executive summary. All of the authors contributed substantially to the conception and design and to the interpretation of the guideline findings, revised the manuscript for important intellectual content and approved the final version to be published.
We extend special thanks to Core Health Services Inc., Toronto, Ont., for providing logistic assistance in organizing meetings and conference calls and for distribution of the guidelines; Barbara Kermode-Scott, medical writer, for writing and editing services; and Valerie Crosbie for her editorial assistance in the preparation of these guidelines.
Competing interests: None declared for James Douketis and Katherine Morrison. David Lau owns common shares in GlaxoSmithKline and Eli Lilly. He is a consultant to Abbott Laboratories, Ltd., AstraZeneca Canada Inc., Merck Frosst Canada Inc., Bristol-Myers Squibb Canada, Eli Lilly Canada Inc., Oryx Pharmaceuticals Inc., Pfizer Canada Inc., sanofi-aventis Canada Inc., Servier Canada Inc. and Solvay Pharma Inc.; and has received speaker fees from Abbott Laboratories, Ltd., AstraZeneca Canada Inc., GlaxoSmithKline, Merck Frosst Canada Inc., Merck/Schering, Eli Lilly Canada Inc., sanofi-aventis Canada Inc. and Novo Nordisk Canada Inc.; research grants from AstraZeneca Canada Inc., Bristol Myers Squibb, Dainippon Pharmaceuticals, GlaxoSmithKline, Pfizer Canada Inc., and sanofi-aventis Canada Inc.; and travel assistance to attend international meetings from Abbott Laboratories, Ltd., AstraZeneca Canada Inc. and sanofi-aventis Canada Inc. Irene Hramiak is a consultant to GlaxoSmithKline Inc. and is on a National Advisory Board for Abbott Laboratories Ltd., Eli Lilly, Novo Nordisk, sanofi-aventis Canada Inc. and GlaxoSmithKline Inc. She has received honoraria for speaking engagements from Merck Frosst Canada Ltd., GlaxoSmithKline Inc. and Novo Nordisk and has received a travel grant from Novo Nordisk. Arya Sharma is a consultant to Abbott Laboratories Ltd., Boehringer Ingelheim, Novartis, sanofi-aventis Canada Inc. and Merck Frosst Canada Ltd. He has received speaker fees from Abbott Laboratories Ltd., Boehringer Ingelheim, AstraZeneca Canada Inc., Novartis and Merck Frosst Canada Ltd. and travel assistance from Abbott Laboratories Ltd., Boehringer Ingelheim, Merck Frosst Canada Ltd., Novartis and sanofi-aventis Canada Inc. Ehud Ur has received speaker fees from sanofi-aventis Canada Inc., Abbott Laboratories, Ltd., GlaxoSmithKline and Novo Nordisk; research grants from GlaxoSmithKline and Novo Nordisk; and travel assistance from sanofi-aventis Canada Inc., Abbott Laboratories, Ltd.
Correspondence to: Dr. David C.W. Lau, Departments of Medicine, Biochemistry and Molecular Biology, Julia McFarlane Diabetes Research Centre, Diabetes and Endocrine Research Group, University of Calgary, 2521–3330 Hospital Dr. NW, Calgary AB T2N 4N1; dcwlau@ucalgary.ca
REFERENCES
- 1.Shields M. Measured obesity: overweight Canadian children and adolescents. In: Nutrition: findings from the Canadian Community Health Survey; issue 1; 2005 (cat no 82-620-MWE2005001). Available: www.statcan.ca/english/research/82-620-MIE/2005001/pdf/cobesity.pdf (accessed 2007 Jan 9).
- 2.Willms JD, Tremblay MS, Katzmarzyk PT. Geographic and demographic variation in the prevalence of overweight Canadian children. Obes Res 2003;11:668-73. [DOI] [PubMed]
- 3.Birmingham CL, Muller JL, Palepu A, et al. The cost of obesity in Canada. CMAJ 1999;160:483-8. [PMC free article] [PubMed]
- 4.The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. Bethesda Md.: National Institutes of Health; 2000. Publ. no. 00-4084. Available: www.nhlbi.nih.gov/guidelines/obesity/prctgd_b.pdf (accessed 2006 Dec 27).
- 5.Dickey RA, Bartuska DG, Bray GW, et al. ACCE/ACE position statement on the prevention, diagnosis, and treatment of obesity. Endocr Pract 1998;4(5):297-330. Available: www.aace.com/pub/pdf/guidelines/obesityguide.pdf (accessed 2007 Jan 20).
- 6.Lyznicki JM, Young DC, Riggs JA, et al. Obesity: assessment and management in primary care. Am Fam Physician 2001;63(11):2185-96. [PubMed]
- 7.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;(246):1-190. [PubMed]
- 8.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:933-49. [DOI] [PubMed]
- 9.Leiter LA, Abbott D, Campbell NR, et al. Lifestyle modifications to prevent and control hypertension. 2. Recommendations on obesity and weight loss. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ 1999;160(9 Suppl):S7-12. [PMC free article] [PubMed]
- 10.Genest J, Frohlich J, Fodor G, et al. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: summary of the 2003 update. CMAJ 2003;169:921-4. [PMC free article] [PubMed]
- 11.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96. [DOI] [PubMed]
- 12.Wetterling T. Body weight gain with atypical antipsychotics. A comparative review. Drug Saf 2001;24:59-73. [DOI] [PubMed]
- 13.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation 2005;111:1999-2012. [DOI] [PubMed]
- 14.Australian National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in children and adolescents. Canberra, Commonwealth of Australia: Australian National Health and Medical Research Council; 2003. Available: www.health.gov.au/internet/wcms/Publishing.nsf/Content/obesityguidelines-guidelines-children.htm/$FILE/children.pdf (accessed 2006 Dec 27).
- 15.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2003:27(Suppl 2):S1-152. [DOI] [PubMed]
- 16.Kavey RE, Daniels SR, Lauer RM, et al. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 2003;107:1562-6. [DOI] [PubMed]
- 17.Chagnac A, Weinstein T, Herman M, et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 2003;14:1480-6. [DOI] [PubMed]
- 18.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001;59:1498-509. [DOI] [PubMed]
- 19.Van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000;152: 514-27. [DOI] [PubMed]
- 20.Roberts RE, Kaplan GA, Shema SJ, et al. Are the obese at greater risk for depression? Am J Epidemiol 2000;152:163-70. [DOI] [PubMed]
- 21.Roberts RE, Deleger S, Strawbridge WJ, et al. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord 2003;27:514-21. [DOI] [PubMed]
- 22.Pine DS, Goldstein RB, Wolk S, et al. The association between childhood depression and adulthood body mass index. Pediatrics 2001;107:1049-56. [DOI] [PubMed]
- 23.Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics 2002;110:497-504. [DOI] [PubMed]
- 24.DiPietro L, Anda RF, Williamson DF, et al. Depressive symptoms and weight change in a national cohort of adults. Int J Obes Relat Metab Disord 1992;16:745-53. [PubMed]
- 25.Richardson LP, Davis R, Poulton R, et al. A longitudinal evaluation of adolescent depression and adult obesity. Arch Pediatr Adolesc Med 2003;157:739-45. [DOI] [PubMed]
- 26.Ziegelstein RC, Fauerbach JA, Stevens SS, et al. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000;160:1818-23. [DOI] [PubMed]
- 27.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for non-compliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101-7. [DOI] [PubMed]
- 28.Linde JA, Jeffery RW, Levy RL, et al. Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int J Obes Relat Metab Disord 2004;28:418-25. [DOI] [PubMed]
- 29.Sherwood NE, Jeffery RW, Wing RR. Binge status as a predictor of weight loss treatment outcome. Int J Obes Relat Metab Disord 1999;23:485-93. [DOI] [PubMed]
- 30.Unutzer J, Katon W, Williams JW Jr, et al. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care 2001;39:785-99. [DOI] [PubMed]
- 31.Williams JW Jr, Katon W, Lin EH, et al. The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med 2004; 140: 1015-24. [DOI] [PubMed]
- 32.Sikand G, Kondo A, Foreyt JP, et al. Two-year follow-up of patients treated with a very-low-calorie diet and exercise training. J Am Diet Assoc 1988;88:487-8. [PubMed]
- 33.Pavlou KN, Krey S, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am J Clin Nutr 1989;49(5 Suppl):1115-23. [DOI] [PubMed]
- 34.Eliakim A, Kaven G, Berger I, et al. The effect of a combined intervention on body mass index and fitness in obese children and adolescents — a clinical experience. Eur J Pediatr 2002;161:449-54. [DOI] [PubMed]
- 35.Saelens BE, Sallis JF, Wilfley DE, et al. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res 2002;10:22-32. [DOI] [PubMed]
- 36.Nemet D, Barkan S, Epstein Y, et al. Short-and long-term beneficial effects of a combined dietary-behavioral-physical activity intervention for the treatment of childhood obesity. Pediatrics 2005;115:e443-9. [DOI] [PubMed]
- 37.Epstein LH, McCurley J, Wing RR, et al. Five-year follow-up of family-based behavioral treatments for childhood obesity. J Consult Clin Psychol 1990;58:661-4. [DOI] [PubMed]
- 38.Torjesen PA, Birkeland KI, Anderssen SA, et al. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care 1997;20:26-31. [DOI] [PubMed]
- 39.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346: 393-403. [DOI] [PMC free article] [PubMed]
- 40.Lindstrom J, Eriksson JG, Valle TT, et al. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish diabetes prevention study: results from a randomized clinical trial. J Am Soc Nephrol 2003;14(Suppl 2):S108-13. [DOI] [PubMed]
- 41.Wood PD, Stefanick ML, Williams PT, et al. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med 1991;325:461-6. [DOI] [PubMed]
- 42.Stefanick ML, Mackey S, Sheehan M, et al. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med 1998;339:12-20. [DOI] [PubMed]
- 43.Wing RR, Jeffery RW. Outpatient treatments of obesity: a comparison of methodology and clinical results. Int J Obes 1979;3:261-79. [PubMed]
- 44.Bennett GA. Behaviour therapy for obesity: a quantitative review of the effects of selected treatment characteristics on outcome. Behav Ther 1986;17:554-62.
- 45.Summerbell CD, Ashton V, Campbell KJ, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev 2003;(3):CD001872. [DOI] [PubMed]
- 46.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. [DOI] [PubMed]
- 47.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001;134:1-11. [DOI] [PubMed]
- 48.Kuller LH, Simkin-Silverman LR, Wing RR, et al. Women's Healthy Lifestyle Project: a randomized clinical trial: results at 54 months. Circulation 2001;103:32-7. [DOI] [PubMed]
- 49.Wonderlich SA, de Zwaan M, Mitchell JE, et al. Psychological and dietary treatments of binge eating disorder: conceptual implications. Int J Eat Disord 2003;34:S58-73. [DOI] [PubMed]
- 50.Pendleton VR, Goodrick GK, Poston WS, et al. Exercise augments the effects of cognitive-behavioral therapy in the treatment of binge eating. Int J Eat Disord 2002;31:172-84. [DOI] [PubMed]
- 51.Gorin AA, Le Grange D, Stone AA. Effectiveness of spouse involvement in cognitive behavioral therapy for binge eating disorder. Int J Eat Disord 2003;33:421-33. [DOI] [PubMed]
- 52.Agras WS, Telch CF, Arnow B, et al. One-year follow-up of cognitive-behavioral therapy for obese individuals with binge eating disorder. J Consult Clin Psychol 1997;65:343-7. [DOI] [PubMed]
- 53.Eldredge KL, Stewart Agras W, Arnow B, et al. The effects of extending cognitive-behavioral therapy for binge eating disorder among initial treatment nonresponders. Int J Eat Disord 1997;21:347-52. [DOI] [PubMed]
- 54.Brehm BJ, Seeley RJ, Daniels SR, et al. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab 2003;88:1617-23. [DOI] [PubMed]
- 55.Astrup A, Grunwald GK, Melanson EL, et al. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord 2000;24:1545-52. [DOI] [PubMed]
- 56.Due A, Toubro S, Skov AR, et al. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord 2004;28:1283-90. [DOI] [PubMed]
- 57.Ashley JM, St Jeor ST, Perumean-Chaney S, et al. Meal replacements in weight intervention. Obes Res 2001;9:312S-20S. [DOI] [PubMed]
- 58.Heaney RP, Davies KM, Barger-Lux MJ. Calcium and weight: clinical studies. J Am Coll Nutr 2002;21:152S-5S. [DOI] [PubMed]
- 59.Bravata DM, Sanders L, Huang J, et al. Efficacy and safety of low-carbohydrate diets: a systematic review. JAMA 2003;289:1837-50. [DOI] [PubMed]
- 60.Raben A. Should obese patients be counselled to follow a low-glycaemic index diet? No. Obes Rev 2002;3:245-56. [DOI] [PubMed]
- 61.Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA 2003;290:1323-30. [DOI] [PubMed]
- 62.Pritchard JE, Nowson CA, Wark JD. A worksite program for overweight middle-aged men achieves lesser weight loss with exercise than with dietary change. J Am Diet Assoc 1997;97:37-42. [DOI] [PubMed]
- 63.Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr 1995;49:1-10. [PubMed]
- 64.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord 1997;21:941-7. [DOI] [PubMed]
- 65.Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain — a systematic review. Obes Rev 2000;1:95-111. [DOI] [PubMed]
- 66.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE — a randomized controlled study. Arch Intern Med 2004;164:31-9. [DOI] [PubMed]
- 67.Reilly JJ, McDowell ZC. Physical activity interventions in the prevention and treatment of paediatric obesity: systematic review and critical appraisal. Proc Nutr Soc 2003;62:611-9. [DOI] [PubMed]
- 68.Epstein LH, Valoski AM, Vara LS, et al. Effects of decreasing sedentary behavior and increasing activity on weight change in obese children. Health Psychol 1995;14:109-15. [DOI] [PubMed]
- 69.Epstein LH, Paluch RA, Gordy CC, et al. Decreasing sedentary behaviors in treating pediatric obesity. Arch Pediatr Adolesc Med 2000;154:220-6. [DOI] [PubMed]
- 70.Epstein LH, Wing RR, Koeske R, et al. A comparison of lifestyle exercise, aerobic exercise, and calisthenics on weight loss in obese children. Behav Ther 1985; 16:345-56.
- 71.Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155-61. [DOI] [PubMed]
- 72.Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab 2002;4:415-23. [DOI] [PubMed]
- 73.Finer N, James WP, Kopelman PG, et al. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord 2000;24:306-13. [DOI] [PubMed]
- 74.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA 1999;281:235-42. [DOI] [PubMed]
- 75.Sjostrom L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 1998;352:167-72. [DOI] [PubMed]
- 76.James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet 2000;356:2119-25. [DOI] [PubMed]
- 77.Bakris G, Calhoun D, Egan B, et al. Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. J Hypertens 2002;20:2257-67. [DOI] [PubMed]
- 78.Kelley DE, Bray GA, Pi-Sunyer FX, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 2002;25:1033-41. [DOI] [PubMed]
- 79.Miles JM, Leiter L, Hollander P, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care 2002;25:1123-8. [DOI] [PubMed]
- 80.Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care 1998;21:1288-94. [DOI] [PubMed]
- 81.McNulty SJ, Ur E, Williams G. A randomized trial of sibutramine in the management of obese type 2 diabetic patients treated with metformin. Diabetes Care 2003;26:125-31. [DOI] [PubMed]
- 82.Sanchez-Reyes L, Fanghanel G, Yamamoto J, et al. Use of sibutramine in overweight adult hispanic patients with type 2 diabetes mellitus: a 12-month, randomized, double-blind, placebo-controlled clinical trial. Clin Ther 2004;26:1427-35. [DOI] [PubMed]
- 83.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [DOI] [PubMed]
- 84.Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg 2001;234:279-89. [DOI] [PMC free article] [PubMed]
- 85.Paranjpe P, Patki P, Patwardhan B. Ayurvedic treatment of obesity: a randomised double-blind, placebo-controlled clinical trial. J Ethnopharmacol 1990;29:1-11. [DOI] [PubMed]
- 86.Karlsson C, Stenlof K, Johannsson G, et al. Effects of growth hormone treatment on the leptin system and on energy expenditure in abdominally obese men. Eur J Endocrinol 1998;138:408-14. [DOI] [PubMed]
- 87.Lacey JM, Tershakovec AM, Foster GD. Acupuncture for the treatment of obesity: a review of the evidence. Int J Obes Relat Metab Disord 2003;27:419-27. [DOI] [PubMed]
- 88.Mhurchu CN, Poppitt SD, McGill AT, et al. The effect of the dietary supplement, Chitosan, on body weight: a randomised controlled trial in 250 overweight and obese adults. Int J Obes Relat Metab Disord 2004;28:1149-56. [DOI] [PubMed]
- 89.Bahadori B, Wallner S, Schneider H, et al. Effect of chromium yeast and chromium picolinate on body composition of obese, non-diabetic patients during and after a formula diet. Acta Med Austriaca 1997;24:185-7. [PubMed]
- 90.Boozer CN, Daly PA, Homel P, et al. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int J Obes Relat Metab Disord 2002;26:593-604. [DOI] [PubMed]
- 91.Pasman WJ, Westerterp-Plantenga MS, Saris WH. The effectiveness of long-term supplementation of carbohydrate, chromium, fibre and caffeine on weight maintenance. Int J Obes Relat Metab Disord 1997;21:1143-51. [DOI] [PubMed]
- 92.Johannsson G, Marin P, Lonn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 1997;82:727-34. [DOI] [PubMed]
- 93.Albert SG, Mooradian AD. Low-dose recombinant human growth hormone as adjuvant therapy to lifestyle modifications in the management of obesity. J Clin Endocrinol Metab 2004;89:695-701. [DOI] [PubMed]
- 94.Herrmann BL, Berg C, Vogel E, et al. Effects of a combination of recombinant human growth hormone with metformin on glucose metabolism and body composition in patients with metabolic syndrome. Horm Metab Res 2004;36:54-61. [DOI] [PubMed]
- 95.Pittler MH, Abbot NC, Harkness EF, et al. Randomized, double-blind trial of chitosan for body weight reduction. Eur J Clin Nutr 1999;53:379-81. [DOI] [PubMed]
- 96.Bray GA, Greenway FL. Current and potential drugs for treatment of obesity. Endocr Rev 1999;20:805-75. [DOI] [PubMed]
- 97.Ernst E, Pittler MH. Chitosan as a treatment for body weight reduction: a meta-analysis. Perfusion 1998;11:461-5.
- 98.Jordan J, Sharma AM. Potential for sibutramine-yohimbine interaction? Lancet 2003;361:1826. [DOI] [PubMed]
- 99.Gross PA, Greenfield S, Cretin S, et al. Optimal methods for guideline implementation: conclusions from Leeds Castle meeting. Med Care 2001;39(Suppl 2):II85-92. [PubMed]
- 100.Chalmers J. Implementation of guidelines for management of hypertension. Clin Exp Hypertens 1999;21:647-57. [DOI] [PubMed]
- 101.Connor H, Annan F, Bunn E, et al. The implementation of nutritional advice for people with diabetes. Diabet Med 2003;20:786-807. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



