TO THE EDITOR
Corticotropin-releasing hormone (CRH), a 41-amino-acid peptide, is a main regulator of the systemic response that suppresses immune response on a central level (Chrousos and Gold, 1992). In the peripheral sites, including the epidermis, CRH acts as potent proinflammatory agent (Karalis et al., 1991; Theoharides et al., 2004; Slominski et al., 2006a, b). In normal keratinocytes, CRH activates the master regulator of inflammation NF-κB (Zbytek et al., 2004) and stimulates the expression of ICAM-1 and HLA-DR (Quevedo et al., 2001).
Epidermal keratinocytes take part in inflammatory responses initiated by various stressors (Barker et al., 1991). Induction of production of inflammatory cytokines in normal keratinocytes by bacteria occurs via toll-like receptor (TLR)-2 and TLR-4 (Nagy et al., 2005). Therefore, there is a need for the functional study of the link between bacteria, CRH, and inflammatory cytokines in human keratinocytes.
Normal human epidermal adult keratinocytes (purchased from Cascade Biologics, Portland, OR) were incubated with CRH, lipopolysaccharide (LPS; TLR-4 agonist, Sigma, St Louis, MO) or PAM3CSK4 (TLR-2 agonist, EMC microcollections, Tuebingen, Germany) in EpiLife medium with epilife defined growth supplement (EDGS) supplement and antibiotics (Cascade Biologics, Portland, OR). Thereafter, supernatants were collected and RNA extracted from the cells. Supernatants were concentrated on C18 SEP-COL-UMNS (Peninsula Laboratories, San Carlos, CA) and CRH was measured with ELISA (Phoenix Pharmaceuticals, Belmont, CA). CRH mRNA and 18SrRNA amounts were quantitated with real-time PCR using Taqman reagents (Applied Biosystems, Foster City, CA) as described previously (Zbytek et al., 2006). Cytokine mRNAs were quantitated by the following ABI reagents: Hs00174128_m1, Hs00174097_m1, Hs00174131_m1, and Hs00174086_m1. Small interfering RNA (siRNA) (CRH-R1-specific and scrambled, Ambion, Austin, TX) was transfected into the cells as described (Slominski et al., 2005). The medical ethical committee of University of Tennessee approved all described studies.
LPS (TLR-4 agonist) but not PAM3CSK4 (TLR-2 agonist) stimulated the expression of CRH mRNA (Figure 1a and b). The effect was most significant at 1 hour at 1,000 ng/ml being 5.4-fold higher than in control. LPS also stimulated the release of the peptide to the supernatant (Figure 1c). At 24 hours, LPS at 1,000 ng/ml stimulated it 14.9- fold. As regards the physiological significance of effect of LPS on CRH production, it was previously shown that cells that initiate stress response in the organism (hypothalamic neurons) produce CRH upon LPS stimulation (Chrousos and Gold, 1992; Wei et al., 2002). We provide data showing that similar phenomenon takes place in the epidermal keratinocytes.
Figure 1. LPS stimulates CRH in normal human adult epidermal keratinocytes.
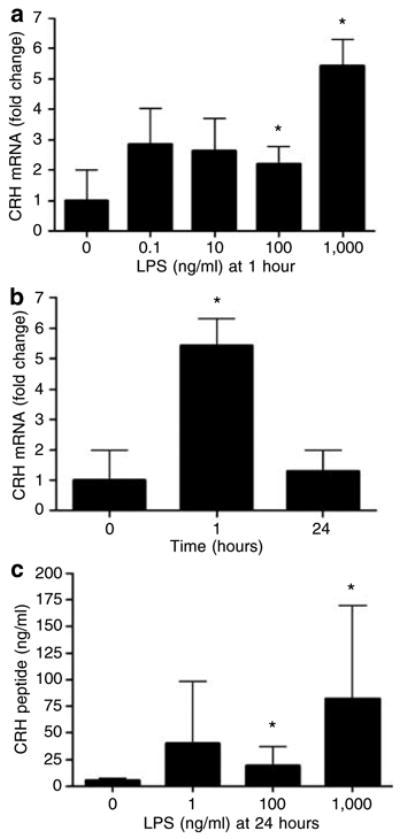
Cells were incubated (a) with LPS (0–1,000 ng/ml) for 1 hour and (b) with LPS (1,000 ng/ml) for 1–24 hours. Cells were lysed, total RNA extracted, and real-time PCRs (n=3) performed for CRH. (c) Cells were incubated with LPS (0–1,000 ng/ml). After 24 hours, the supernatants were collected, concentrated on C18 SEP-COLUMNS, and CRH determined by ELISA (n=4). Data are presented as mean ± SEM. *P< 0.05 between untreated and LPS-treated cells.
Having determined that LPS has stimulated production and release of CRH, we decided to test the effects of CRH on the production of selected cytokines that play a role in the regulation of the mode of immune response (T-helper cell 1 versus T-helper cell 2). The cytokines tested were tumor necrosis factor-α (TNF-α), IL-1β, IL-6, and IL-10. CRH stimulated the expression of IL-1β, IL-6, and TNF-α mRNAs, but did not stimulate the expression of IL-10 mRNA (whose amount was below the limit of detection) (Figure 2a–c). The most significant stimulatory effects, reached at 1 hour, were 12.9-fold (TNF-α), 8.5-fold (IL-1β), and 7.3-fold (IL-6). Hitherto, CRH stimulated the production of inflammatory and T-helper cell 1- (TNF-α) rather than T-helper cell 2- (IL-10) upregulating cytokines. This effect of CRH is consistent with its previously reported proinflammatory (Theoharides et al., 2004) and selective T-helper cell 1-enhancing effects (Benou et al., 2005).
Figure 2. CRH and CRH-R1 participate in the LPS-mediated stimulation of inflammatory cytokine mRNA expression in normal human adult epidermal keratinocytes.
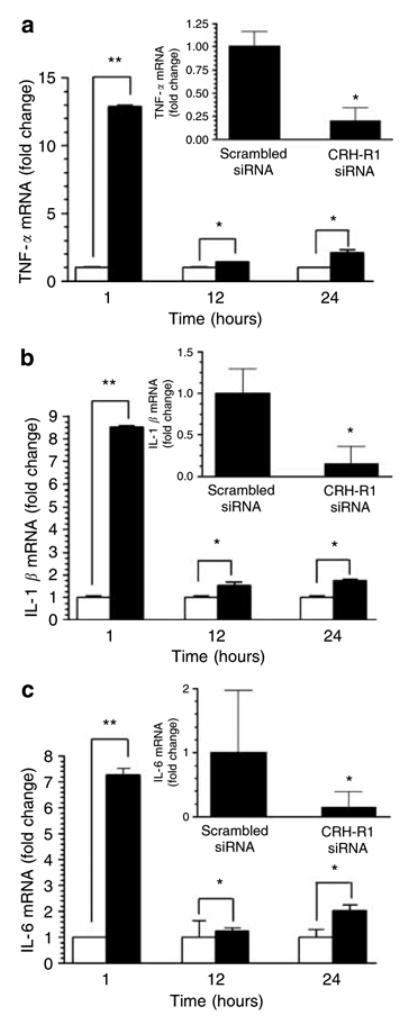
Main panels: Keratinocytes were incubated with or without 100 nM CRH for 1–24 hours. Insets: Keratinocytes were transfected with either scrambled (control) or CRH-R1-specific siRNA. Forty-eight hours after transfection, media were changed and cells stimulated with LPS (1,000 ng/ml) for 1 hour. After incubations, cells were lysed, total RNA extracted, and real-time PCRs performed for (a) TNF-α, (b) IL-1β, and (c) IL-6. Data are presented as mean ± SEM (n=3). *P<0.05, **P<0.005 between untreated and CRH-treated cells or cells transfected with scrambled or CRH-R1 siRNA (insets).
To further document a role of CRH-CRH- R1 signaling in the response of keratinocytes to bacteria, we transfected keratinocytes with CRH-R1 siRNA or scrambled (control) siRNA. After 48 hours, the cells were stimulated with LPS, and then, after 1 hour, levels of cytokine mRNA measured. CRH-R1 siRNA-transfected cells responded with diminished production of TNF-α (by 80%), IL-1β (by 84%), and IL-6 (by 84%) as compared to cells transfected with control siRNA (insets in Figure 2a–c). This indicates that enhanced production of inflammatory cytokines is dependent on CRH-R1 expression.
Locally released CRH may play a role in inflammatory and immunemediated skin diseases such as psoriasis, alopecia areata or acne (O’Kane et al., 2006). We have previously reported that CRH stimulates epidermal keratinocyte differentiation (Zbytek and Slominski, 2005), which exclusively express CRH-R1 (Slominski et al., 2006b). Zouboulis et al. (2002) reported that CRH induces synthesis of sebaceous lipids. UV was shown to upregulate CRH release (Zbytek et al., 2006). This report shows that CRH may serve as a local autocrine mediator that amplifies inflammatory responses to bacterial antigens and hence may be an important target for the treatment of several skin conditions.
Acknowledgments
The work was supported by National Institutes of Health (Grant No. AR047079 to A.S.) and Johnson and Johnson Skin Research Training Grant (B.Z., A.S. as mentor). Real-time reverse transcription- PCR was performed on ABI Prism 7770 in the Molecular Resource Center, University of Tennessee, Memphis, TN. This work was performed in Memphis, TN.
Abbreviations
- CRH
corticotropin-releasing hormone
- LPS
lipopolysaccharide
- siRNA
small interfering RNA
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–4. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Benou C, Wang Y, Imitola J, VanVlerken L, Chandras C, Karalis KP, et al. Corticotropin- releasing hormone contributes to the peripheral inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2005;174:5407–13. doi: 10.4049/jimmunol.174.9.5407. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropinreleasing hormone in vivo. Science. 1991;254:421–3. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–8. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- O’Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immunemediated cutaneous inflammatory disease. Exp Dermatol. 2006;15:143–53. doi: 10.1111/j.1600-0625.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Quevedo ME, Slominski A, Pinto W, Wei E, Wortsman J. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim. 2001;37:50–4. doi: 10.1290/1071-2690(2001)037<0050:peocrh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006a;206:780–91. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006b;11:2230–48. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–8. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Wei R, Phillips TM, Sternberg EM. Specific up-regulation of CRH or AVP secretion by acetylcholine or lipopolysaccharide in inflammatory susceptible Lewis rat fetal hypothalamic cells. J Neuroimmunol. 2002;131:31–40. doi: 10.1016/s0165-5728(02)00251-5. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J Endocrinol. 2004;181:R1–7. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Slominski AT. Corticotropin- releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–26. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Wortsman J, Slominski A. Characterization of a UVB induced corticotropin releasing hormone - proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–47. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, et al. Corticotropinreleasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci USA. 2002;99:7148–53. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]


