Abstract
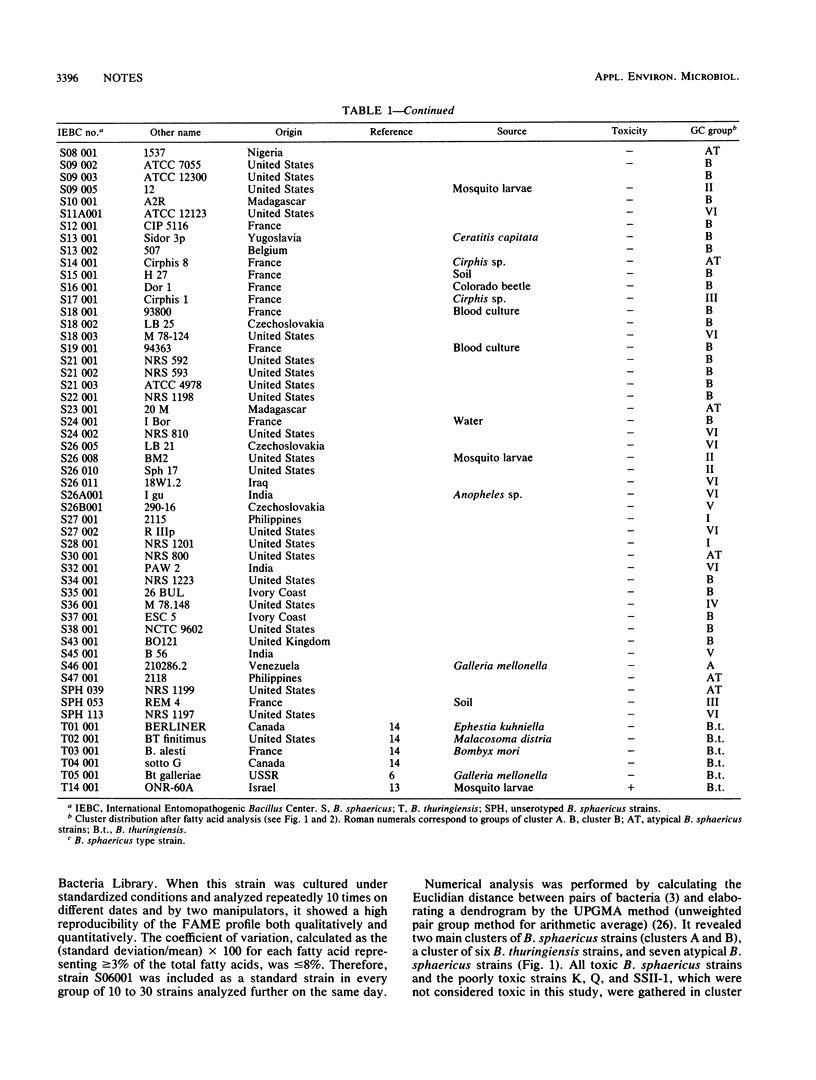
Gas-liquid chromatography of fatty acid methyl esters and numerical analysis were carried out with 114 Bacillus sphaericus strains. Since only two clusters harbored mosquitocidal strains, this technique could be developed in screening programs to limit bioassays on mosquito larvae. It also allows differentiation of highly homologous strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry C., Jackson-Yap J., Oei C., Hindley J. Nucleotide sequence of two toxin genes from Bacillus sphaericus IAB59: sequence comparisons between five highly toxinogenic strains. Nucleic Acids Res. 1989 Sep 25;17(18):7516–7516. doi: 10.1093/nar/17.18.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann L., Baumann P. The 42- and 51-kilodalton mosquitocidal proteins of Bacillus sphaericus 2362: construction of recombinants with enhanced expression and in vivo studies of processing and toxicity. J Bacteriol. 1990 May;172(5):2217–2223. doi: 10.1128/jb.172.5.2217-2223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøe B., Gjerde J. Fatty acid patterns in the classification of some representatives of the families Enterobacteriaceae and Vibrionaceae. J Gen Microbiol. 1980 Jan;116(1):41–49. doi: 10.1099/00221287-116-1-41. [DOI] [PubMed] [Google Scholar]
- Dees S. B., Powell J., Moss C. W., Hollis D. G., Weaver R. E. Cellular fatty acid composition of organisms frequently associated with human infections resulting from dog bites: Pasteurella multocida and groups of EF-4, IIj, M-5, and DF-2. J Clin Microbiol. 1981 Dec;14(6):612–616. doi: 10.1128/jcm.14.6.612-616.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIMPEL A. M., ANGUS T. A. The taxonomy of insect pathogens related to Bacillus cereus Frankland and Frankland. Can J Microbiol. 1958 Oct;4(5):531–541. doi: 10.1139/m58-058. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol Rev. 1977 Jun;41(2):391–418. doi: 10.1128/br.41.2.391-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellen W. R., Clark T. B., Lindegren J. E., Ho B. C., Rogoff M. H., Singer S. Bacillus sphaericus Neide as a pathogen of mosquitoes. J Invertebr Pathol. 1965 Dec;7(4):442–448. doi: 10.1016/0022-2011(65)90120-5. [DOI] [PubMed] [Google Scholar]
- Lambert M. A., Patton C. M., Barrett T. J., Moss C. W. Differentiation of Campylobacter and Campylobacter-like organisms by cellular fatty acid composition. J Clin Microbiol. 1987 Apr;25(4):706–713. doi: 10.1128/jcm.25.4.706-713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko O., Davidson E. W., Lacey L. A., Yousten A. A. Five new mosquito larvicidal strains of Bacillus sphaericus from non-mosquito origins. J Am Mosq Control Assoc. 1985 Sep;1(3):369–371. [PubMed] [Google Scholar]
- Mukwaya G. M., Welch D. F. Subgrouping of Pseudomonas cepacia by cellular fatty acid composition. J Clin Microbiol. 1989 Dec;27(12):2640–2646. doi: 10.1128/jcm.27.12.2640-2646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. Insecticidal activity of recent bacterial isolates and their toxins against mosquito larvae. Nature. 1973 Jul 13;244(5411):110–111. doi: 10.1038/244110a0. [DOI] [PubMed] [Google Scholar]
- Weiser J. A mosquito-virulent Bacillus sphaericus in adult Simulium damnosum from northern Nigeria. Zentralbl Mikrobiol. 1984;139(1):57–60. [PubMed] [Google Scholar]
- Yousten A. A., de Barjac H., Hedrick J., Cosmao Dumanoir V., Myers P. Comparison between bacteriophage typing and serotyping for the differentiation of Bacillus sphaericus strains. Ann Microbiol (Paris) 1980 Nov-Dec;131B(3):297–308. [PubMed] [Google Scholar]
- de Barjac H., Bonnefoi A. Essai de classification biochimique de 64 Bacillus des groupes 2 et 3 représentant 11 espèces différentes. Ann Inst Pasteur (Paris) 1972 Mar;122(3):463–473. [PubMed] [Google Scholar]
- de Barjac H., Thiery I., Cosmao-Dumanoir V., Frachon E., Laurent P., Charles J. F., Hamon S., Ofori J. Another Bacillus sphaericus serotype harbouring strains very toxic to mosquito larvae: serotype H6. Ann Inst Pasteur Microbiol. 1988 May-Jun;139(3):363–377. doi: 10.1016/0769-2609(88)90028-2. [DOI] [PubMed] [Google Scholar]
- de Barjac H., Véron M., Cosmao Dumanoir V. Caractérisation biochimique et sérologique de souches de Bacillus sphaericus pathogènes ou non pour les moustiques. Ann Microbiol (Paris) 1980 Sep-Oct;131B(2):191–201. [PubMed] [Google Scholar]



