Abstract
We discuss a simple approach to enhance sensitivity for 13C high-resolution solid-state NMR for proteins in microcrystals by reducing 1H T1 relaxation times with paramagnetic relaxation reagents. It was shown that 1H T1 values can be reduced from 0.4-0.8 s to 60-70 ms for ubiquitin and lysozyme in D2O in the presence of 10 mM Cu(II)Na2EDTA without substantial degradation of the resolution in 13C CPMAS spectra. Faster signal accumulation using the shorter 1H T1 attained by paramagnetic doping provided sensitivity enhancements of 1.4-2.9 for these proteins, reducing the experimental time for a given signal-to-noise ratio by a factor of 2.0-8.4. This approach presented here is likely to be applicable to various other proteins in order to enhance sensitivity in 13C high-resolution solid-state NMR spectroscopy.
Keywords: solid-state NMR, protein microcrystal, T1 relaxation, paramagnetic doping, 13C CPMAS
Introduction
Over the past years, significant progress has been achieved in solid-state NMR (SSNMR) spectroscopy of biomolecules such as peptides and proteins.[1-16] Particularly, use of protein micro-/nano-crystals[17] has significantly improved resolution in high-resolution SSNMR of dilute spins such as 13C and 15N, permitting signal assignment and structural determination of various uniformly 13C and/or 15N-labeled proteins by SSNMR.[18-25] However, restricted sensitivity in 13C and 15N SSNMR has been still one of the major limiting factors in SSNMR analysis of proteins. In an experimental time required for 13C SSNMR of proteins, more than 95 % is typically consumed for recycle delays to retrieve spin polarization by 1H T1 relaxation, to protect a probe from arcing due to RF irradiation, or to avoid sample degradation due to heating. The latter two problems have been addressed by improving designs of MAS probes to minimize sample heating[26] and tolerate handling of relatively high RF power,[27] or by using low-power 1H decoupling.[28; 29] Doping with paramagnetic metal ions such as Cu(II) has been utilized to reduce 1H T1 relaxation times in 13C CPMAS of proteins/peptides in cryogenic conditions.[30] However, the effects of paramagnetic doping on resolution and T1 relaxation have not been fully examined for biomolecular SSNMR, in particular, for 13C CPMAS of protein micro-crystals, which generally provides excellent resolution.
In this study, we experimentally investigate the effects of paramagnetic ion doping to reduce 1H T1 values in 1D 13C CPMAS for microcrystals of two model proteins: ubiquitin and lysozyme using a Cu(II)Na2EDTA complex (Cu-EDTA) as a relaxation reagent. In addition to 1H T1 values, we examine resolution and line positions in 13C CPMAS spectra for these proteins in the presence of Cu-EDTA. Motivations and prospects of this approach for sensitivity enhancements in biomolecular SSNMR are presented.
Materials and Methods
D2O was purchased from Cambridge isotope (Andover, MA). Ubiquitin from bovin red blood cells (ubiquitin), lysozyme from chicken egg white (lysozyme), and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Purified water (double deionized and distilled) was prepared using a High-Q 103 S water still system (High-Q Corp., Wilmette, IL). The purified water was used for preparation of all the protein microcrystals in H2O.
We prepared protein microcrystals in D2O or H2O following the protocols by Martin and Zilm for preparing protein nanocrystals[17] with minor modifications. Unless otherwise mentioned, all the data were collected for samples prepared in D2O for potential applications to partially deuterated proteins in D2O. As will be discussed, protein microcrystals prepared in D2O have longer 1H T1 values than those in H2O. Thus, the effects of paramagnetic doping are more notable for proteins in D2O, although this approach is also effective for samples prepared in H2O. An equal volume mixture of a protein stock solution (25 mg protein/ml) and a crystallization solution was concentrated to approximately half the starting volume by using a SpeedVac concentrator (Savant, Farmingdale, NY).[17] For preparation of the protein stock solution, lysozyme was dissolved in a 100 mM sodium acetate buffer (pH 4.5) while ubiquitin was dissolved in pure D2O/H2O. The crystallization solution for ubiquitin contained 25% w/v PEG 8000 and 200 mM cadmium acetate in a 50 mM sodium HEPES buffer (pH 7); the solution for lysozyme contained 12.5% w/v PEG 2000 and 75 mM sodium chloride in a 100 mM sodium acetate buffer (pH 4.5). The concentrated protein solution of ∼0.5 mL was kept in a microtube at 4 °C for 10-12 hours to produce protein crystals. Then, the solution containing crystals was centrifuged at 1.5 × 103 g for 5 min using an Eppendorf 5414D micro-centrifuge (Eppendorf, Westbury, NY).
A Cu-EDTA complex was selected as a relaxation reagent that minimizes undesired interactions between metal ions and proteins.[31] To prepare protein crystal samples containing Cu-EDTA, about 0.4 mL of the mother liquor was separated as a supernatant from the protein crystals after the centrifugation. Then, 1.0-14.9 mg of Cu-EDTA was dissolved in the mother liquor. This solution was kept at 4 °C for 2-4 hours, and centrifuged to remove any precipitated proteins due to the salts. After the pH was adjusted to an appropriate value for the particular protein, the mother liquor containing Cu-EDTA was reintroduced to the protein crystals, and left at 4 °C for another 10-12 hours to dope Cu-EDTA into protein crystals. The samples which do not contain Cu-EDTA were prepared in the same manner for a control, but without the addition of Cu-EDTA. Then, the sample was centrifuged for 5 min, and the collected protein crystals were packed into a MAS rotor by centrifugation. The concentration of Cu-EDTA was estimated from the amount of Cu-EDTA used and the total volume of the mother liquor and the protein microcrystals. Formation of the protein crystals were confirmed under an optical microscope. The images of the crystals were obtained at ×32 magnification using a CCD camera (CoolSnap, Roper, Trenton, NJ) attached to a Carl Zeiss Axiovert 25 inverted microscope (Carl Zeiss MicoImaging, Thornwood, NY).
SSNMR experiments were performed at 9.4 T (1H NMR frequency of 400.2 MHz) with a Varian InfinityPlus 400 NMR spectrometer. For experiments at the spinning of 10 kHz, a Varian T3 3.2-mm MAS double-resonance NMR probe or a home-built 2.5-mm MAS double-resonance probe was used. The signals were collected during an acquisition period of 10 ms at the spinning speed of 10,000 ± 5 Hz with cooling air at −10 °C supplied through a Varian VT stack at a flow rate of ∼140 standard-cubic-feet per hour (scfh). For experiments at the spinning of 40 kHz, we used a 2.0-mm MAS double-resonance probe developed in Dr. Samoson's lab.[32-34] Unless otherwise mentioned, the signals were collected during an acquisition period of 20 ms at the spinning speed of 40,000 ± 10 Hz with cooling air at −5 °C supplied through the Varian VT stack at a flow rate of ∼140 scfh and cooled bearing air (1 °C). The data were processed with Varian Spinsight software. The spectra in Fig 2 were processed with Gaussian line broadening of 15 Hz; other spectra were processed with Gaussian line broadening of 25 Hz. 1H T1 values were calculated from the data collected by 1H inversion recovery experiments detected by 13C CPMAS, where a π-pulse to 1H spins and the following inversion recovery delay were added prior to the conventional CPMAS sequence with ramped CP[35] and TPPM decoupling[36]. The signal intensities were measured for the highest signals in the 13CO (160-190 ppm), 13Cα (40-65 ppm), 13CH (10-30 ppm) regions to estimate 1H T1; the average of the 1H T1 values estimated for the three regions was used as 1H T1 of the sample. For proteins without Cu-EDTA, some variations in 1H T1 values (∼10 %) were observed from batch to batch. We also noticed that 1H T1 is gradually reduced (10-25 %) over the course of experiments after a week for the proteins without Cu-EDTA. The values for 1H T1 in Table 1 were measured within a week after the sample was packed in a rotor.
Figure 2.

(a-d) 13C CPMAS spectra of protein micro crystals for (a, b) lysozyme and (c, d) ubiquitin prepared in D2O obtained (a, c) with and (b, d) without 10 mM Cu-EDTA at 13C NMR frequency of 100.6 MHz. The recycle delays were (a) 0.18 s, (b) 1.50 s, (c) 0.18 s, and (d) 2.50 s. The spectra were acquired at a spinning speed of 40 kHz with π-pulse-train decoupling. In the decoupling scheme, a π-pulse with the width of 2.5 μs was rotor-synchronously applied at the end of every rotor cycle (25 μs) with the XY-8 phase cycle.[51] Signals were accumulated during acquisition periods of 20 ms with (a) 36,000, (b) 4,800, (c) 72,000, and (d) 5,680 scans in a common total experimental time of (a, b) 2 hours or (c, d) 4 hours. During the CP period of 1.0 ms, the 13C RF field was swept from 53 kHz to 71 kHz, while the 1H RF field was kept at 102 kHz. All the spectra were processed with Gaussian line broadening of 15 Hz.
Table 1.
1H T1 values of ubiquitin and lysozyme in the presence and absence of Cu-EDTA with their approximate crystal sizes measured from microscopic images.
Results and Discussion
In Fig. 1(a-d), we demonstrate sensitivity enhancements in 13C CPMAS by faster signal accumulation using short 1H T1 optimized with Cu-EDTA doping for (a, b) lysozyme and (c, d) ubiquitin microcrystals. These spectra were collected (a, c) with 10 mM Cu-EDTA and (b, d) without Cu-EDTA in a common experimental time of 4 hours. Inspections of the microscopic images in Fig. 1(e-h) for the crystals (e, g) with and (f, h) without Cu-EDTA revealed that there were no noticeable changes in the crystal shapes or sizes due to the introduction of Cu-EDTA for (e, f) lysozyme and (g, h) ubiquitin. Hence, it is not likely that the addition of Cu-EDTA damaged the protein crystals. The recycle delays for the lysozyme samples were (a) 0.5 s (1H T1 = 59 ms) and (b) 1.1 s (1H T1 = 350 ms) while the delays for the ubiquitin samples were (c) 1.0 s (1H T1 = 73 ms) and (d) 2.5 s (1H T1 = 820 ms). As will be discussed later, increasing the Cu-EDTA concentration further reduces 1H T1 values. For comparison, the spectra were scaled so that the spectra for the same protein display a common noise level. Faster signal accumulation using the shorter 1H T1 in the presence of Cu-EDTA clearly yielded considerable sensitivity enhancements by factors of 1.4 and 1.6 in Fig. 1(a) and (c), respectively. It is also clear that no major changes in the line positions or line-widths were induced by the presence of 10 mM Cu-EDTA. Since line positions in 13C CPMAS spectra were not altered by the Cu-EDTA doping, major structural changes in the proteins due to Cu-EDTA doping are not likely. 1H T1 values were successfully reduced by a factor of 6-11. On the other hand, we were able to speed up the experiment by only up to 2.5 times to protect a probe from arcing under high-power 1H RF decoupling. We also tested decoupling by fast MAS at 20-30 kHz, which was successfully employed in our recent studies for paramagnetic systems.[37-39] However, the resolution without 1H RF decoupling is considerably limited for the diamagnetic poly-crystalline proteins.
Figure 1.

(a-d) 13C CPMAS spectra of protein crystals for (a, b) lysozyme and (c, d) ubiquitin prepared in D2O obtained (a, c) with and (b, d) without 10mM Cu-EDTA at 13C NMR frequency of 100.6 MHz. The recycle delays were set to (a) 0.5 s, (b) 1.1 s, (c) 1.0 s, and (d) 2.5 s. The spectra were acquired at a spinning speed of 10 kHz under 1H TPPM decoupling of (a, b) 71 kHz and (c, d) 90 kHz. Signals were accumulated during acquisition periods of 10 ms with (a) 28160, (b) 12800, (c) 14336, and (d) 5736 scans in a common total experimental time of 4 hours. During the CP period of 1.0 ms, 13C RF field was swept (a, b) from 56 kHz to 76 kHz and (c, d) from 52 kHz to 72 kHz, while 1H RF field was kept at (a, b) 76 kHz and (c, d) 72 kHz. All the spectra were processed with Gaussian line broadening of 25 Hz. At the right of the spectra in (a-d), microscope images of the corresponding protein micro/nano-crystals used for the NMR experiments are displayed in (e-h). The images were obtained at 32× magnification.
To overcome this problem, we employed a rotor-synchronous π-pulse train decoupling under ultra-fast MAS condition (40 kHz), which was recently proposed by Hafner and coworkers at Varian.[40] We found that this decoupling permits narrowing comparable to TPPM decoupling of 1H RF irradiation at 200 kHz under MAS at 40 kHz. An equivalent decoupling sequence was successfully applied for 1H decoupling for paramagnetic systems[39] and 19F decoupling for fluoro-polymers[41] under fast MAS (≥ 20 kHz). Figure 2 shows CPMAS spectra at a spinning speed of 40 kHz for (a, b) lysozyme and (c, d) ubiquitin microcrystals (a, c) with and (b, d) without 10 mM Cu-EDTA doping. The recycle delays for the lysozyme samples were (a) 0.18 s (1H T1 = 60 ms) and (b) 1.5 s (1H T1 = 500 ms) while the delays for the ubiquitin samples were (c) 180 ms (1H T1 = 60 ms) and (d) 2.5 s (1H T1 = 820 ms). We confirmed that faster signal accumulation with the low-duty-facor 1H decoupling sequence at a spinning speed of 40 kHz further enhanced sensitivity in the 13C CPMAS spectra by a factor of 2.7-2.9, which speeds up our experiments 7-8 fold. The resolution of the CPMAS spectra obtained with fast recycling in the presence of Cu-EDTA was comparable to that without Cu-EDTA. It is noteworthy that the effective RF duty factor due to 1H decoupling is only ∼1.1 % in the experiment with a recycle delay of 180 ms and an acquisition period of 20 ms. Because of the low RF duty factor and high efficiency of the 1H RF circuit of the fast MAS probe, we could collect the signals up to 30 ms of acquisition periods without any arcing problems. Thus, significant sensitivity enhancements with uncompromised resolution in this approach are possible under the fast MAS condition.
We prepared crystals in D2O and H2O without Cu-EDTA to examine solvent effects on 1H T1 values. Considerably longer 1H T1 was observed in D2O for ubiquitin and, to a lesser degree, for lysozyme, as shown in Table 1. The sensitivity enhancements for the proteins in H2O are less, particularly for ubiquitin. Nevertheless, considerable sensitivity gains (∼2) are still expected under the fast MAS and decoupling condition used for Fig. 2. Martin and Zilm reported that 1H T1 of unlabeled ubiquitin nanocrystals in H2O is 0.5 s.[17] Zilm and coworkers more recently reported average 1H T1 values of 300-400 ms for uniformly 2D- and 15N-labeled ubiquitin nanocrystal samples for which amide protons are back-exchanged in H2O, as well as for unlabeled ubiquitin.[42] Thus, the long 1H T1 observed for our ubiquitin sample in D2O cannot be simply explained by lower 1H density. Understanding this solvent dependence of 1H T1 requires more systematic work, and a fuller study is outside of the scope of this study.
Figure 3 shows 13C CPMAS spectra of ubiquitin microcrystals acquired at different 1H inversion recovery delays (a) without and (b) with 10 mM Cu-EDTA. This result clearly shows that the 1H T1 value of lysozyme is reduced by the introduction of Cu-EDTA in a uniform manner for different chemical groups and residues. After 50 ms, the 13C CPMAS spectra in (b) display a null signal in the presence of Cu-EDTA, while the spectra in (a) show a signal close to the null only after 800 ms without Cu-EDTA. 1H T1 values obtained from the experiments are (a) 830 ms and (b) 73 ms. In a recent study on uniformly 13C labeled ubiquitin by Igumenova et. al., it has been reported that a 2D 13C/13C chemical-shift correlation experiment at 1H frequency of 800 MHz required about 36 hours with recycle delay of 1.5 s.[43] Assuming that the RF-duty factor and the sample stability are not the limiting factor, it is possible to speed up this experiment up to seven times with a shorter 1H T1 value of ∼70 ms in the paramagnetic doping approach.
Figure 3.
1H inversion recovery delay (τIR) dependence of 13C CPMAS spectra of ubiquitin microcrystals prepared in D2O (a) without and (b) with 10 mM Cu-EDTA obtained at 10 kHz MAS. The values of τIR used in the experiments are indicated in the figure.
Figure 4 shows Cu-EDTA concentration dependence of the longitudinal relaxation rate 1/T1 for the lysozyme sample. Figure 4 clearly demonstrates that 1/T1 is not linearly proportional to the concentration of Cu-EDTA. The slope is the largest at the lower Cu-EDTA concentration (5-10 mM). At higher concentration, the relaxation rate increases more slowly for a given increase in the Cu-EDTA concentration. This is probably because 1H T1 relaxation due to the paramagnetic reagents is mediated by a 1H-1H spin diffusion mechanism.[44] Since Cu-EDTA is hydrophilic, it is most likely that 1H polarization is retrieved more quickly by paramagnetic T1 relaxation around the water-accessible protein surface, where Cu-EDTA is easily accessible. The recovered polarization can be transferred across the molecule by 1H-1H spin diffusion. We noticed that there is minor solvent dependence of 1H T1 even in the presence of Cu-EDTA. Slightly lower 1H 1/T1 rates in D2O may be attributed to slower 1H-1H spin diffusion in D2O, in which amide hydrogens are exchanged for 2D. In spite of the difference, we found that in both H2O and D2O solvents, 1H T1 for the protein microcrystal samples can be reduced to 20-30 ms in the presence of 75 mM Cu-EDTA.
Figure 4.

Cu-EDTA concentration dependence of the 1H relaxation rate (1/T1) for lysozyme microcrystals prepared in D2O (triangle) and H2O (square). The 1H T1 values were measured by the inversion recovery experiment shown in Fig. 3. The error bars indicate standard errors in the measurements of 1/T1.
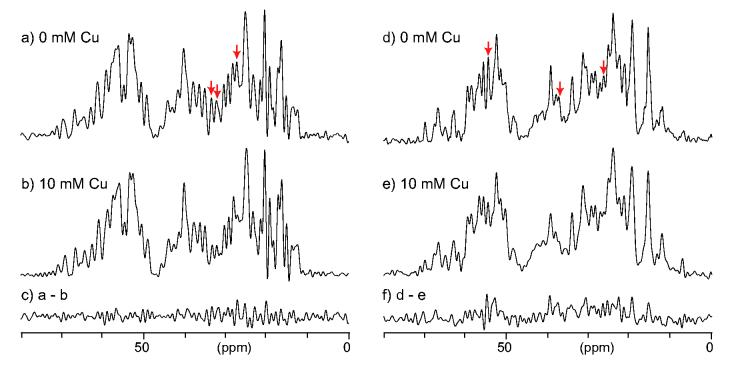
In Fig. 5, we examined resolution of 13C CPMAS spectra for (a, b) lysozyme and (d, e) ubiquitin microcrystals (a, d) without and (b, e) with 10 mM Cu-EDTA, where only the aliphatic region of the spectra are displayed for clarity. The spectra in (c, f) show the difference between the spectra without and with Cu-EDTA for (c) lysozyme and (f) ubiquitin. As discussed above, in (b, e), no major changes were observed by the addition of 10 mM Cu-EDTA. Although 1H T1 values were reduced by factors of (b) 8.3 and (e) 13.7, the line broadening is only subtle (10-20 %). However, we observed that a few resonances observed in Fig. 5(a, d) are substantially reduced in intensity in the spectra in (b, e) (indicated by arrows). The quenched signals may be assigned to residues exposed to the protein surface, at which 13C spins can be subject to much faster paramagnetic T2 relaxation due to Cu-EDTA in water phase. It is known that paramagnetic 13C T2 relaxation rates are proportional to 1/R6, where R is the distance between a 13C spin and a paramagnetic ion.[45; 46] Hong et al. reported that the paramagnetic quenching can be utilized to measure distances of 13C sites in membrane bound peptides from the membrane surface with ranging Mn(II) concentration in water phase.[46] Thus, it is probable that signals for residues exposed to the protein surface are selectively quenched by the paramagnetic effects, while the majority of signals for other residues are unaffected. Further studies are needed to examine the possibilities of using the effects for structural analysis. The difference spectra in (c, f) also suggest that the effects of paramagnetic quenching are relatively minor; the integral intensities of (c) and (f) are only 2 and 11 % of those for (a) and (d), respectively. Considering that the amount of the proteins may differ by 5-10 % between (d) and (e) (or (a) and (b)), the overall signal quenching due to the Cu(II) addition is less than the significant level. We also found that the resolution in 13C CPMAS spectrum of lysozyme was not substantially degraded by the addition of 75 mM Cu-EDTA (data not shown). The 1H T1 value in this condition is about 27 ms, which is one tenth of the 1H T1 for the same protein without Cu-EDTA. Therefore, for the majority of the signals, further sensitivity enhancement is possible with more optimized pulse sequences for this purpose.
Figure 5.
13C CPMAS spectra of (a, b) lysozyme and (d, e) ubiquitin protein crystals with Cu-EDTA concentrations of (a, d) 0 mM, (b, e) 10 mM. The spectra in (c, f) are the difference between the spectra obtained with and without Cu-EDTA for the same sample. The spectra were obtained at 13C NMR frequency of 100.6 MHz at a spinning speed of 40 kHz with 1H π pulse-train decoupling during the acquisition periods of 10 ms. The shorter acquisition periods were adopted to minimize the noise level for comparison. The recycle delays were (a) 1.50 s, (b) 0.18 s, (d) 2.50 s, and (e) 0.18 s. All the spectra were processed with Gaussian line broadening of 25 Hz.
Conclusion
In this study, we demonstrated that 1H T1 values of the two model proteins, lysozyme and ubiquitin, in microcrystals can be reduced to ∼60 ms by Cu-EDTA doping without major degradation in the resolution of their 13C CPMAS spectra. We also demonstrated that significant sensitivity enhancements of 1D 13C CPMAS spectra were attained for these proteins by faster signal repetitions using the reduced 1H T1 values under fast MAS. Although the full potential of this approach for sensitivity enhancement is still restricted only in the fast MAS condition, our study presented a new opportunity to gain significant sensitivity enhancements using paramagnetic doping in biomolecular SSNMR. In this communication, we focused on testing this approach in 1D 13C CPMAS for unlabeled ubiquitin and lysozyme microcrystals. It is probable that the present approach can be adopted in CPMAS or static experiments of other insoluble proteins. The successful reduction of 1H T1 relaxation times in the present experiments will also open new possibilities of sensitivity enhancements in more advanced experiments such as multi-dimensional 13C SSNMR of uniformly 13C-labeled proteins[18-25] and various distance measurements[3; 11; 13; 47-50] for selectively 13C-labeled protein samples in our future studies.
Acknowledgement
We thank Dr. Siegfried Hafner at Varian Germany for helpful discussion on the π-pulse decoupling scheme under fast MAS. This work was supported in part by the Alzheimer's Association (NIRG 035123), the Dreyfus Foundation Teacher-Scholar Award program, the NSF CAREER program (CHE 449952), the NIH/NIA RO1 grant (1R01 AG028490-01) for YI, and EU PF6 UPMAN project for AS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Opella SJ. NMR and membrane proteins. Nat. Struct. Biol. 1997;4:845–848. [PubMed] [Google Scholar]
- 2.Wildman KAH, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Weliky DP. Solid-state nuclear magnetic resonance evidence for parallel and antiparallel strand arrangements in the membrane-associated HIV-1 fusion peptide. Biochemistry. 2003;42:11879–11890. doi: 10.1021/bi0348157. [DOI] [PubMed] [Google Scholar]
- 4.Opella SJ, Marassi FM. Structure determination of membrane proteins by NMR spectroscopy. Chem. Rev. 2004;104:3587–3606. doi: 10.1021/cr0304121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant CV, Frydman V, Harwood JS, Frydman L. Co-59 solid-state NMR as a new probe for elucidating metal binding in polynucleotides. J. Am. Chem. Soc. 2002;124:4458–4462. doi: 10.1021/ja012353j. [DOI] [PubMed] [Google Scholar]
- 6.Griffin RG. Dipolar recoupling in MAS spectra of biological solids. Nat. Struct. Biol. 1998;5:508–512. doi: 10.1038/749. [DOI] [PubMed] [Google Scholar]
- 7.McDermott AE. Structural and dynamic studies of proteins by solid-state NMR spectroscopy: rapid movement forward. Curr. Opin. Struct. Biol. 2004;14:554–561. doi: 10.1016/j.sbi.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Baldus M. Solid-state NMR spectroscopy: Molecular structure and organization at the atomic level. Angew. Chem. Int. Edit. 2006;45:1186–1188. doi: 10.1002/anie.200503223. [DOI] [PubMed] [Google Scholar]
- 9.Hong M. Resonance assignment of C-13/N-15 labeled solid proteins by two- and three-dimensional magic-angle-spinning NMR. J. Biomol. NMR. 1999;15:1–14. doi: 10.1023/a:1008334204412. [DOI] [PubMed] [Google Scholar]
- 10.Antzutkin ON, Balbach JJ, Leapman RD, Rizzo NW, Reed J, Tycko R. Multiple quantum solid-state NMR indicates a parallel, not antiparallel, organization of beta-sheets in Alzheimer's beta-amyloid fibrils. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13045–13050. doi: 10.1073/pnas.230315097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii Y. 13C-13C dipolar recoupling under very fast magic angle spinning in solid-state NMR: Applications to distance measurements, spectral assignments, and high-throughput secondary-structure elucidation. J. Chem. Phys. 2001;114:8473–8483. [Google Scholar]
- 12.Petkova A, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. A structural model for Alzheimer's b-amyloid peptide fibrils based on experimental constraints from solid-state NMR spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gullion T, Kishore R, Asakura T. Determining dihedral angles and local structure in silk peptide by C-13-H-2 REDOR. J. Am. Chem. Soc. 2003;125:7510–7511. doi: 10.1021/ja0342345. [DOI] [PubMed] [Google Scholar]
- 14.Chimon S, Ishii Y. Capturing intermediate structures of Alzheimer's b-amyloid, Ab(1-40), by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2005;127:13472–13473. doi: 10.1021/ja054039l. [DOI] [PubMed] [Google Scholar]
- 15.Heise H, Hoyer W, Becker S, Andronesi OC, Riedel D, Baldus M. Molecular-level secondary structure, polymorphism, and dynamics of full-length alpha-synuclein fibrils studied by solid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15871–15876. doi: 10.1073/pnas.0506109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siemer AB, Ritter C, Ernst M, Riek R, Meier BH. High-resolution solid-state NMR spectroscopy of the prion protein HET-s in its amyloid conformation. Angew. Chem. Int. Edit. 2005;44:2441–2444. doi: 10.1002/anie.200462952. [DOI] [PubMed] [Google Scholar]
- 17.Martin RW, Zilm KW. Preparation of protein nanocrystals and their characterization by solid state NMR. J. Magn. Reson. 2003;165:162–174. doi: 10.1016/s1090-7807(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 18.Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H. Structure of a protein determined by solid-state magic-angle- spinning NMR spectroscopy. Nature. 2002;420:98–102. doi: 10.1038/nature01070. [DOI] [PubMed] [Google Scholar]
- 19.Bockmann A, Lange A, Galinier A, Luca S, Giraud N, Juy M, Heise H, Montserret R, Penin F, Baldus M. Solid state NMR sequential resonance assignments and conformational analysis of the 2 × 10.4 kDa dimeric form of the Bacillus subtilis protein Crh. J. Biomol. NMR. 2003;27:323–339. doi: 10.1023/a:1025820611009. [DOI] [PubMed] [Google Scholar]
- 20.Marulanda D, Tasayco ML, McDermott A, Cataldi M, Arriaran V, Polenova T. Magic angle spinning solid-state NMR spectroscopy for structural studies of protein interfaces. Resonance assignments of differentially enriched Escherichia coli thioredoxin reassembled by fragment complementation. J. Am. Chem. Soc. 2004;126:16608–16620. doi: 10.1021/ja0464589. [DOI] [PubMed] [Google Scholar]
- 21.Paulson EK, Morcombe CR, Gaponenko V, Dancheck B, Byrd RA, Zilm KW. High-sensitivity observation of dipolar exchange and NOEs between exchangeable protons in proteins by 3D solid-state NMR spectroscopy. J. Am. Chem. Soc. 2003;125:14222–14223. doi: 10.1021/ja037559u. [DOI] [PubMed] [Google Scholar]
- 22.Franks WT, Zhou DH, Wylie BJ, Money BG, Graesser DT, Frericks HL, Sahota G, Rienstra CM. Magic-angle spinning solid-state NMR spectroscopy of the beta 1 immunoglobulin binding domain of protein G (GB1): N-15 and C-13 chemical shift assignments and conformational analysis. J. Am. Chem. Soc. 2005;127:12291–12305. doi: 10.1021/ja044497e. [DOI] [PubMed] [Google Scholar]
- 23.Pauli J, Baldus M, van Rossum B, de Groot H, Oschkinat H. Backbone and side-chain C-13 and N-15 signal assignments of the alpha-spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 tesla. Chembiochem. 2001;2:272–281. doi: 10.1002/1439-7633(20010401)2:4<272::AID-CBIC272>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Giraud N, Bockmann A, Lesage A, Penin F, Blackledge M, Emsley L. Site-specific backbone dynamics from a crystalline protein by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2004;126:11422–11423. doi: 10.1021/ja046578g. [DOI] [PubMed] [Google Scholar]
- 25.Lesage A, Emsley L, Penin F, Bockmann A. Investigation of dipolar-mediated water-protein interactions in microcrystalline Crh by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2006;128:8246–8255. doi: 10.1021/ja060866q. [DOI] [PubMed] [Google Scholar]
- 26.Stringer JA, Bronnimann CE, Mullen CG, Zhou DHH, Stellfox SA, Li Y, Williams EH, Rienstra CM. Reduction of RF-induced sample heating with a scroll coil resonator structure for solid-state NMR probes. J. Magn. Reson. 2005;173:40–48. doi: 10.1016/j.jmr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Martin RW, Paulson EK, Zilm KW. Design of a triple resonance magic angle sample spinning probe for high field solid state nuclear magnetic resonance. Review of Scientific Instruments. 2003;74:3045–3061. [Google Scholar]
- 28.Ernst M, Samoson A, Meier BH. Low-power decoupling in fast magic-angle spinning NMR. Chem. Phys. Lett. 2001;348:293–302. [Google Scholar]
- 29.Ishii Y, Tycko R. Sensitivity enhancement in solid state 15N NMR by indirect detection with high-speed magic angle spinning. J. Magn. Reson. 2000;142:199–204. doi: 10.1006/jmre.1999.1976. [DOI] [PubMed] [Google Scholar]
- 30.Long HW, Tycko R. Biopolymer conformational distributions from solid-state NMR: alpha-helix and 3(10)-helix contents of a helical peptide. J. Am. Chem. Soc. 1998;120:7039–7048. [Google Scholar]
- 31.Iwahara J, Anderson DE, Murphy EC, Clore GM. EDTA-derivatized deoxythymidine as a tool for rapid determination of protein binding polarity to DNA by intermolecular paramagnetic relaxation enhancement. J. Am. Chem. Soc. 2003;125:6634–6635. doi: 10.1021/ja034488q. [DOI] [PubMed] [Google Scholar]
- 32.Samoson A. Extended magic-angle spinning. In: Grant DM, Harris RK, editors. Encyclopedia of Nuclear Magnetic Resonance. John Wiley & Sons, Ltd; New York: 2002. pp. 59–64. [Google Scholar]
- 33.Samoson A, Tuherm T, Past J. Rotation sweep NMR. Chem. Phys. Lett. 2002;365:292–299. [Google Scholar]
- 34.Samoson A, Tuherm T, Past J, Reinhold A, Anupold T, Heinmaa N. New horizons for magic-angle spinning NMR. In: J. K, editor. New Techniques in Solid-State Nmr. Springer-Verlag; Berlin Heidelberg: 2005. pp. 15–31. [DOI] [PubMed] [Google Scholar]
- 35.Metz G, Wu X, Smith SO. Ramped-amplitude cross polarization in magic-angle-spinning NMR. J. Magn. Reson. A. 1994;110:219–27. [Google Scholar]
- 36.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995;103:6951–8. [Google Scholar]
- 37.Ishii Y, Chimon S, Wickramasinghe NP. A new approach in 1D and 2D 13C high resolution solid-state NMR spectroscopy of paramagnetic organometallic complexes by very fast magic-angle spinning. J. Am. Chem. Soc. 2003;125:3438–3439. doi: 10.1021/ja0291742. [DOI] [PubMed] [Google Scholar]
- 38.Wickramasinghe NP, Ishii Y. Sensitivity enhancement, assignment, and distance measurement in 13C solid-state NMR spectroscopy for paramagnetic systems under fast magic angle spinning. J. Magn. Reson. 2006;181:233–243. doi: 10.1016/j.jmr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Ishii Y, Wickramasinghe NP. 1H and 13C high-resolution solid-state NMR of paramagnetic compounds under Very Fast Magic Angle Spinning. In: Webb G, editor. Modern Magnetic Resonances; Springer, Berlin: In press. [Google Scholar]
- 40.Hafner S, Palmer A, Cormos M. Pulsed Heteronuclear Decoupling under Ultra-Fast Magic-Angle Spinning. ENC 2006; Asilomar, CA: 2006. [Google Scholar]
- 41.Liu SF, Schmidt-Rohr K. High-resolution solid-state C-13 NMR of fluoropolymers. Macromolecules. 2001;34:8416–8418. [Google Scholar]
- 42.Morcombe CR, Gaponenko V, Byrd RA, Zilm KW. C-13 CPMAS spectroscopy of deuterated proteins: CP dynamics, line shapes, and T-1 relaxation. J. Am. Chem. Soc. 2005;127:397–404. doi: 10.1021/ja045581x. [DOI] [PubMed] [Google Scholar]
- 43.Igumenova TI, McDermott AE, Zilm KW, Martin RW, Paulson EK, Wand AJ. Assignments of carbon NMR resonances for microcrystalline ubiquitin. J. Am. Chem. Soc. 2004;126:6720–6727. doi: 10.1021/ja030547o. [DOI] [PubMed] [Google Scholar]
- 44.Abragam A. Principles of nuclear magnetism. Oxford University Press; New York: 1961. [Google Scholar]
- 45.Solomon I. Relaxation processes in a system of two spins. Phys. Rev. 1995;99:559–565. [Google Scholar]
- 46.Buffy JJ, Hong T, Yamaguchi S, Waring AJ, Lehrer RI, Hong M. Solid-state NMR investigation of the depth of insertion of protegrin-1 in lipid bilayers using paramagnetic Mn2+ Biophys. J. 2003;85:2363–2373. doi: 10.1016/s0006-3495(03)74660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gullion T, Schaefer J. Rotational-echo double resonance NMR. J. Magn. Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Long JR, Shaw WJ, Stayton PS, Drobny GP. Structure and dynamics of hydrated statherin on hydroxyapatite as determined by solid-state NMR. Biochemistry. 2001;40:15451–15455. doi: 10.1021/bi010864c. [DOI] [PubMed] [Google Scholar]
- 49.Antzutkin ON, Leapman RD, Balbach JJ, Tycko R. Supramolecular structural constraints on Alzheimer's beta-amyloid fibrils from electron microscopy and solid-state nuclear magnetic resonance. Biochemistry. 2002;41:15436–15450. doi: 10.1021/bi0204185. [DOI] [PubMed] [Google Scholar]
- 50.Jaroniec CP, Tounge BA, Herzfeld J, Griffin RG. Frequency selective heteronuclear dipolar recoupling in rotating solids: Accurate C-13-N-15 distance measurements in uniformly C-13,N-15-labeled peptides. J. Am. Chem. Soc. 2001;123:3507–3519. doi: 10.1021/ja003266e. [DOI] [PubMed] [Google Scholar]
- 51.Gullion T. The effect of amplitude imbalance on compensated Carr-Purcell Sequence. J. Magn. Reson. A. 1993;101:320–323. [Google Scholar]