Abstract
We report the expression of endogenous CRF1 in COS-7 cells (African green monkey origin). Cloning of the coding region of CRF1 gene identified three alternatively spliced isoforms with nucleotide and predicted amino acid sequences corresponding to the membrane bound α and c and soluble e isoforms. DNA sequencing of the main isoform CRF1α showed homologies of 99%, 97% and 91% with the rhesus monkey, human and rodent genes, respectively; the deduced protein sequence differed in only one amino acid with Rhesus monkey and human. Western blot analysis with antibodies against human CRF1 demonstrated immunoreactive proteins with MW of 37, 52, 70 and 80–85 in crude membrane or cytoplasm preparation; two additional species of 40 and 60 kDa were detected only in the cytoplasmic fraction. On immunocytochemistry CRF1 was localized to both the cell surface and intracellularly. The receptor was functional, e.g., addition of CRF to COS-7 cells inhibited cell proliferation and stimulated release of arachidonic acid; nevertheless, it was poorly coupled to cAMP production (its stimulation was minimal in native cells). In conclusion, COS cells that are routinely used for the study of transfected CRF receptors do express endogenous CRF1 mRNA with splicing behavior similar to that reported in human and rodent cells, and translated into functional CRF1 receptors.
Keywords: CRF receptors, alternative splicing, western blot, immunocytochemistry, cell proliferation
1. Introduction
Corticotropin releasing factor (CRF) and related peptides coordinate the complex array of behavioral, autonomic and endocrine responses to systemic stress at the central level (Vale et al., 1981; Chrousos, Gold, 1992; Perrin, Vale, 1999; Hillhouse, Grammatopoulos, 2006). Moreover, the same peptides are also expressed in peripheral organs where they act locally to regulate homeostasis in situ (Chrousos, Gold, 1992; Perrin, Vale, 1999; Linton et al., 2001; Slominski et al., 2001; Hillhouse, Grammatopoulos, 2006; Slominski et al., 2006b). These diverse phenotypic effects are mediated through interaction with G protein coupled membrane bound CRF receptors (CRF1 and CRF2) (Perrin, Vale, 1999; Hillhouse, Grammatopoulos, 2006). The gene for mammalian CRF1 has been cloned in humans, rat, mouse, hamster, sheep, bovine, tree shrew, chicken and rhesus monkey (Chen et al., 1993; Vita et al., 1993; Yu et al., 1996; Myers et al., 1998; Palchaudhuri et al., 1998; Perrin, Vale, 1999; Pisarchik, Slominski, 2002; Oshida et al., 2004; Hillhouse, Grammatopoulos, 2006); and more specifically, the genes for human and rodent CRF1 contain 14 and 13 exons, respectively (Tsai-Morris et al., 1996; Sakai et al., 1998; Parham et al., 2004), and produce several alternatively spliced isoforms (reviewed in (Hillhouse, Grammatopoulos, 2006; Slominski et al., 2006b)).
The properties of CRF receptors (CRF-Rs) have been widely defined in COS-7 cells, an African green monkey kidney fibroblast-like line, in transfection experiments using constructs containing the appropriate vectors. Thus, vast information on CRF-Rs molecular biology, biochemistry and pharmacology is based on the studies performed in this cell line. We therefore used COS-7 cells to study the properties of alternatively spliced CRF1 isoforms recently cloned in skin cells (Pisarchik, Slominski, 2001; Pisarchik, Slominski, 2002; Pisarchik, Slominski, 2004). Unexpectedly, we found functionally active endogenous CRF1 in the COS-7 cells, which had properties similar to those described in human cells expressing the CRF1 (Slominski et al., 2006b). We also cloned the coding region of the African green monkey CRF1 gene and sequenced its alternatively spliced isoforms.
2. Materials and Methods
2.1. Cell culture
COS-7 cells (gift of Dr L. Pfeffer, University of Tennessee) were grown in Ham’s F10 medium as described previously; the media were supplemented with 10% fetal bovine serum (FBS) and antibiotics (GIBCO BRL) (Pisarchik, Slominski, 2004). Cultures with 90–100% of confluency were detached with trypsin, washed with PBS and cell pellets were frozen in –70°C until used.
2.2. Gene cloning and RT-PCR assays
Total RNA was prepared using RNA extraction kit (Qiagen, Valencia, CA) supplemented with RNAse-free DNAse Set (Qiagen, Valencia, CA). Two μg of total RNA was reversely transcribed with SuperScript First-Strand Synthesis System (Applied Biosystems, Foster City, CA). Quality and quantity of all samples were standardized by amplification of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S rRNA subunit as described previously (Pisarchik, Slominski, 2001; Zbytek, Slominski, 2005). PCR reactions were carried out as described previously (Pisarchik, Slominski, 2001).
A full-length coding region of Cercopithecus aethiops CRF1 mRNA was obtained by RT-PCR of COS cDNA using methodology similar to that reported previously (Pisarchik, Slominski, 2001; Pisarchik, Slominski, 2002). The RT-PCR primers were based on the published sequence of the human gene as a closely related species. The sequencing strategy is presented in the Fig 1. The coding sequence spanning human exons 2–14 was amplified by PCR using primers MZ038 and MZ041, whereas fragments spanning human exons: 1–7 (Primers: MZ045 and MZ039) and 9–14 (Primers: MZ040 and MZ046) were amplified separately. Primers MZ038, MZ039, MZ040 and MZ041 were identical as previously reported: P112, P113, P114 and P115, respectively (Pisarchik, Slominski, 2001). Sequences of primers MZ045 and MZ046 are 5-ATGTCCGTAGGACCCGGGCA-3 and 5-GCTCTTTGGGGGCTGCTCCAT-3, respectively. All PCR fragments were extracted from the agarose gel and purified by GFX PCR DNA and Gel Band Purification Kit (Amersham Bioscience, Little Chalfont, Buckinghamshire, England) and sequenced from both ends using RT-PCR primers. Sequencing was performed in the Molecular Resource Center at the University of Tennessee HSC (Memphis, TN) using the Applied Biosystems 3100 Genetic Analyzer and BigDye Terminator Kit. CRF1 sequence was assembled and analyzed with Clone Manager Suite 7 (Scientific & Educational Software, Cary, NC).
Figure 1. Cloning of African green monkey CRF1.
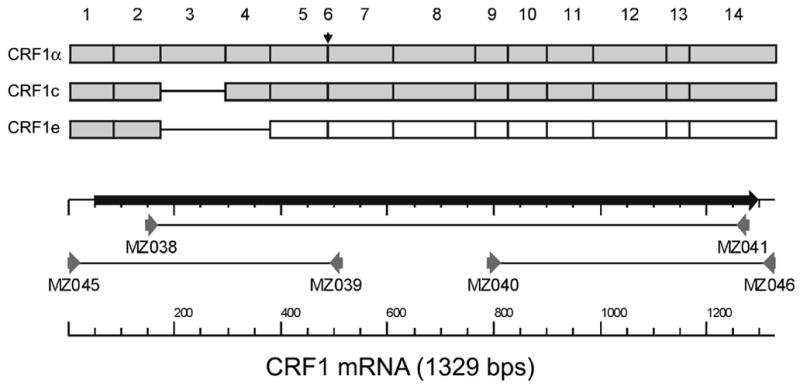
Shadow boxes = translated exons; open boxes = exons situated after the frame shift, small horizontal arrow indicates position of exon 6, which is absent from endogenous CRF1 mRNA isolated from COS-7 cells. Numbers above the picture indicate exon counting order. Solid lines show position of the missing exons. Arrows indicate position of primers.
2.3. Western blot analyses
Cell pellets were lysed in 0.2 % NaCl and subjected to four freeze/thaw cycles in liquid nitrogen and buffer A (10 mM Tris/HCl 7.2, 1 mM EDTA supplemented with Protease cocktail (Sigma, St. Louis, MO)). The whole lysates were either used for SDS-PAGE or further fractionated to obtain cytoplasmic or membrane fractions. In the latter protocol, cell debris and nuclear fractions were removed by centrifugation at 4°C, 600 g for 10 min. The supernatant was recentrifuged at 4°C, 40,000 g for 1 hour. The pellet containing the crude membrane fraction was resuspended in buffer A, while the supernatants were separated and collected as the cytoplasmic fraction. Twenty micrograms of protein were separated on 12% SDS-PAGE, transferred to immobilion-P poly(vinylidene difluoride) membrane (Millipore Corp, Bedford, MA) and blocked overnight in 5% non fat powdered milk in TBST (50 mM Tris, pH 7.5, 150 mM NaCl, 0.01% Tween-20). The membranes were incubated for 3-h with rabbit anti-CRF1 antibodies (dilution 1: 2000, gift from Dr E. Linton, Oxford University), or with goat anti-CRHR1 antibody (sc-1757, Santa Cruz Biotechnology, Santa Cruz, Ca) at dilution 1:250, washed, and incubated for 1 h with secondary anti-rabbit or anti-goat antibodies coupled to horseradish peroxidase, respectively, at dilutions 1 : 4000 or 1:1000 (Santa Cruz Biotechnology, Santa Cruz, CA). After two washes in TBST and one in TBS, the bands were visualized by Super Signal West Pico according to the manufacturer’s instructions (Pierce, Rockford, IL).
2.4. Immunohistochemical detection of CRF1 in COS-7 cells
Subconfluent cultures were grown on 8 well chambered coverslides, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 (in PBS), blocked with 1% BSA and immunostained with goat anti-CRHR1 antibody (sc-1757, Santa Cruz Biotechnology, Santa Cruz, Ca) at dilution 1:100 as described (Slominski et al., 2006a). Briefly, washed slides were incubated with FITC-conjugated anti-goat antibody (1:500 in 1% BSA in PBS), washed again and mounted in VECTASHIELD mounting medium with propidium iodide (Vector Laboratories, Burlingame, CA). Images were collected with NIKON Eclipse TE300 microscope (Melville, NY), recorded, and analyzed with MetaVue software. Cells incubated with nonimmune goat serum were used as nonspecific immunostaining controls.
2.5. CRF treatment and cAMP and arachidonic acid release assays
COS-7 cells were grown on 96 well plates to 75% confluence, and twelve hours prior to the experiments media were replaced with DMEM containing 5% fetal bovine serum. Cells to be tested were then incubated for one hour with DMEM containing 5% fetal bovine serum, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), and serial added dilutions of CRF (Sigma, St. Louis, MO), at 37°C and 5% CO2. Cyclic AMP concentration in the cells was measured using a cAMP functional assay kit (Packard BioScience, Meriden, CT, USA) as described previously (Pisarchik, Slominski, 2004; Slominski et al., 2006a). In control experiments COS cells were transiently transfected with plasmid containing human CRF1α and cAMP production after CRF addition was measured as described previously (Pisarchik, Slominski, 2004).
To measure arachidonic acid release COS-7 cells were seeded in 24-well plates in DMEM supplemented with 10% FBS to 50% confluence. After overnight incubation, media were changed to serum-free DMEM (500 μl/well) containing CRF (10−7 M) or vehicle (control) and 0.2 μCi of [3H]arachidonic acid (Amersham, NEN, 1 mCi/ml, specific activity- 214 Ci/mmol). This dose was optimal as defined in separate dose–dependent experiments performed in HaCaT keratinocytes. After 24 hours, media were discarded, the cells washed 2 times with PBS and DMEM (200 μl/well) was added. After 2, 3, 4 and 5 minutes of incubation conditioned media (200 μl per condition) was removed and transferred into mini-vials containing scintillation fluid (5 ml) for liquid scintillation spectrometry analysis.
2.6. Cell proliferation
COS cells were seeded (2,000 cells per well) in 96 wells and incubated in 200 μl media (DMEM containing 5% FBS). Next day media were discarded and changed to serum free DMEM or DMEM supplemented with 5% FBS media and then CRF was added at graded concentrations (10−10-10−6M) together with (3H) thymidine (1 μCi/ml; Amersham). After 24 hours media were removed, washed twice with PBS, and the cells lysed with 200 μl of 1N NaOH. Two hundreds μl of lysates were removed from each well and transferred into mini-vials containing scintillation fluid and radioactivity was measured by liquid scintillation spectrometry.
2.7. Statistical analyses
Data were tested for statistical significance with Student’s t-test using Prism 4.00 (GraphPad Software, San Diego). Data are presented as the mean ± SEM; for n=3–24. Dose–response curve fitting and EC50 calculations were performed with the Prism 4.0 software.
3. Results and Discussion
3.1. Cloning of African green monkey (Cercopithecus aethiops) CRF1 in COS-7 cells
We assumed the organization of exons in the green monkey CRF1 gene to be similar to humans, and thus primers based on the human sequence were used for amplification, sequencing and cloning of CRF1 from COS-7 cells. The strategy is presented in Fig. 1 and described in detail in Materials and Methods. The resulting full coding sequence was deposited in GenBank (GeneBank accession no. DQ086864). Alignment of this cDNA sequence with the exonal organization of the corresponding human gene showed that the African green monkey sequence lacked an homologue of the human exon 6 (Fig. 1). Thus, the cloned sequence of CRF1 derived from COS-7 cells corresponded to the human isoform CRF1α (composed of 13 exons), and it was then used to prepare the phylogeny dendrogram of CRF1 family according to the distance-based tree-building method using the neighbor-joining algorithm (Fig. 2A). Overall, the African green monkey CRF1 coding sequence (GeneBank accession no. DQ086864) was very similar to other already cloned CRF1α coding sequences; it showed 99% cDNA homology with rhesus monkey (Macaca mulatto, GeneBank accession no. AB078141), 97% with humans (Homo sapiens, GeneBank accession no. AY457172), 91% with mouse (Mus musculus, GeneBank accession no. NM_007762) and 91% with hamster (Mesocricetus auratus, GeneBank accession no. AY034599). The deduced protein sequence (GeneBank accession no. AAY87929) differed in only one amino acid with rhesus monkey (GeneBank accession no. BAD02831) and with humans (GeneBank accession no. AAR19768) (Fig. 2B). These differences were larger for the mouse (Mus musculus, GeneBank accession no. NP_031788) and hamster (Mesocricetus auratus, GeneBank accession no. AAK59707), with both protein sequences showing 97% homology.
Figure 2. CRF1 protein sequence analysis.

A. Phylogeny dendrogram of CRF1 family.
The dendrogram was constructed with a distance-based tree-building method using the neighbor-joining algorithm and Clone Manager Software. Multi-way alignments were used as data source. Protein sequences with accession number for the comparison are as follows: Human (Homo sapiens - GeneBank accession no. AAR19768), Rhesus monkey (Macaca mulatta - GeneBank accession no. BAD02831), Green monkey (Cercopithecus aethiops - GeneBank accession no. AAY87929), Shrew (Tupaia belangeri - GeneBank accession no. CAD19577), Hamster (Mesocricetus auratus - GeneBank accession no. AAK59707), Mouse (Mus musculus - GeneBank accession no. NM_007762), Rat (Rattus norvegicus - GeneBank accession no. AAA16441), Bovine (Bos taurus GeneBank accession no. NP_776712), Sheep (Ovis aries - GeneBank accession no. NP_001009727), Chicken (Gallus gallus - GeneBank accession no. NP_989652), Bullfrog (Rana catesbeiana - GeneBank accession no. BAD36783), Claw frog (Xenopus laevis - GeneBank accession no. CAA74363), Salmon (Oncorhynchus keta - GeneBank accession no. CAC81753), Catfish (Ameiurus nebulosus - GeneBank accession no. AAK01068).
B. Comparison of protein sequences of CRF1α.
Alignment of predicted sequences of CRF1α in human (Homo sapiens GeneBank accession no. AAR19768), rhesus monkey (Macaca mulatta, GeneBank accession no. BAD02831), green monkey (Cercopithecus aethiops, GeneBank accession no. AAY87929), hamster (Mesocricetus auratus, GeneBank accession no. AAK59707) and mouse (Mus musculus, GeneBank accession no. NM_007762). Exons 1 to 4 are presented as E1-E4, while transmembrane regions TM-1 to TM-7 are indicated above alignment. Identical amino acids are shown in green.
Further analysis showed that, similar to other species, > 90% of nucleotide substitutions in African green monkey did not affect amino acid sequence, which suggests the existence of stabilizing selection. Although nucleotide substitutions were evenly spread across the mRNA sequence, those resulting in changes in amino acid sequence were predominantly located in the extracellular domain (substrate binding domain). This is in agreement with the known increased susceptibility to mutations changing the amino acid sequence in the N-terminal domain of CRF1 involved in CRF binding (Perrin, Vale, 1999; Wille et al., 1999; Hillhouse, Grammatopoulos, 2006).
3.2. Alternative splicing of green monkey CRF1
Nested RT-PCR with primers spanning exons 2–7 (Line 1) and 9–14 (Line 2) were used to detect CRF1 splicing variants (Fig 3). Sequence analysis of the cloned fragments with subsequent correlation with the exonal organization of human CRF1 demonstrated that green monkey CRF1 gene is alternatively spliced (Fig. 1) producing isoforms α (fragments of 369 bp in line 1 and 336 bp in line 2; GeneBank accession no. DQ086864) and e (fragment of 163 bp in line 1; GeneBank accession no. DQ291148) (Fig. 3). The presence of fragments of 249 bp in line 1 and of 336 bp in line 2 indicates that these represent the alternatively spliced isoform c, in analogy to the human, mouse and hamster genes (Ross et al., 1994; Grammatopoulos et al., 1998; Pisarchik, Slominski, 2001; Pisarchik, Slominski, 2002; Hillhouse, Grammatopoulos, 2006) (cf. Fig. 1).
Figure 3. Alternative splicing of CRF1 in COS cells.
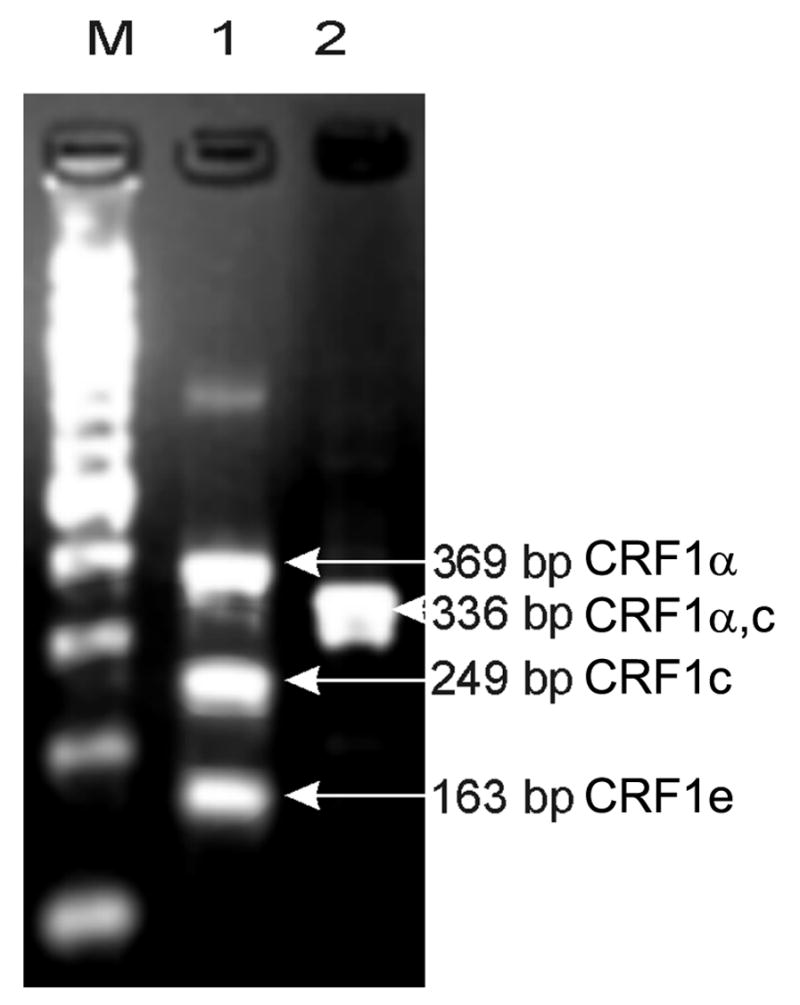
Nested RT-PCR with primers spanning exons 2–7 (Line 1) and 9–14 (Line 2) were used to detect CRF1 splicing variants.
Line 1: fragments of 369 bp corresponding to isoform α, 249 bp isoform c and 163 bp – isoform e.
Line 2: fragment of 336 bp corresponding to isoforms α, c or e. 100 kB DNA ladder (Line M).
Thus, we have identified CRF1α, e and presumably c isoforms but not isoform β in COS-7 cells. Since the CRF1α isoform (lacking exon 6) is considered the most efficient at transducing CRF signal into second messengers (Perrin, Vale, 1999; Hillhouse, Grammatopoulos, 2006), its expression is considered necessary for the development of phenotypic effects of CRF peptides in COS cells. The CRF1β isoform (has all 14 exons), codes for a protein with both decreased affinity for CRF and decreased receptor coupling to G proteins. This isoform is rarely expressed in human tissues (Perrin, Vale, 1999; Hillhouse, Grammatopoulos, 2006), absent in rodents (Perrin, Vale, 1999; Hillhouse, Grammatopoulos, 2006) and not detected in COS cells. As regards CRF1c, this isoform lacks exons 3 and 6, and is expected to play only a minor role in signal transduction, because of poor ligand binding (Ross et al., 1994; Perrin, Vale, 1999; Hillhouse, Grammatopoulos, 2006).
CRF1e has spliced off exons 3, 4 and 6 leading to frame shift; accordingly, CRF1e mRNA has two potential open reading frames (ORFs) (Pisarchik, Slominski, 2001; Slominski et al., 2006b). In COS-7 cells the first CRF1e ORF contains two in-frame exons of the original receptor and can be translated into a 108 aa peptide, where aa 1–40 are derived from CRF1α and include the signal peptide (aa 1–23) (Fig. 4A). Sequence analysis of the CRF1 C-terminus shows a point mutation introducing a stop codon that is present in green and rhesus monkeys and leads to earlier translation termination, compared to humans or rodents (Fig. 4A, lower panel). A second potential ORF codes for a 240 aa peptide (CRF1e2; predicted MW 28 kD) that would contain the transmembrane domains 3–7 (TM3-7), and would have 100% homology with rhesus and humans (Fig. 4B). Because of different translation starting point this peptide lacks the N-terminal fragment coding for the transmembrane fragments 1–2 (TM1-2) present in mouse and hamster (both code for a 309 aa protein with predicted MW of 36 kD (Pisarchik, Slominski, 2001; Pisarchik, Slominski, 2002; Slominski et al., 2006b). It must be noted that all of the presented CRF1e2 isoforms lack a signal peptide and most of the N-terminus and thus, localization to the cell membrane is questionable and substrate binding should be impaired.
Figure 4.
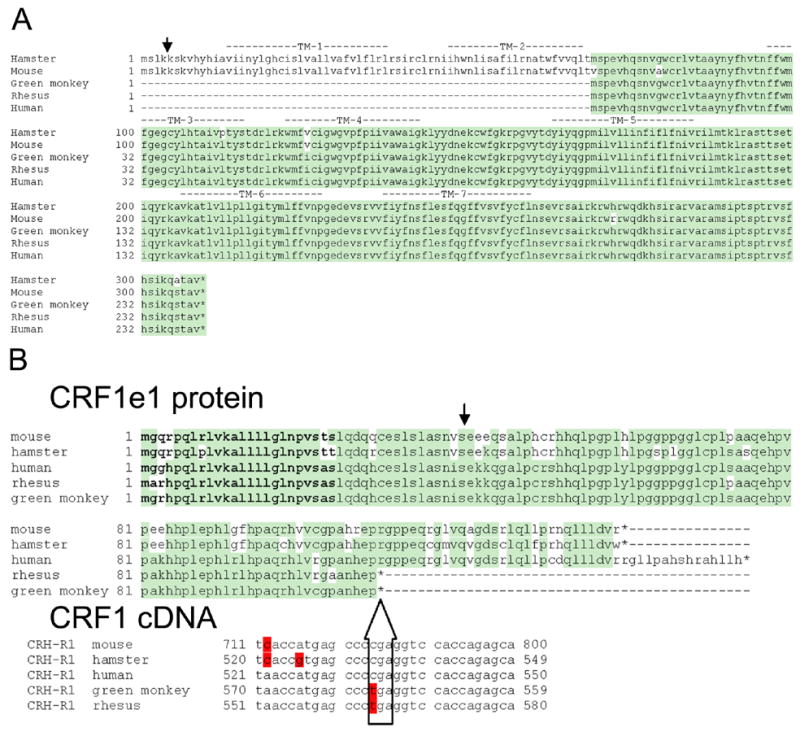
Comparison of the predicted protein sequences of the two products of CRF1e coded by open reading frames (ORF) 1 and 2. Alignment of predicted sequences human, hamster, mouse and green monkey (GeneBank accession nos. AAL46631, AF387669, AF369655 and DQ291148, respectively), while the CRF1e sequence for rhesus monkey was obtained from theoretical prediction of splicing (GeneBank accession no. BAD02831).
A. Predicted sequences of CRF1e1 proteins.
Identical amino acids are shown in green. Splicing of corresponding mRNA is shown with arrow. Signal peptide is in bold setting. *- stop codon. Lower panel shows alignment of CRF1 cDNA fragments where stop codon is introduced in alternatively spliced monkey CRF1e. Differing nucleotides are shown in red.
B. Predicted sequences of CRF1e2 proteins.
Transmembrane regions TM-1 to TM-7 are indicated above alignment. Splicing sites in mRNA are shown with arrow. Identical amino acids are shown in green.
*- stop codon.
3.3. Detection of CRF1 proteins in COS-7 cells
Using Western blot and immunofluorescence analyses we detected expression of the CRF1 protein in COS cells (Figs. 5). Thus, immunoreactive proteins of 37, 52, 70 and 80–85 kDa were detected in preparations of membranes and in cytoplasm (40,000g supernatants) fractions (Fig. 5A), and in the latter we detected two additional species of 40 and 60 kDa. The predicted mass of unprocessed CRF1α is 47.7 kDa, which decreases to 45 kDa after cleavage of the signal peptide (Chen et al., 1993; Vita et al., 1993; Perrin, Vale, 1999; Hillhouse, Grammatopoulos, 2006). Thus, the species of 52, 60 and 70 kDa likely represent glycosylated forms of CRF1 (α and/or c), while the 80–85 kDa protein might represent a dimer, in agreement with reports of varying CRF1 mobility on SDS-PAGE (Grigoriadis, De Souza, 1988; Grigoriadis, De Souza, 1989; Ruhmann et al., 1996; Hauger et al., 2000; Slominski et al., 2006a; Slominski et al., 2006b). In fact, glycosylated forms of CRF1 of 70 kDa and 53 kDa were respectively detected in pituitary and brain tissues and skin cells (Grigoriadis, De Souza, 1988; Grigoriadis, De Souza, 1989; Slominski et al., 2006a), whereas multiple CRF1 bands were detected in the AtT20 and Y-79 cell lines, and in rat pituitary and human skin cells (Sydow et al., 1997; Slominski et al., 2006a; Slominski et al., 2006b). As regards bands of lower MW (37 and 40 kDa), these could represent products of proteolytic processing of CRF1. Not unexpectedly, the observed pattern of endogenous CRF1 expression in COS cells is very similar to that described in the same cells transiently expressing the CRF1α and c isoforms (Pisarchik, Slominski, 2004). Moreover, the relative concentration and expression pattern of CRF1 in COS-7 cells are similar to those of human skin cells (Fig. 5B). To confirm the significance of these findings we performed further experiments using commercially available anti-CRH-R1 antibody (C20, Santa Cruz) and tested lysates of COS-7 cells and human brain. We found similar levels of expression of the 45 kDa CRF1 protein in COS-7 cells and human brain (Fig. 5C). Preincubation of the antibodies with the corresponding synthetic (blocking) peptide (sc-1757 P, Santa Cruz) abolished the CRF1 signal in both COS-7 and brain extracts (lane C in Fig. 5C), confirming the specificity of the detection.
Figure 5. Detection of CRF1 immunoreactivity in COS-7 cells.


A. Western blot analysis.
Twenty μg of crude membrane or cytoplasm fractions of COS-7 cells (40,000g pellet or supernatant, respectively) were separated using 12.5% SDS-PAGE. CRF1 isoforms were detected with specific CRF1 antibodies as indicated on the right side of the panel.
B. Comparison of CRF1 expressionaccross cell lines.
Fifty μg of whole cells lysate were separated using 12.5 % SDS-PAGE. CRF1 isoforms were detected as described in panel A. MW – protein ladder, C –COS-7, K –human adult normal keratiocytes (passage 4), H –immortalized human keratinocytes (HaCaT), F- human adult dermal fibroblasts (passage 4), M – melanoma WM98, O –osteosarcoma MG63.
C. CRF1expression in COS-7 cells and human brain.
Fifty μg of whole cells lysate were separated using 12.5 % SDS-PAGE. CRF1 isoforms were detected with specific anti-CRF1 antibody (C20, Santa Cruz). MW – protein ladder, C – control, COS - COS-7 cells, B – brain. To test specificity of CRF1 antibody (lane C) anti-CRF1 antibody was incubated with 5 times excess of blocking peptide (sc-1757 P, Santa Cruz) for 2 hours at 4º C prior the immunodetection (cf. materials and methods)
D. Immunocytochemical detection.
Left panel (a): CRF1 immunoreactivity was detected in COS-7 cells using goat anti- CRF1 antibody, followed by fluorescent detection with FITC-conjugated anti-goat antibody. Right panel (b): Control staining after omission of primary antibody. Nuclei were stained with propidium iodide. Bar – 10 μm.
The current unavailability of antibodies recognizing the isoform e precludes further analysis on the translatability of this isoform in native (untransfected) COS-7 cells. It must be nevertheless noted that COS cells can produce CRF1e proteins after transient transfection with the corresponding plasmid (Pisarchik, Slominski, 2004). Lastly, localization by immunocytochemistry did confirm distribution along the cell surface and intracellularly for the CRF1 antigens (Fig. 5D). Thus, COS-7 cells do produce endogenous CRF1 protein(s) that undergoes intracellular processing through posttranslational modification on its way to the cell surface and/or to degradation.
3.4. Activation of endogenous CRF1 in COS-7 cells has minimal or no effect on cAMP production
CRF produced inconsistent increases of cAMP formation, which did not fit concentration-dependence; for example, the effect was 5% above the basal level (p<0.05; Fig. 6A), or absent (p>0.05; not shown). In contrast, CRF stimulated cAMP production in COS cells transfected with human CRF1α by 10–20 fold, and in dose dependent manner (Fig. 6B)(Pisarchik, Slominski, 2004). Similar effects have been described in skin cells (Slominski et al., 2006a). Thus, the above findings indicate poor coupling of endogenous CRF1α to the cAMP generating system (adenylate cyclase); perhaps, because of unique properties of COS-7 cells. This may be similar to the case of normal epidermal keratinocytes, where expression of CRF1α is not associated with response to the ligand in terms of cAMP accumulation (Slominski et al., 2006a). Alternatively, since COS cells co-express the isoforms c and e, it is also possible that these isoforms could interfere with CRF1α signaling or peptide binding to the receptor.
Figure 6.

Effects of CRF on cAMP production in COS-7 cells expressing solely native (endogenous) receptors (A) or transiently transfected with human CRF1α (B)(Pisarchik, Slominski, 2004). Data represent mean ± SEM; n=3–4. * p<0.05;**p<0.005.
3.5. CRF1 in COS-7 cells is functionally active
In contrast to the cAMP response, CRF at dose 10−7 M stimulates significantly the release of arachidonic acid (AA) after a 2–5 minutes incubation (Fig. 7), indicating phenotypic activity for CRF1. Although the mechanism for AA increase by CRF in COS cells remains to be clarified, a similar effect has been noted in HaCaT keratinocytes (not shown). As regards the level of stimulation, the effect was similar to that of interleukin 1 or bradykinin B1 receptor agonists in other cell types (Rauk, Chiao, 2000; Schaeffer et al., 2001).
Figure 7. CRF stimulated release of arachidonic acid from COS-7 cells.
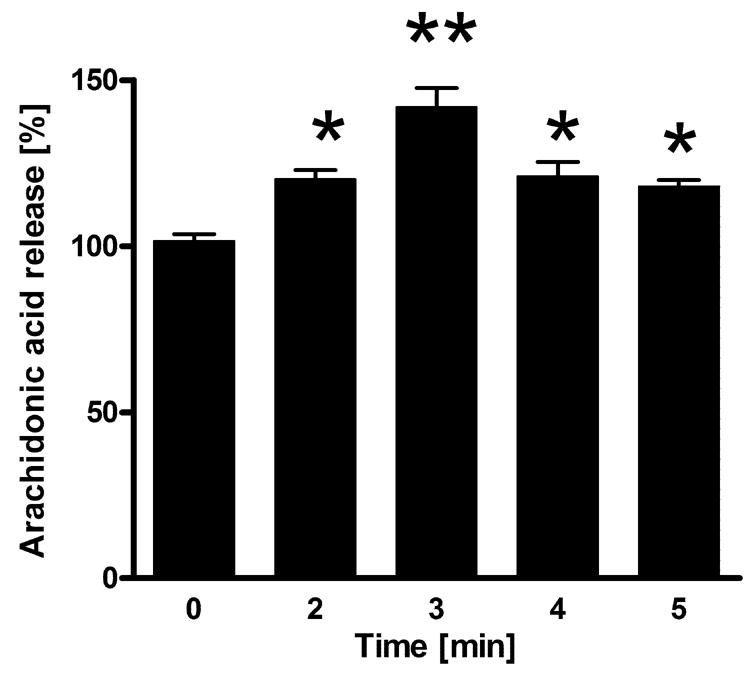
Data represent mean ± SEM; n=4–6. *p<0.05;**p<0.001.
Testing for effects of CRF in native COS-7 cells demonstrated inhibition of proliferation in cells cultured in either serum free medium or, in medium containing 5% serum (Fig. 8A, B). This dose dependent inhibitory effects of CRF are similar to those observed in COS-7 cells overexpressing the human CRF1α Fig. 8C, D), and is also in agreement with the inhibitory effects on cell growth reported for other cell types (Zbytek, Slominski, 2005; Slominski et al., 2006a). Taken together, the data on stimulation of AA release and inhibition of cell proliferation by CRF confirm that the endogenous CRF1 of COS cells is in fact functional.
Figure 8. CRF inhibits DNA synthesis in COS-7 cells cultured in medium containing serum (B and D) or in serum free medium (A and C).

COS-7 cells used expressed solely native receptors (A and B) or, were transiently transfected with human CRF1α (C and D)(Pisarchik, Slominski, 2004).
Data represent mean ± SEM; n=16–24.*p<0.05;**p<0.005, ***p<0.001, ****p<0.0005, *****p<0.00005, ******p<0.0000005.
3.6. Concluding remarks
The above results provide strong evidence supporting alternative splicing of CRF1 as a general trait expressed across many species (Hillhouse, Grammatopoulos, 2006; Slominski et al., 2006b). More specifically, the splicing pattern in the COS-7 cells is similar to that of human and rodent cells, which generates both membrane bound and soluble forms of the receptor. Besides the scientific interest, there are additional implications for the findings in COS-7 cells that do express endogenous CRF1 that are functional. Since COS-7 cells are routinely used to study the characteristic of artificially overexpressed CRF receptors, the interference by endogenous receptors must be taken into account in studies addressed at characterizing overexpression of the receptor. Of note, despite their similarities to human cells in functional properties, the CRF1α of COS-7 cells show poor coupling to cAMP production.
In summary we have cloned African green monkey CRF1 cDNA from COS-7 cells and identified alternatively spliced isoforms including membrane bound CRF1α and c forms, and the soluble form e. We also found that the resulting mRNAs are translated into functional CRF1 receptors.
Acknowledgments
The project was supported by NIH grant #AR047079 to AS.
Abbreviations
- AA
arachidonic acid
- cAMP
cyclic adenosine monophosphate
- CRF
corticotropin releasing factor
- CRF1
CRF receptor type 1; receptor aliases: CRH1, CRFR1, CRF-R1
- DMEM
Dulbecco's Modified Eagle's Medium
- DNA
deoxyribonucleic acid
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IBMX
3-isobutyl-1-methylxanthine
- PBS
phosphate buffered saline
- RT-PCR
reverse transcription polymerase chain reaction
- RNA
ribonucleic acid
- TBS
Tris-buffered saline
- TTBS
Tris-buffered saline, Tween20
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967–71. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- Grammatopoulos D, Dai Y, Chen J, Karteris E, Papadopoulou N, Easton AJ, Hillhouse EW. Human corticotropin-releasing hormone receptor: differences in subtype expression between pregnant and nonpregnant myometria. J Clin Endocrinol Metab. 1998;83:2539–44. doi: 10.1210/jcem.83.7.4985. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, De Souza EB. The brain corticotropin-releasing factor (CRF) receptor is of lower apparent molecular weight than the CRF receptor in anterior pituitary. Evidence from chemical cross-linking studies. J Biol Chem. 1988;263:10927–31. [PubMed] [Google Scholar]
- Grigoriadis DE, De Souza EB. Corticotropin-releasing factor (CRF) receptors in intermediate lobe of the pituitary: biochemical characterization and autoradiographic localization. Peptides. 1989;10:179–88. doi: 10.1016/0196-9781(89)90095-8. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid Agonist-Induced Phosphorylation of the Human CRF Receptor, Type 1: A Potential Mechanism for Homologous Desensitization. Biochem Biophys Res Commun. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–86. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Linton EA, Woodman JR, Asboth G, Glynn BP, Plested CP, Bernal AL. Corticotrophin releasing hormone: its potential for a role in human myometrium. Exp Physiol. 2001;86:273–281. doi: 10.1113/eph8602183. [DOI] [PubMed] [Google Scholar]
- Myers DA, Trinh JV, Myers TR. Structure and function of the ovine type 1 corticotropin releasing factor receptor (CRF1) and a carboxyl-terminal variant. Mol Cell Endocrinol. 1998;144:21–35. doi: 10.1016/s0303-7207(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Oshida Y, Ikeda Y, Chaki S, Okuyama S. Monkey corticotropin-releasing factor1 receptor: Complementary DNA cloning and pharmacological characterization. Life Sci. 2004;74:1911–24. doi: 10.1016/j.lfs.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri MR, Wille S, Mevenkamp G, Spiess J, Fuchs E, Dautzenberg FM. Corticotropin-releasing factor receptor type 1 from Tupaia belangeri--cloning, functional expression and tissue distribution. Eur J Biochem. 1998;258:78–84. doi: 10.1046/j.1432-1327.1998.2580078.x. [DOI] [PubMed] [Google Scholar]
- Parham KL, Zervou S, Karteris E, Catalano RD, Old RW, Hillhouse EW. Promoter analysis of human corticotropin-releasing factor (CRF) type 1 receptor and regulation by CRF and urocortin. Endocrinology. 2004;145:3971–83. doi: 10.1210/en.2004-0194. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Pisarchik A, Slominski A. Corticotropin releasing factor receptor type 1: molecular cloning and investigation of alternative splicing in the hamster skin. J Invest Dermatol. 2002;118:1065–72. doi: 10.1046/j.1523-1747.2002.01770.x. [DOI] [PubMed] [Google Scholar]
- Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Europ J Biochem. 2004;271:2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- Rauk PN, Chiao JP. Oxytocin signaling in human myometrium is impaired by prolonged exposure to interleukin-1. Biol Reprod. 2000;63:846–50. doi: 10.1095/biolreprod63.3.846. [DOI] [PubMed] [Google Scholar]
- Ross PC, Kostas CM, Ramabhadran TV. A variant of the human corticotropin-releasing factor (CRF) receptor: cloning, expression and pharmacology. Biochem Biophys Res Commun. 1994;205:1836–42. doi: 10.1006/bbrc.1994.2884. [DOI] [PubMed] [Google Scholar]
- Ruhmann A, Kopke AK, Dautzenberg FM, Spiess J. Synthesis and characterization of a photoactivatable analog of corticotropin-releasing factor for specific receptor labeling. Proc Natl Acad Sci U S A. 1996;93:10609–13. doi: 10.1073/pnas.93.20.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Yamada M, Horiba N, Wakui M, Demura H, Suda T. The genomic organization of the human corticotropin-releasing factor type-1 receptor. Gene. 1998;219:125–30. doi: 10.1016/s0378-1119(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Schaeffer P, Laplace MC, Savi P, Prabonnaud V, Salel V, Herbert JM. Detection of bradykinin B1 receptors in rat aortic smooth muscle cells. Biochem Pharmacol. 2001;61:291–8. doi: 10.1016/s0006-2952(00)00554-2. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006a;206:780–91. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006b;11:2230–48. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow S, Radulovic J, Dautzenberg FM, Spiess J. Structure-function relationship of different domains of the rat corticotropin-releasing factor receptor. Brain Res Mol Brain Res. 1997;52:182–93. doi: 10.1016/s0169-328x(97)00256-8. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Buczko E, Geng Y, Gamboa-Pinto A, Dufau ML. The genomic structure of the rat corticotropin releasing factor receptor. A member of the class II G protein-coupled receptors. J Biol Chem. 1996;271:14519–25. doi: 10.1074/jbc.271.24.14519. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, Le Fur G, Caput D, Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335:1–5. doi: 10.1016/0014-5793(93)80427-v. [DOI] [PubMed] [Google Scholar]
- Wille S, Sydow S, Palchaudhuri MR, Spiess J, Dautzenberg FM. Identification of amino acids in the N-terminal domain of corticotropin-releasing factor receptor 1 that are important determinants of high-affinity ligand binding. J Neurochem. 1999;72:388–95. doi: 10.1046/j.1471-4159.1999.0720388.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Xie LY, Abou-Samra AB. Molecular cloning of a type A chicken corticotropin-releasing factor receptor with high affinity for urotensin I. Endocrinology. 1996;137:192–7. doi: 10.1210/endo.137.1.8536612. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–26. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]


