Abstract
Binding of IL-2 to its specific receptor induces activation of two members of Jak family protein tyrosine kinases, Jak1 and Jak3. An IL-2R-reconstituted NIH 3T3 fibroblast cell line proliferates in response to IL-2 only when hematopoietic lineage-specific Jak3 is ectopically expressed. However, the mechanism of Jak3-dependent proliferation in the fibroblast cell line is not known. Here, I showed that Jak3 expression is dispensable for IL-2-induced activation of Jak1 and Stat proteins and expression of nuclear proto-oncogenes in the IL-2R-reconstituted fibroblast cell line. However, Jak3 expression markedly enhanced these IL-2-induced signaling events. In contrast, Jak3 expression was essential for induction of cyclin genes involved in the G1-S transition. These data suggest a critical role of Jak3 in IL-2 signaling in the fibroblast cell line and may provide further insight into the cell type-specific mechanism of cytokine signaling.
Keywords: IL-2, fibroblast, Jak kinase, Stat protein, proto-oncogene, Cyclin
Introduction
The high-affinity interleukin-2 (IL-2) receptor (IL-2R) is composed of three subunits; the IL-2Rα, IL-2Rβ, and common cytokine receptor γ (γc) chains [1-5]. Two members of the Jak kinase family protein tyrosine kinases, Jak1 and Jak3, are associated with IL-2Rβ and γc, respectively, and activated upon IL-2 stimulation [6-8]. Activation of Jak1 and Jak3 induces activation of Stat5/Stat3 and other signaling pathways to initiate cell proliferation [9-11]. Although IL-2 can transmit signals in IL-2R-reconstituted fibroblast cell lines [12, 13], they do not proliferate in response to IL-2. However, IL-2-induced cell cycle progression was achieved by the additional ectopic expression of Jak3 in NIH 3T3-derived 3T3αβγ cells expressing the reconstituted IL-2R (and endogenous Jak1) [6]. It is not shown how ectopic expression of Jak3 supports IL-2-induced proliferation in the fibroblast cell line.
Here, I showed that IL-2 induces activation of Jak1 and Stat proteins and expression of nuclear proto-oncogenes in the absence of Jak3 in 3T3αβγ. On the other hand, Jak3 expression markedly enhanced IL-2-induced activation of Jak1 and Stat proteins and induction of protooncogenes in this cell line. In contrast, Jak3 expression was indispensable for induction of cyclin genes involved in the G1-S transition. These data suggest a critical role of Jak3 in IL-2 signaling in the fibroblast cell line and may provide further insight into the cell type-specific mechanism of cytokine signaling.
Materials and Methods
Cell lines
3T3αβ is an NIH 3T3-derived cell line ectopically expressing the human IL-2Rα and IL-2Rβ chains [12]. 3T3αβγ was derived from 3T3αβ and expresses human γc ectopically [12]. J3 was derived from 3T3αβγ and expresses mouse Jak3 ectopically [6]. These cell lines were maintained in Dulbecco Modified Eagle's Medium supplemented with 10% (v/v) fetal calf serum (FCS).
Immunoprecipitation and immunoblot Analysis
FCS-depleted cells were unstimulated or stimulated with human IL-2 (10 nM) or mouse IFNγ (25 U/ml) for 10 min. Preparation of whole cell lystates, immunoprecipitation, and immunoblot analyses were done as described previously [10, 14-18].
Electrophoretic mobility shift assay (EMSA)
FCS-depleted cells were unstimulated or stimulated with human IL-2 (10 nM) or mouse IFNγ (25 U/ml) for 10 min. Preparation of whole cell lysates and EMSA were performed as described previously [10, 14].
Northern blot analysis
FCS-depleted cells were unstimulated or stimulated with human IL-2 (10 nM). At indicated times, cells were harvested and total RNA (10 μg) were analyzed by Northern blot analysis as previously described [12, 19].
Results and Discussion
Ectopic expression of Jak3 enhances IL-2-induced tyrosine phosphorylation of Jak1 in an IL-2R-reconstituted NIH 3T3-derived cell line
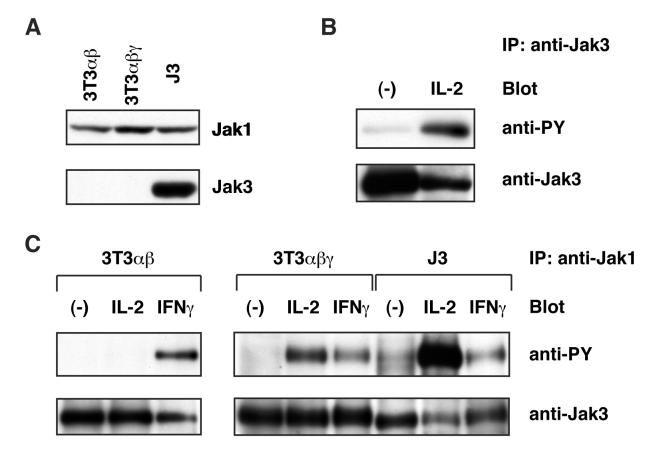
3T3αβγ is derived from NIH 3T3 fibroblast cell line and has the reconstituted high affinity IL-2R [12]. As shown in Fig. 1A, 3T3αβγ cells did not express Jak3 [6], whose expression is detected only in the hematopoietic cell lineages [20, 21]. In contrast, this cell line expressed Jak1 (Fig. 1A), whose expression is observed ubiquitously [22]. 3T3αβγ does not proliferate in response to IL-2 stimulation [6]. Interestingly, IL-2-induced cell cycle progression was achieved in 3T3αβγ-derived J3 cells, in which Jak3 is ectopically expressed by DNA transfection [6]. In J3 cells, IL-2 stimulation induced rapid tyrosine-phosphorylation of Jak3 as expected (Fig. 1B). To elucidate how ectopic expression of Jak3 supports IL-2-induced proliferation in 3T3αβγ, I first examined tyrosine-phosphorylation of Jak1. It has been shown that activation of Jak1 is well correlated with its tyrosine-phosphorylation levels [23]. As shown in Fig. 1C, IL-2 stimulation induced tyrosine-phosphorylation of Jak1 in 3T3αβγ. Since IL-2 failed to induce tyrosine-phosphorylation of Jak1 in 3T3αβ expressing IL-2Rα and IL-2Rβ but not γc, tyrosine-phosphorylation of Jak1 by IL-2 was mediated by the reconstituted heterotrimeric IL-2R. These results showed that Jak1 could be activated in the absence of Jak3 in 3T3αβγ. This result is in clear contrast with data from a transformed B cell line derived from a Jak3-deficient SCID patient, in which IL-2-induced Jak1 activation was not observed [24]. It is possible that activation of Jak1 in the B cell line was under the threshold of sensitivity of the experimental condition. Alternatively, it is possible that dependence of Jak1 activation on Jak3 is differentially regulated in different cell types.
Figure 1.
Jak3 enhances activation of Jak1 in an IL-2R-reconstituted NIH 3T3-derived cell line. (A) Expression of Jak1 and Jak3 in 3T3αβγ and J3. Cell lysates of these cells were separated by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-Jak1 or anti-Jak3 antibody (Ab). (B) Activation of Jak3 by IL-2 in J3 cells. FCS-depleted J3 cells were unstimulated or stimulated with human IL-2 (10 nM) for 10 min. Cells were lysed, and Jak3 was immunoprecipitated with anti-Jak3 Ab. Immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis with anti-phosphotyrosine (anti-PY) Ab (upper panel). Jak3 protein levels were determined by immunoblot analysis with anti-Jak3 Ab (lower panel). (C) Expression of Jak3 enhances activation of Jak1. FCS-depleted 3Tαβ, 3T3αβγ and J3 were unstimulated or stimulated with human IL-2 (10 nM) or mouse IFNγ (25 U/ml) for 10 min. Cells were lysed, and Jak1 was immunoprecipitated with anti-Jak1 Ab. Immunoprecipitates were separated by SDS-PAGE and subjected to immunoblot analysis with anti-PY Ab (upper panel). Jak1 protein levels were determined by immunoblot analysis with anti-Jak1 Ab (lower panel).
Ectopic expression of Jak3 markedly enhanced IL-2-induced tyrosine-phosphorylation of Jak1 (J3 cells, Fig. 1C). This result further confirmed the critical importance of Jak3 for maximal activation of Jak1 and the concept of cross-phosphorylation/activation among receptor-associated Jak kinases [1-5, 23].
Ectopic expression of Jak3 enhanced IL-2-induced Stat activation in the IL-2R-reconstituted NIH 3T3-derived cell line
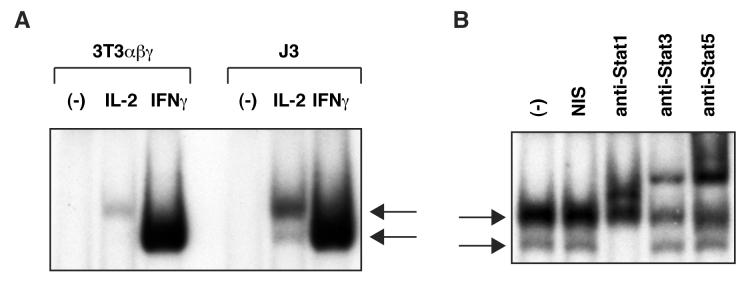
Next, I examined activation of Stat proteins in the IL-2R-reconstituted NIH 3T3-derived cell lines by EMSA using the interferon regulatory factor-1 (IRF-1) interferon-γ (IFNγ) activated site (GAS) probe. As shown in Fig. 2A, IL-2 stimulation induced rapid DNA-binding activities in 3T3αβγ. This result suggests that the basal activation of Jak1 can mediate IL-2-induced Stat activation in the absence of Jak3 in this cell line. In J3 cells, IL-2 stimulation induced appearance of two distinct DNA-binding activities. The size of the upper band corresponded to that observed in 3T3αβγ, and its intensity was markedly increased in J3 cells. The lower band was not detected in 3T3αβγ. As shown in Fig. 2B, the upper DNA-binding complexes contained Stat3 and Stat5, whereas the lower one contained Stat1. These results indicate that Jak1 activation can induce activation of Stat5/Stat3 and Jak3 enhances IL-2-mediated Stat5/Stat3 activation in the NIH 3T3-derived cells. Interestingly, Stat1 activation by IL-2 was totally dependent on expression of Jak3. It has been reported that IL-2 can activate Stat1 in addition to Stat5/Stat3 and Stat1 activation was only observed in the presence of high concentrations of IL-2 [9]. This finding is consistent with my observation that Stat1 activation is detected only when activation of Jak3 and maximal activation of Jak1 are achieved by ectopic expression of Jak3 in 3T3αβγ.
Figure 2.
Jak3 enhances activation of Stat proteins in the IL-2R-reconstituted NIH 3T3-derived cell line. (A) 3T3αβγ and J3 were mock-stimulated or stimulated with human IL-2 (10 nM) or mouse IFNγ (25 U/ml) for 10 min. Whole cell lysates were incubated with 32P-labeled IRF-1 GAS probe. Complexes were resolved on 4% acrylamide gels and detected by autoradiography. The upper arrow indicates the position of DNA-binding complex detected in IL-2-stimulated 3T3αβγ and J3. The lower arrow indicates the position of DNA-binding complex detected in IL-2-stimulated J3 and IFNγ-stimulated cells. (B) Supershifts of IL-2-induced DNA-binding complex. Whole cell lysates were preincubated for 1 hr at 4°C with non-immune serum (NIS) or Abs specific to Stat1, Stat3, or Stat5. The upper arrow indicates the position of DNA-binding complex containing Stat3 and Stat5. The lower arrow indicates the position of DNA-binding complex containing Stat1.
Ectopic expression of Jak3 enhances IL-2-induced nuclear proto-oncogene expression in the IL-2R-reconstituted NIH 3T3-derived cell line
It has been shown that induction of nuclear proto-oncogenes by growth-promoting cytokines plays important roles in cell proliferation [19, 25]. IL-2 stimulation induces expression of protooncogenes including c-fos and c-myc in 3T3αβγ (Fig. 3A) as previously described [12]. It is likely that IL-2-induced activation of Jak1 and Stat5/Stat3 mediates the proto-oncogene induction in 3T3αβγ. I examined how IL-2-induced proto-oncogene expression is affected by ectopic expression of Jak3 by Northern blot analysis. As shown in Fig. 3A, induction of c-myc and c-fos was significantly enhanced by ectopic expression of Jak3. Furthermore, c-myc induction was sustained in J3 cells (Fig. 3B). These results showed that although Jak3 is dispensable for proto-oncogene expression in 3T3αβγ, it enhances expression of nuclear protooncogenes by IL-2. These results also suggest that quantitative regulation of these protooncogenes is critical for cell proliferation in NIH 3T3-derived cells.
Figure 3.
Enhanced proto-oncogene induction by Jak3 expression. (A) Enhanced IL-2-induced expression of c-fos and c-myc by ectopic expression of Jak3. FCS-depleted cells were unstimulated or stimulated with human IL-2 (10 nM). At indicated times, cells were harvested and total RNA (10 μg) were analyzed by Northern blot analysis. The filters were stained with methylene blue to show total RNA (18S ribosomal RNA). (B) Sustained IL-2-induced expression of c-myc mRNA in J3. FCS-depleted cells were unstimulated or stimulated with human IL-2 (10 nM). At indicated times, cells were harvested and total RNA (10 μg) were analyzed by Northern blot analysis. The filters were stained with methylene blue to show total RNA (28S ribosomal RNA).
Essential role of Jak3 in the induction of cyclin genes in IL-2R-reconstituted NIH 3T3-derived cell lines
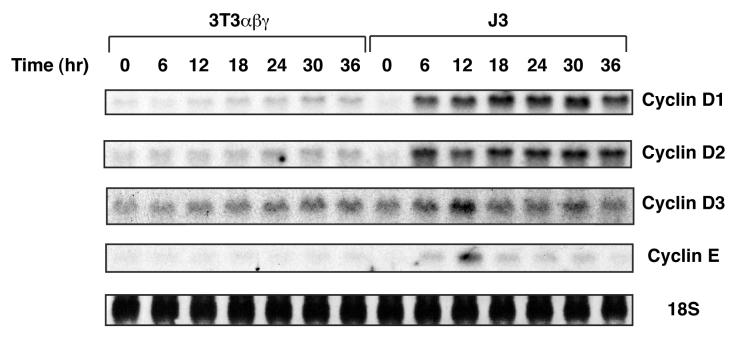
Cyclins play essential roles in cell cycle progression, and their expression is induced by growth-promoting stimuli [26]. Among others, D-type Cyclins and Cyclin E have been shown to be critical for the G1-S transition [27]. To examine how cyclin mRNA induction is affected by ectopic expression of Jak3 in IL-2R-reconstituted NIH 3T3-derived cells, I performed Northern blot analysis using specific cDNA probes. In 3T3αβγ, IL-2 stimulation marginally upregulated expression of cyclin D1, D2, D3, and E genes (Fig. 4). In contrast, expression of these cyclin genes was significantly induced by IL-2 in J3 cells, indicating an essential role of Jak3 in the induction of the cyclin genes.
Figure 4.
IL-2-induced expression of the G1-S cyclin genes by extopic expression of Jak3. FCS-depleted cells were unstimulated or stimulated with human IL-2 (10 nM). At indicated times, cells were harvested and total RNA (10 μg) were analyzed by Northern blot analysis. The filters were stained with methylene blue to show total RNA (18S ribosomal RNA).
In conclusion, I showed that IL-2 induces activation of Jak1 in the absence of Jak3 in an IL-2R-reconstituted NIH 3T3-derived cell line, 3T3αβγ (Fig. 1). This result was unexpected because Jak3 is indispensable for IL-2-induced Jak1 activation in human B cells [24]. It is an interesting future issue how Jak1 activation is regulated in a cell type-specific manner. In 3T3αβγ, Stat5/Stat3 activation and induction of proto-oncogenes were observed in the absence of Jak3 expression (Fig. 2 and 3). In contrast, IL-2-induced Stat1 activation was dependent on expression of Jak3 (Fig. 2). Although IL-2-induced activation of Jak1 and Stat5/Stat3 as well as induction of proto-oncogenes were observed in 3T3αβγ, IL-2 stimulation failed to induce the G1-S cyclin genes (Fig. 4) and the S-phase transition [6, 28]. Jak3 expression augmented activation of Jak1 and Stat5/Stat3 and induction levels of nuclear proto-oncogenes (Fig. 1-3). Furthermore, induction of the G1-S cyclin genes and cell cycle progression were observed in J3 cells but not in 3T3αβγ (Fig. 4) [6, 28]. These results suggest the importance of enhanced signaling by Jak3 expression for cell cycle progression. It is also suggested that cell cycle progression requires signaling strength above a certain threshold. Alternatively, it cannot be excluded at this stage whether Jak3 transmits signaling distinct from that induced by Jak1 activation. This is an important future issue.
Acknowledgements
I thank Dr. T. Taniguchi for IL-2R-reconstituted cell lines. This work is supported by NIH grant R01 AI059315 (H. F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leonard WJ. In: Type I Cytokines and Interferons and their receptors, in Fundamental Immunology. Paul WE, editor. Lippincott Williams & Wilkins; Philadelphia, PA: 2003. pp. 701–747. [Google Scholar]
- 2.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 3.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu. Rev. Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 4.Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 5.O'Shea JJ. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 7.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, Goldman AS, Schmalstieg FC, Ihle JN, O'Shea JJ, Leonard WJ. Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: Implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 8.Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, D'Andrea AD, Ritz J, Nadler LM. Prevention of T cell anergy by signaling through the γc chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 9.Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2:321–329. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 10.Fujii H, Nakagawa Y, Schindler U, Kawahara A, Mori H, Gouilleux F, Groner B, Ihle JN, Minami Y, Miyazaki T, Taniguchi T. Activation of Stat5 by interleukin 2 requires a carboxyl-terminal region of the interleukin 2 receptor β chain but is not essential for the proliferative signal transmission. Proc. Natl. Acad. Sci. USA. 1995;92:5482–5486. doi: 10.1073/pnas.92.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J-X, Migone T-S, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, Leonard WJ. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 12.Minami Y, Oishi I, Liu Z-J, Nakagawa S, Miyazaki T, Taniguchi T. Signal transduction mediated by the reconstituted IL-2 receptor: evidence for a cell type specific function of IL-2 receptor β chain. J. Immunol. 1994;152:5680–5690. [PubMed] [Google Scholar]
- 13.Asao H, Takeshita T, Ishii N, Kumaki S, Nakamura M, Sugamura K. Reconstitution of the functional IL-2 receptor complexes on fibroblastoid cells: Involvement of the cytoplasmic domain of the γ chain in two distinct signaling pathways. Proc. Natl. Acad. Sci. USA. 1993;90:4127–4131. doi: 10.1073/pnas.90.9.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii H, Ogasawara K, Otsuka H, Suzuki M, Yamamura K.-i., Yokochi T, Miyazaki T, Suzuki H, Mak TW, Taki S, Taniguchi T. Functional dissection of the cytoplasmic subregions of the IL-2 receptor βc chain in primary lymphocyte populations. EMBO J. 1998;17:6551–6557. doi: 10.1093/emboj/17.22.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujino S, Di Santo JP, Takaoka A, McKernan TL, Noguchi S, Taya C, Yonekawa H, Saito T, Taniguchi T, Fujii H. Differential requirement of the cytoplasmic subregions of γc chain in T cell development and function. Proc. Natl. Acad. Sci. USA. 2000;97:10514–10519. doi: 10.1073/pnas.180063297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsujino S, Miyazaki T, Kawahara A, Maeda M, Taniguchi T, Fujii H. Critical role of the membrane-proximal, proline-rich motif of the interleukin-2 receptor γc chain in the Jak3-independent signal transduction. Genes Cells. 1999;4:363–373. doi: 10.1046/j.1365-2443.1999.00266.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino A, Matsumura S, Kondo K, Hirst JA, Fujii H. Inducible translocation trap: a system for detecting inducible nuclear translocation. Mol. Cell. 2004;15:153–159. doi: 10.1016/j.molcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino A, Saint Fleur S, Fujii H. Regulation of Stat1 protein expression by phenylalanine 172 in the coiled-coil domain. Biochem. Biophys. Res. Commun. 2006;346:1062–1066. doi: 10.1016/j.bbrc.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibuya H, Yoneyama M, Ninomiya-Tsuji J, Matsumoto K, Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992;70:57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura M, McVicar DW, Johnston JA, Blake TB, Chen Y-Q, Lal BK, Lloyd AR, Kelvin DJ, Staples JE, Ortaldo JR, O'Shea JJ. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc. Natl. Acad. Sci. USA. 1994;91:6374–6378. doi: 10.1073/pnas.91.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witthuhn BA, Silvennnoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 22.Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zürcher G, Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol. Cell Biol. 1991;11:2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy DE, Darnell JE., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 24.Oakes SA, Candotti F, Johnston JA, Chen Y-Q, Ryan JJ, Taylor N, Liu X, Hennighausen L, Notarangelo LD, Paul WE, Blaese RM, O'Shea JJ. Signaling via IL-2 and IL-4 in Jak3-deficient severe combined immunodeficiency lymphocytes: Jak3-dependent and independent pathways. Immunity. 1996;5:605–615. doi: 10.1016/s1074-7613(00)80274-5. [DOI] [PubMed] [Google Scholar]
- 25.Roussel MF, Cleveland JL, Shurtleff SA, Sherr CJ. Myc rescue of a mutant CSF-1 receptor impaired in mitogenic signaling. Nature. 1991;353:361–363. doi: 10.1038/353361a0. [DOI] [PubMed] [Google Scholar]
- 26.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 27.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Takaoka A, Nogueira L, Dikic I, Fujii H, Tsujino S, Mitani Y, Maeda M, Schlessinger J, Taniguchi T. Pyk2 is a downstream mediator of the IL-2 receptor-coupled Jak signaling pathway. Genes Dev. 1998;12:770–775. doi: 10.1101/gad.12.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]