Abstract
Heart failure frequently involves diastolic dysfunction that is characterized by a prolonged relaxation. This prolonged relaxation is typically the result of a decreased rate of intracellular Ca2+ sequestration. No effective treatment for this decreased Ca2+ sequestration rate currently exists. As an approach to possibly correct diastolic dysfunction, we hypothesized that expression of the Ca2+ binding protein parvalbumin in cardiac myocytes would lead to increased rates of Ca2+ sequestration and mechanical relaxation. Parvalbumin, which is normally absent in cardiac tissue, is known to act as a soluble relaxing factor in fast skeletal muscle fibers by acting as a delayed Ca2+ sink. As a test of the hypothesis, gene transfer was used to express parvalbumin in isolated adult cardiac myocytes. We report here that expression of parvalbumin dramatically increases the rate of Ca2+ sequestration and the relaxation rate in normal cardiac myocytes. Importantly, parvalbumin fully restored the relaxation rate in diseased cardiac myocytes isolated from an animal model of human diastolic dysfunction. These findings indicate that parvalbumin gene transfer offers unique potential as a possible direct treatment for diastolic dysfunction in failing hearts.
Heart failure is a leading cause of hospitalization in the U.S., diagnosed in ≈700,000 new patients annually (1). About a third of all heart failure involves diastolic dysfunction (2), which frequently is the result of an inappropriate prolongation of the intracellular Ca2+ transients that trigger cardiac muscle contraction (3–6). Consequently, the heart fails to relax appropriately between contractions, leading to an increased stiffness of the myocardium during diastole and thereby generating excessive resistance to filling. Currently there is no clinical treatment that directly ameliorates this prolonged Ca2+ transient. Recent research into alleviating this form of diastolic dysfunction has centered primarily on increasing the rate of Ca2+ uptake by the sarcoplasmic reticulum (SR), either through increased SR Ca2+ pumping rates or increased expression of the SR pump. These are energy-dependent approaches, however, and energy production in failing hearts also is frequently impaired (5, 6). As a different approach, we used adenoviral-mediated gene transfer to express the soluble relaxing factor parvalbumin in cardiac myocytes. Parvalbumin, which is not naturally found in cardiac tissue, is a Ca2+ binding protein whose Ca2+ affinity is intermediate between the thin filament regulatory protein troponin C and the SR Ca2+ pump. This intermediate Ca2+ affinity allows parvalbumin to enhance the rate of relaxation in fast skeletal muscle by acting as a Ca2+ sink to temporarily store Ca2+ before SR uptake (7–9). Relaxation thus becomes dependent on the rate of Ca2+ binding to parvalbumin, independent of energy status, rather than dependent on the slower, energy requiring SR Ca2+ uptake (see Fig. 2A, Inset). Parvalbumin is thought to be complexed to Mg2+ at rest, hence Ca2+ released by the SR initially binds to troponin C because Ca2+ binding to parvalbumin is delayed by the Mg2+ off rate (10, 11). Because parvalbumin acts as a delayed Ca2+ sink, there has been some controversy over the ability of parvalbumin to alter the kinetics of a single twitch in skeletal muscle (10–12). Therefore, it is of great interest to determine whether parvalbumin can produce an increase in relaxation rate in cardiac muscle and thereby improve diastolic function.
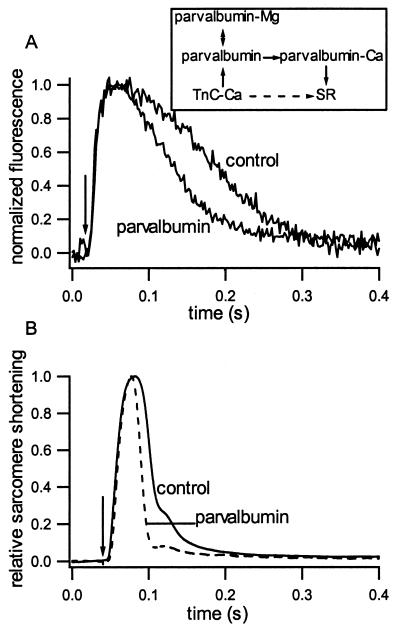
Figure 2.
Fluo-3 fluorescence and mechanical properties in cardiac myocytes. (A) Example of fluorescence intensity vs. time at 37°C. Arrow indicates time of electrical stimulation. (Inset) Schematic of Ca2+ movements during relaxation. Normally, Ca2+ moves from Troponin C (TnC) directly to the SR (dashed arrow). In the presence of parvalbumin, Ca2+ coming off TnC exchanges with Mg2+ bound to parvalbumin where it is temporarily stored before uptake by the SR (solid arrows). (B) Sarcomere movement vs. time as measured by first-order laser diffraction at 37°C from representative control and parvalbumin-expressing myocytes.
Materials and Methods
Myocyte Isolation, Gene Transfer, and Electrical Stimulation.
Intact myocytes were isolated from normal and hypothyroid adult female Sprague–Dawley rats by enzymatic digestion as described (13). Rats were made hypothyroid by adding 0.6% propylthiouracil to the drinking water for a minimum of 4 weeks before myocyte isolation. Adult cardiac myocytes were plated on laminin-coated glass coverslips in DMEM + 5% serum + 50 units/ml penicillin, 50 μg/ml streptomycin (P/S) at 1 × 105 cells/ml for 2 hr. Myocytes then were incubated with adenovirus at a multiplicity of infection (moi) of 500 (pfu/cell) diluted in serum-free DMEM, P/S. Optimal parvalbumin expression occurred with 500 mois AdαParv on day 4 after cell isolation, and these conditions were adopted for all experiments. Recombinant adenovirus construction and purification was as described (13). Myocytes were electrically stimulated in media 199 supplemented with 0.2 mg/ml BSA, P/S, and 5 mM glutathione, which was replaced every 8–12 hr. The stimulation chamber consisted of a series of wells milled into a plastic slab with a glass bottom, and field stimulation was provided by platinum electrodes. Myocyte stimulation began the morning after isolation and was continuous with a 2.5-ms pulse at 0.5 Hz at a voltage that produced twitches in >50% of the myocytes.
Detection of Parvalbumin Expression.
Western blot analysis was performed as described (13) with a parvalbumin antibody (PARV19, Sigma) titer of 1:1,000 or anti actin (5c5, Sigma) at 1:100,000. For indirect immunofluorescence, cultured myocytes were fixed in 3% paraformaldehyde/PBS for 30 min, washed, and incubated in PBS + 50 mM NH4Cl for 30 min. Primary (PARV19, 1:500, Sigma) and secondary (anti-mouse IgG-Texas Red, 1:100, Molecular Probes) antibodies were diluted in 2% goat serum in PBS + 0.5% Triton X-100. Nonspecific binding was blocked by incubation in 20% normal goat serum/PBS +0.5% Triton X-100. To visualize actin, FITC-phalloidin was dissolved in DMSO and applied to myocytes for 20 min at a dilution of 1:1,000. Samples were examined on a Leitz Aristoplan microscope outfitted with a Sony digital camera.
Ca2+ Transient Measurements.
Fluo-3 AM solutions were made fresh by dissolving 50 μg fluo-3 AM (Molecular Probes) in 10 μl DMSO and diluting to 5 μM in Krebs-Henseleit buffer (KHB) containing 5 mM glutathione, 1.8 mM CaCl2, 118 mM NaCl, 4.8 mM KCl, 25 mM Hepes, 1.2 mM KH2PO4, 1.2 mM MgSO4, and 11 mM glucose. Coverslips with plated myocytes were mounted in a chamber similar to that used for the laser diffraction studies. After mounting, the coverslip was washed briefly with KHB followed by the fluo-3 AM solution for loading at 37°C. Myocytes were loaded with fluo-3 AM for 10 min and washed with KHB, and an additional 20 min was allowed for de-esterification. Twitches were generated by a 5-ms square pulse electric field stimulus. Fluorescence was stimulated by an argon laser at 488 nm and collected through a 500/25 barrier pass filter. Images were collected at 480 Hz on a Noran Instruments (Middleton, WI) confocal imaging system. The fluorescence intensity was averaged over the entire myocyte in each image and plotted vs. time. A signal average from three stimulations was analyzed for each myocyte.
Laser Diffraction and Mechanical Measurements.
The diffraction chamber consisted of a temperature-controlled microscope stage, and a coverslip containing cultured myocytes formed the bottom of the chamber through which the output of a 10-mW HeNe laser was focused by using an achromatic lens. Platinum electrodes provided a 5-ms square pulse electrical stimulation. A myocyte was positioned in the laser beam and the first-order diffraction line focused with a cylindrical lens onto a linear position detector (LSC 5D or 30D, United Detector Technology). The output of the detector was amplified and recorded on a digital oscilloscope at 5,000 Hz. The average movement from 10 twitches per myocyte was used for subsequent analysis. After the above protocol, the detector was removed and a viewing screen inserted in the beam path. The distance from the zero to the first-order diffraction pattern was used to estimate the initial sarcomere length and, when possible, the amplitude of the sarcomere length change after electrical stimulation. The time from stimulation to maximum shortening (tpeak) and from maximum shortening to 1/2 relengthening (t1/2R) were measured from the average trace for each myocyte. The maximum rate of shortening and relengthening (+ and − dl/dtmax, respectively) were calculated and normalized to the maximum shortening amplitude.
Results and Discussion
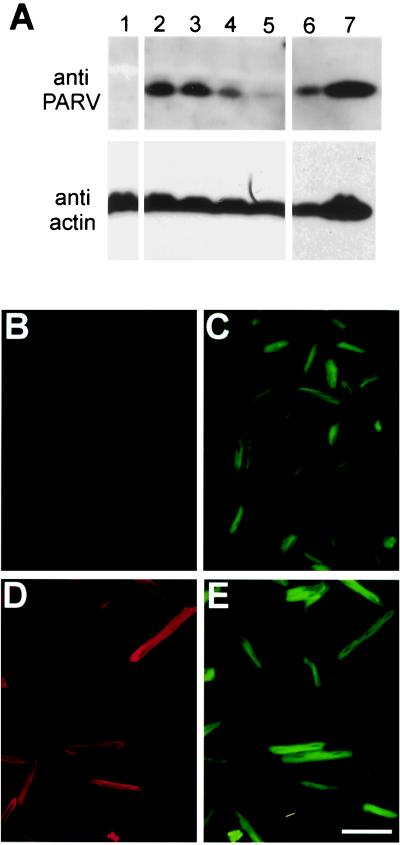
An adenoviral vector (AdαParv) containing a parvalbumin expression cassette was used for gene transfer into isolated adult cardiac myocytes. The expression cassette consisted of a cytomegalovirus promoter driving expression of a full-length human α-parvalbumin cDNA with a simian virus 40 polyadenylation signal. Isolated adult cardiac myocytes were plated, treated with AdαParv or AdLacZ (500 mois), and electrically stimulated in primary culture for 4 days. Western blots from myocytes collected on day 4 after gene transfer are shown in Fig. 1A. Quantitative analysis of the parvalbumin/actin ratio indicates that AdαParv-treated myocytes express ≈50% of the parvalbumin content of rat fast twitch superior vastus lateralis (rat superior vastus lateralis expresses ≈0.45 mM parvalbumin; ref. 14). This parvalbumin/actin ratio puts parvalbumin expression in these myocytes in the range of published parvalbumin expression levels for the rat fast twitch extensor digitorum longus (14). Immunofluorescence microscopy showed parvalbumin expression was detected at a similar level in ≈95% of myocytes with uniform expression throughout the cell (Fig. 1D). This pattern of parvalbumin expression is as expected for a cytoplasmic protein. The high level of uniform expression indicates that individual myocytes selected for mechanical study had a high probability of expressing parvalbumin at a level similar to that observed on the Western blots.
Figure 1.
Parvalbumin expression in adult cardiac myocytes after AdαParv gene transfer. (A) Western blot showing parvalbumin expression in cardiac myocytes. Lane 1: control. Lanes 2–5: dose response with 500, 150, 50, and 15 mois AdαParv. Lanes 6 and7: superior vastus lateralis at two dilutions. Relative loading indicated by stripping and reprobing blots with actin antibody. (B–E) Indirect immunofluorescence of control myocytes (B and C) and myocytes treated with AdαParv at 500 mois (D and E). Cells have been stained with anti-parvalbumin followed by goat anti-mouse IgG-Texas Red secondary antibody (B and D). FITC phalloidin was used to visualize actin (C and E). (Scale bar = 100 μm.)
Discrepancies in the literature regarding the ability of parvalbumin to enhance relaxation from a single twitch in fast skeletal muscle (10–12), and known differences in Ca2+ handling between skeletal and cardiac muscle (15), necessitated the direct testing of the effect of parvalbumin expression on the Ca2+ transient by using the fluorescent Ca2+ indicator fluo-3. The rate of fluorescence decay at 37°C was dramatically faster in myocytes expressing parvalbumin than in control (Fig. 2A) or AdLacZ-treated myocytes, with the time from peak to 50% fluorescence decay decreasing from 136.0 ± 5.7 ms (n = 18) in control and 129.2 ± 7.5 with lacZ (n = 13) to 74.6 ± 7.6 ms (n = 10) with parvalbumin. Additionally, parvalbumin expression significantly decreased the time from stimulus to peak Ca2+ in these myocytes from 54.5 ± 3.2 ms in control and 63.8 ± 4.9 with lacZ to 39.8 ± 2.6 ms with parvalbumin expression. Also, no change was observed in the amplitude of the Ca2+ transient with either parvalbumin or lacZ gene transfer (data not shown). This is evidence that parvalbumin can act as a delayed Ca2+ sink during a single twitch in cardiac myocytes to increase the Ca2+ transient decay rate.
Although parvalbumin increases the Ca2+ transient decay rate, parvalbumin also must improve the mechanical relaxation of the myocyte to be a potential treatment for diastolic dysfunction in heart failure. The mechanical response to stimulation was studied by using laser diffraction to follow sarcomere motion. An example of the change in the first-order diffraction position in response to a single stimulation is shown in Fig. 2B, and values for tpeak, t1/2R, +dl/dtmax, and −dl/dtmax are given in Table 1. Parvalbumin expressing myocytes showed a significantly faster relaxation (t1/2R, −dl/dtmax) than control myocytes, whereas the shortening parameters (tpeak, +dl/dtmax) were not significantly changed. The shortening amplitude at 37°C also was unaffected by parvalbumin expression (data not shown). Thus, parvalbumin expression enhances the mechanical relaxation in adult cardiac myocytes without compromising the shortening capacity of the cell. The response at 25°C also was examined to verify that parvalbumin is responsible for the change in kinetics. This analysis is possible because the temperature sensitivity of the SR Ca2+ uptake [Q10 = 3–5 (estimated from rat soleus and rabbit skeletal muscle), refs. 17–19] is greater than that of Ca2+ binding to parvalbumin (as limited by the Mg2+ off rate, Q10 = ≈1.9; ref. 16). Therefore, by comparing mechanical experiments at both 25°C and 37°C, it is possible to verify that relaxation is caused by the specific Ca2+ removal mechanism. Comparison of the results with different temperatures (Table 1) indicates that t1/2R and −dl/dtmax become less temperature sensitive in myocytes expressing parvalbumin than in control myocytes (with Q10 for t1/2R decreasing from 3.0 to 2.4 in control and parvalbumin-expressing myocytes, respectively). Given the differences in temperature sensitivity between the rat SR Ca2+ pump and human parvalbumin, the temperature results provide further evidence that the twitch relaxation becomes dominated by Ca2+ binding to parvalbumin instead of being taken up by the SR (Fig. 2A, Inset).
Table 1.
Parameters of sarcomere shortening and relengthening as measured by laser diffraction
| tpeak, ms | t1/2R, ms | +dl/dtmax | −dl/dtmax | n | |
|---|---|---|---|---|---|
| 37°C | |||||
| Control | 47.1 ± 1.7 | 27.9 ± 1.5 | 12.51 ± 0.76 | 7.74 ± 0.52 | 48 |
| Parvalbumin | 43.0 ± 2.2 | 21.1 ± 1.7* | 12.77 ± 0.67 | 10.61 ± 0.64* | 43 |
| lacZ | 51.9 ± 2.0 | 34.1 ± 2.9 | 13.47 ± 0.77 | 7.77 ± 0.75 | 32 |
| 25°C | |||||
| Control | 148.5 ± 5.7 | 105.7 ± 6.4 | 5.16 ± 0.29 | 2.48 ± 0.15 | 40 |
| Parvalbumin | 138.9 ± 5.4 | 61.3 ± 3.2* | 4.58 ± 0.24 | 3.77 ± 0.18* | 45 |
| lacZ | 146.0 ± 7.9 | 100.9 ± 11.6 | 4.94 ± 0.45 | 3.02 ± 0.26 | 24 |
Values given as mean ± SEM.
Significantly different from control and lacZ (one-way ANOVA, p < 0.05).
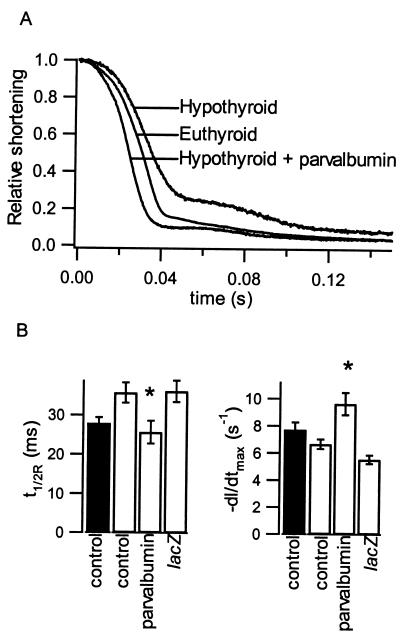
The increased relaxation kinetics seen in normal myocytes demonstrates the potential for parvalbumin expression to ameliorate the compromised diastolic function in diseased states. To test this possibility more directly, we expressed parvalbumin in myocytes isolated from hypothyroid adult rats. The hypothyroid state leads to diastolic dysfunction characterized by a decrease in the SR Ca2+ pump expression and, consequently, a significantly prolonged Ca2+ transient and twitch duration (20, 21). Thus, the hypothyroid rat is an excellent model for human diastolic dysfunction. Additionally, the hypothyroid rat model is uncomplicated by structural defects, such as fibrosis, which could obscure the Ca2+ response. As expected by an increase in the duration of the Ca2+ transient (20, 21), myocytes from hypothyroid rats exhibited a significantly slower t1/2R than euthyroid myocytes at 37°C (Fig. 3 A and B). The tpeak and +dl/dtmax also are slowed, likely the result of the myosin isoform shift produced by hypothyroidism (20, 21). Most importantly, parvalbumin expression in myocytes isolated from hypothyroid rats leads to a substantial decrease in t1/2R and an increase in −dl/dtmax, fully correcting the diastolic dysfunction in these myocytes (Fig. 3B).
Figure 3.
Effects of parvalbumin expression on diseased cardiac myocyte relaxation rates. (A) Sarcomere relaxation records from representative control and parvalbumin-expressing myocytes isolated from euthyroid and hypothyroid rats. (B) Relaxation parameters from control and parvalbumin-expressing myocytes from hypothyroid rats (open bars) at 37°C. * indicates significantly different from hypothyroid control and lacZ. Parameters from control euthyroid rat myocytes (filled bars) included for comparison. Differences in sarcomere shortening parameters for hypothyroid myocytes were qualitatively similar to euthyroid rat myocyte parameters (Table 1) with tpeak = 68.1 ± 2.9, 53.9 ± 3.2*, 78.7 ± 3.4*, and +dl/dtmax = 7.36 ± 0.43, 8.98 ± 0.44, 6.58 ± 0.49 (mean ± SEM) for control (n = 26), parvalbumin- (n = 20), and lacZ- (n = 32) expressing hypothyroid myocytes. * indicates significantly different from hypothyroid control (P < 0.05).
The ability of parvalbumin expression to correct diastolic dysfunction, as demonstrated in this report, should not be limited to the hypothyroid rat model used here. Although parvalbumin is not expected to fully correct dysfunction caused by fibrosis or other structural abnormalities, parvalbumin may be expected to be an effective new therapeutic approach in any dysfunction characterized by an increase in diastolic Ca2+ levels. Thus, when coupled with emerging in vivo gene delivery systems, parvalbumin expression has unique potential as a new tool to correct diastolic dysfunction in failing hearts.
Acknowledgments
We thank C. W. Heizmann for the gift of human parvalbumin cDNA and F. Albayya for technical assistance. This work was supported by the National Institutes of Health, the American Heart Association, and the Whitaker Foundation (to J.M.M.). J.M.M. is an Established Investigator of the American Heart Association.
Abbreviations
- SR
sarcoplasmic reticulum
- moi
multiplicity of infection
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Abraham W T, Bristow M R. Circulation. 1997;96:2755–2757. doi: 10.1161/01.cir.96.9.2755. [DOI] [PubMed] [Google Scholar]
- 2.Ruzumna P, Gheorghiade M, Bonow R O. Curr Opin Cardiol. 1996;11:269–275. doi: 10.1097/00001573-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Lorell B H. Annu Rev Med. 1991;42:411–436. doi: 10.1146/annurev.me.42.020191.002211. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt U, Hajjar R J, Helm P A, Kim C S, Doye A A, Gwathmey J K. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 5.Morgan J P. N Engl J Med. 1991;325:625–632. doi: 10.1056/NEJM199108293250906. [DOI] [PubMed] [Google Scholar]
- 6.Liao R, Helm P A, Hahhar R J, Saha C, Gwathmey J K. Yale J Biol Med. 1995;67:247–264. [PMC free article] [PubMed] [Google Scholar]
- 7.Hou T-T, Johnson J D, Rall J A. J Physiol. 1992;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lännergren J, Elzinga G, Stienen G J M. J Physiol. 1993;463:123–140. doi: 10.1113/jphysiol.1993.sp019587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müntener M, Käser L, Weber J, Berchtold M W. Proc Natl Acad Sci USA. 1995;92:6504–6508. doi: 10.1073/pnas.92.14.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillis J M, Thomason D, Lefèvre J, Kretsinger R H. J Muscle Res Cell Motil. 1982;3:377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- 11.Cannell M B, Allen D G. Biophys J. 1984;45:913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson S P, Johnson J D, Potter J D. Biophys J. 1981;34:559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westfall M V, Rust E M, Albayya F, Metzger J M. Methods Cell Biol. 1998;52:307–322. [PubMed] [Google Scholar]
- 14.Green H J, Klug G A, Reichmann H, Seedorf U, Wiehrer W, Pette D. Pflügers Arch. 1984;400:432–438. doi: 10.1007/BF00587545. [DOI] [PubMed] [Google Scholar]
- 15.Bers D M. Med Sci Sports Exercise. 1991;23:1157–1162. [PubMed] [Google Scholar]
- 16.Hou T-T, Johnson J D, Rall J A. J Physiol. 1992;449:399–410. doi: 10.1113/jphysiol.1992.sp019092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein R B, Gordon T, Shriver J. Biophys J. 1982;40:97–107. doi: 10.1016/S0006-3495(82)84464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puglisi J L, Bassani R A, Bassani J W M, Amin J N, Bers D M. Am J Physiol. 1996;270:H1772–H1778. doi: 10.1152/ajpheart.1996.270.5.H1772. [DOI] [PubMed] [Google Scholar]
- 19.Masuda H, De Meis L. J Biol Chem. 1977;252:8567–8571. [PubMed] [Google Scholar]
- 20.Holubarsch Ch, Goulette R P, Litten R Z, Martin B J, Mulieri L A, Alpert N R. Circ Res. 1985;56:78–86. doi: 10.1161/01.res.56.1.78. [DOI] [PubMed] [Google Scholar]
- 21.MacKinnon R, Gwathmey J K, Allen P D, Briggs G M, Morgan J P. Circ Res. 1988;63:1080–1089. doi: 10.1161/01.res.63.6.1080. [DOI] [PubMed] [Google Scholar]