Summary
RNA synthesis and processing are coordinated by proteins that associate with RNA polymerase II (pol II) during transcription elongation. The yeast Paf1 complex interacts with RNA pol II and mediates histone modifications during elongation. To elucidate the functions of this complex, we isolated missense mutations in the gene encoding the Rtf1 subunit and used them to identify functionally interacting proteins. We identified NAB3 as a dosage suppressor of rtf1. Nab3, together with Nrd1, directs 3′ end formation of nonpolyadenylated RNA pol II transcripts, such as snoRNAs. Deletion of Paf1, but not the Set1, Set2, or Dot1 histone methyltransferases, causes accumulation of snoRNA transcripts that are extended at their 3′ ends. The Paf1 complex associates with and facilitates Nrd1 recruitment to the SNR47 gene, suggesting a direct involvement in 3′ end formation. Our results reveal a posttranscriptional function for the Paf1 complex, which appears unrelated to its role in histone methylation.
Introduction
Production of mature transcripts by RNA pol II involves a tight coupling between RNA synthesis and processing. Proteins that cap 5′ ends of nascent transcripts, remove introns from RNA, and modify 3′ ends by RNA cleavage and polyadenylation associate with RNA pol II during transcription elongation (Bentley, 2005; Proudfoot, 2004). The association of these RNA processing factors with RNA pol II requires the carboxy-terminal domain (CTD) on the largest subunit of RNA pol II and is dependent upon the phosphorylation state of the CTD. The RNA pol II CTD consists of tandem repeats of the consensus heptad sequence YSPTSPS, with the serine residues at positions 2 and 5 serving as important sites for phosphorylation (Kobor and Greenblatt, 2002). Changes in the pattern of CTD phosphorylation during the transcription cycle lead to an exchange of RNA pol II-associated factors that aid in RNA synthesis, processing, packaging, and export (Bentley, 2005; Jensen et al., 2003; Proudfoot, 2004).
The importance of the CTD and its modification to RNA processing has been studied extensively. Enzymes that add a 7-methylguanosine cap to the 5′ end of an RNA pol II transcript interact directly with the CTD in its Ser5-phosphorylated state in vitro and associate with the 5′ ends of RNA pol II-transcribed genes in vivo (Cho et al., 1997; Komarnitsky et al., 2000; McCracken et al., 1997a; Schroeder et al., 2000). In yeast, recruitment of capping enzymes to actively transcribed genes is dependent upon Kin28, the TFIIH-associated kinase responsible for Ser5 phosphorylation at the initiation to elongation transition (Komarnitsky et al., 2000; Schroeder et al., 2000). Components of the pre-mRNA splicing machinery also associate with elongating RNA pol II, and in mammalian cells, the CTD is required for efficient splicing (McCracken et al., 1997b). As elongation proceeds across a gene, the pattern of CTD phosphorylation changes from a predominantly Ser5-phosphorylated form to a predominantly Ser2-phosphorylated form (Komarnitsky et al., 2000). Pre-mRNA cleavage and polyadenylation factors are recruited to elongating RNA pol II near the polyA site in a process that requires CTD phosphorylation by Ctk1, the major Ser2 kinase in yeast (Ahn et al., 2004; Kim et al., 2004). Interestingly, a reciprocal regulatory relationship exists between certain RNA processing factors and RNA pol II. Whereas cotranscriptional association of RNA pol II with splicing factors stimulates pre-mRNA splicing, U snRNPs working together with the mammalian transcription elongation factor TAT-SF1 can also stimulate transcription elongation (Fong and Zhou, 2001). Moreover, in both yeast and human cells, capping enzymes regulate early steps in elongation (Bentley, 2005).
In addition to proteins with direct roles in RNA maturation, proteins that regulate the activity of RNA pol II or chromatin structure associate with the polymerase during elongation. These transcription elongation factors include the yeast Spt4-Spt5 complex, which has been shown to both repress and stimulate elongation (Hartzog et al., 2002). The Spt4-Spt5 complex interacts genetically and physically with two other essential elongation factors, Spt6 and FACT, both of which influence chromatin structure during elongation (Hartzog et al., 2002; Kaplan et al., 2003; Krogan et al., 2002; Lindstrom et al., 2003; Mason and Struhl, 2003).
For other factors that associate with elongating RNA pol II, the precise mechanisms by which they influence RNA synthesis are less clear. One such factor is the conserved Paf1 complex. In yeast, this complex is minimally composed of Paf1, Ctr9, Cdc73, Rtf1, and Leo1. Evidence suggests that the Paf1 complex functions during transcription elongation. First, the Paf1 complex physically associates with RNA pol II and the coding regions of actively transcribed genes (Krogan et al., 2002; Mueller and Jaehning, 2002; Simic et al., 2003; Squazzo et al., 2002). Second, members of the Paf1 complex exhibit strong genetic and physical interactions with proteins that regulate transcription elongation, including Spt4-Spt5 and yFACT, or proteins that control the phosphorylation state of RNA pol II (Costa and Arndt, 2000; Krogan et al., 2002; Mueller and Jaehning, 2002; Squazzo et al., 2002). Third, mutations in genes encoding members of the Paf1 complex confer sensitivity to base analogs, such as 6-azauracil (6AU) (Krogan et al., 2002; Riles et al., 2004; Squazzo et al., 2002). Fourth, biochemical assays have revealed a requirement for Paf1 and Cdc73 in the synthesis of long transcripts in vitro (Rondon et al., 2004). However, chromatin immunoprecipitation (ChIP) assays have thus far failed to detect an effect of the Paf1 complex on transcription elongation rate or RNA pol II processivity in vivo (Mason and Struhl, 2005; Mueller et al., 2004).
Several members of the Paf1 complex, Rtf1, Paf1, and Ctr9, are essential for certain histone modifications that occur during transcription. The Paf1 complex is required for the recruitment and activation of the Rad6 ubiquitin conjugase and the Bre1 ubiquitin ligase, which ubiquitylate lysine 123 of histone H2B in yeast (Ng et al., 2003a; Wood et al., 2003; Xiao et al., 2005). Paf1 complex-dependent H2B ubiquitylation and recruitment of the Set1 histone methyltransferase complex are required for subsequent methylation of histone H3 lysine 4 (Krogan et al., 2003a; Ng et al., 2003b). The Paf1 complex is also required for histone H3 K79 methylation by the Dot1 methyltransferase, a modification important for transcriptional silencing (Krogan et al., 2003a; Ng et al., 2003a), and histone H3 K36 methylation by the Set2 methyltransferase during transcription elongation (Krogan et al., 2003b). Whether the Paf1 complex regulates steps in RNA synthesis or maturation by mechanisms unrelated to histone modification remains to be determined.
To identify additional functions of the Paf1 complex, we have isolated point mutations within RTF1 and used these mutations as a foundation for subsequent studies. Our results reveal a previously unrecognized role for the Paf1 complex in regulating 3′ end formation of certain RNA pol II transcripts. Specifically, we show that the Paf1 complex interacts genetically with Nab3 and Nrd1, two essential hnRNPs required for 3′ end formation of nonpolyadenylated RNA pol II transcripts (Steinmetz et al., 2001). This class of noncoding RNAs includes small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs) whose mature 3′ ends are produced by the nuclear exosome complex working in concert with Nab3, Nrd1, and other processing factors. We also show that mutations in genes coding for members of the Paf1 complex lead to the accumulation of snoRNA transcripts extended at their 3′ ends and reduced association of Nrd1 with a snoRNA gene in vivo. These results indicate a requirement for the Paf1 complex in RNA 3′ end formation and expand our understanding of this evolutionarily conserved complex beyond its role in histone modification.
Results
Characterization of Missense Mutations in RTF1
Mutations in RTF1 and genes encoding other members of the yeast Paf1 complex confer sensitivity to 6AU and suppression of transposable element insertion mutations within promoters (Spt phenotype = suppression of Ty) (Krogan et al., 2002; Riles et al., 2004; Squazzo et al., 2002). The Spt− phenotype is associated with defects in both transcription initiation and elongation (Hartzog et al., 2002; Winston and Sudarsanam, 1998). To elucidate the functions of the Paf1 complex, we performed a genetic screen to identify missense mutations in RTF1. We specifically screened for RTF1 mutations that confer stronger 6AUS and Spt− phenotypes at higher growth temperatures (37°C) than at lower growth temperatures (30°C). By screening for conditional mutations, we hoped to avoid the isolation of complete loss-of-function alleles. Three new missense mutations encoding single amino acid substitutions in Rtf1 were identified. The rtf1-100 mutation, which encodes a glutamine 172 to arginine (Q172R) substitution, confers a conditional Spt− phenotype, as indicated by suppression of the his4-912δinsertion mutation and cell growth on media lacking histidine at 37°C, but not at 30°C (Figure 1A). The rtf1-105 (V274D) and rtf1-107 (M289K) alleles both cause conditional sensitivity to 6AU and Spt−phenotypes; however, these mutations also cause weak Spt− phenotypes at 30°C (Figure 1A). The amino acids altered by the mutations lie within a highly conserved region of Rtf1 but are not themselves invariant across species (data not shown).
Figure 1. Characterization of rtf1 Point Mutants.

(A) Equivalent numbers of cells from wild-type (wt) (RTF1) (KY767), rtf1-105 (KKY64), rtf1-100 (KKY56), rtf1-107 (KKY58), and rtf1Δ (KY656) strains were spotted in 10-fold serial dilutions onto the indicated media. Sensitivity to 6AU was examined on SC-Ura medium supplemented with 50 μg/ml 6AU (5 days of incubation). The Spt− phenotype was analyzed on medium lacking histidine (3 days of incubation).
(B) Immunoblot analysis was performed on wt (KY406), rtf1-105 (V274D; KKY37), rtf1-100 (Q172R; KKY40), rtf1-107 (M289K; KKY45), and rtf1Δ (KY410) cells. Cultures were grown to early log phase, divided, and further incubated at 30°C or 37°C for 2 hr. Extract from 1 × 107 cells per sample was analyzed by immunoblotting with anti-Rtf1 and, as a loading control, anti-L3 antisera.
(C) Histone H3 K4 trimethylation was analyzed by immunoblotting of whole-cell extracts prepared from strains grown as in (B).
The conditional phenotypes of the rtf1 point mutants suggest that Rtf1 retains partial function at the permissive temperature (30°C). We performed immunoblot analysis to measure expression of Rtf1 from the conditional alleles. For each of the mutant strains, similar levels of Rtf1 were detected when cells were grown at 30°C or incubated for 2 hr at 37°C (Figure 1B). However, the rtf1-105 and rtf1-107 mutants express Rtf1 to lower levels than the wild-type (wt) control strain (Figure 1B). Steady-state levels of histone H3 K4 trimethylation in the mutant strains correlate with Rtf1 protein levels, indicating that the mutations do not cause a specific defect in this modification (Figure 1C).
NAB3 Is a Dosage Suppressor of the rtf1 Point Mutations
To identify proteins that functionally interact with the Paf1 complex, we performed a genetic screen for genes that suppress the phenotypes associated with the rtf1-107 mutation when overexpressed on yeast 2-micron plasmids. Several high-copy-number suppressors were identified, and one plasmid of particular interest suppressed the Spt− phenotype of rtf1-107 strains and contained the NAB3 gene within a large genomic fragment. Nab3 functions in the Nrd1 pathway for 3′ end formation of RNA pol II transcripts that are not polyadenylated (Conrad et al., 2000; Steinmetz et al., 2001). Together, the essential Nab3 and Nrd1 proteins recognize and bind to specific sequences within snRNA and snoRNA transcripts (Carroll et al., 2004) to promote transcript termination and 3′ end formation in a reaction that also requires the Sen1 helicase (Steinmetz et al., 2001) and components of the APT and CFI cleavage/polyadenylation factor complexes (Cheng et al., 2004; Dheur et al., 2003; Dichtl et al., 2004; Fatica et al., 2000; Morlando et al., 2002; Nedea et al., 2003; Steinmetz and Brow, 2003). Final maturation of snoRNA 3′ ends also requires the 3′ -to-5′ exonuclease activity of the nuclear exosome complex (Allmang et al., 1999; van Hoof et al., 2000). To determine whether NAB3 overexpression was responsible for the suppression, the NAB3 gene was subcloned into a 2-micron vector and transformed into rtf1 point mutant, rtf1Δ, and RTF1+ strains (Figure 2A). Although NAB3 overexpression suppressed the Spt− phenotypes caused by the three rtf1 point mutations (Figure 2A and data not shown), it did not suppress the phenotypes associated with deletion of RTF1 (Figure 2A). Therefore, NAB3 is not a bypass suppressor of rtf1.
Figure 2. Paf1 Complex Members Genetically Interact with NAB3 and NRD1.

(A) Vector (pRS425), the original 2μ library plasmid containing NAB3, and a NAB3 sub-clone (2μ NAB3) were transformed into wt (KY767), rtf1Δ (KY656), and rtf1-107 (KKY58) strains. Suppression of the Spt− phenotype was analyzed by spotting equivalent numbers of cells in 10-fold serial dilutions onto selective medium lacking histidine (5 days incubation).
(B) A plasmid shuffle experiment was performed by transforming pRS314, pRS314-NRD1, or pRS314-nrd1-5 into nrd1Δ RTF1(KKY129), nrd1Δ rtf1Δ (KKY128), or nrd1Δ cdc73Δ (KKY143) cells that contained pRS316-NRD1. Transformants were passaged on 5-FOA to select against the pRS316-NRD1 plasmid. Equivalent numbers of cells were spotted in 10-fold serial dilutions onto YPD (4 days incubation).
Genetic Interactions between Paf1 Complex Members and Nrd1
To test for functional interactions between Nrd1 and the Paf1 complex, we generated double mutant strains containing the nrd1-5 mutation and deletions of individual components of the Paf1 complex. The nrd1-5 allele alters a single amino acid in the RNA recognition motif of Nrd1 (Steinmetz and Brow, 1996) and causes a moderate temperature-sensitive growth phenotype (Figure 2B). However, strains that contain nrd1-5 in combination with a deletion of RTF1, CDC73, PAF1, or CTR9 exhibit strongly enhanced temperature sensitivity (Figure 2B and data not shown). These synthetic interactions between an nrd1 mutation and defects in the Paf1 complex are consistent with our discovery of NAB3 as a dosage suppressor of an rtf1 mutation. Taken together, our genetic results demonstrate a functional connection between the Paf1 complex and factors involved in polyA-independent 3′ end formation.
Defects in the Paf1 Complex Lead to the Accumulation of Transcriptional Readthrough Products at the SNR13 Locus
To investigate a possible role for the Paf1 complex in the 3′ end formation of snoRNAs, we used Northern analysis to detect transcripts produced from the snoRNA gene SNR13 and the downstream protein-coding gene TRS31. For strains defective in 3′ end formation, such as nrd1 and nab3 mutants, a hybridization probe specific for TRS31 detects an SNR13-TRS31 fusion RNA arising from readthrough of the SNR13 termination signals (Figure 3A, top). Northern analysis of RNAs expressed from the SNR13-TRS31 locus was performed on strains lacking members of the Paf1 complex. Whereas the normal TRS31 transcript was detected in all strains (Figure 3A, bottom), rtf1, paf1, and ctr9 mutants express an additional, lower mobility RNA that is also recognized by the TRS31 probe and corresponds to the product of transcriptional readthrough. The migration of this longer transcript is similar to that seen in strains lacking Ctk1 (Steinmetz et al., 2001). When compared to the Paf1 complex mutants, nrd1 and nab3 mutants accumulate higher levels of this 3′ -extended RNA, as might be expected for central components of the termination machinery (Steinmetz et al., 2001). These results indicate that the Paf1 complex plays an important role in the 3′ end formation of snoRNA transcripts.
Figure 3. SNR13 Readthrough Transcripts Accumulate in Paf1 Complex Mutant Strains.
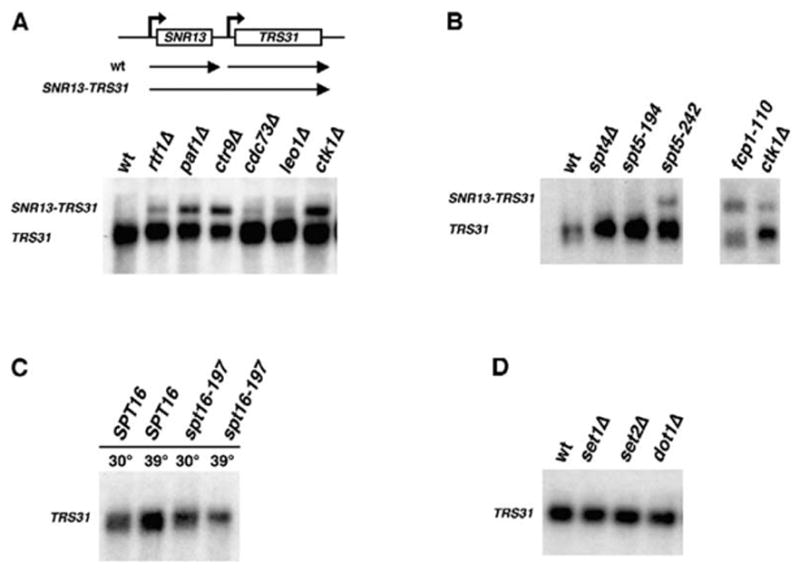
(A) Top, SNR13-TRS31 genomic locus and transcription products. The transcriptional readthrough product is indicated as SNR13-TRS31. (A–D) Northern analysis using a TRS31 probe and RNA isolated from the following yeast strains: (A) wt (KY661), rtf1Δ (KY656), paf1Δ (KY685), ctr9Δ (GHY1094), cdc73Δ (KY689), leo1Δ (GHY250), and ctk1Δ (KY586); (B) wt (FY118), spt4Δ (GHY166), spt5-194 (KY718), spt5-242 (FY1635), fcp1-110 (PCY448), and ctk1Δ (KY586); (C) SPT16 (FY118) and spt16-197 (FY348); and (D) wt (FY118), set1Δ (KY910), set2Δ (KY912), and dot1Δ (KY903). For each strain in (C), one-half of a log phase culture was shifted to 39°C for 80 min to inactivate the spt16-197 gene product (Kaplan et al., 2003). The remaining culture was kept at 30°C for 80 min.
The Paf1 complex physically and genetically interacts with Spt4-Spt5 and yFACT (Costa and Arndt, 2000; Krogan et al., 2002; Lindstrom et al., 2003; Mueller and Jaehning, 2002; Squazzo et al., 2002). To determine whether an effect on snR13 3′ end formation is specific to the Paf1 complex, we performed Northern analysis on strains containing a null mutation in SPT4 or specific point mutations in SPT5 and SPT16. Whereas spt4Δ and spt5-194 cells produce only the normal TRS31 transcript, spt5-242 cells accumulate the SNR13-TRS31 fusion transcript (Figure 3B). Interestingly, the spt5-242 mutation, but not the spt5-194 mutation, can be suppressed by defects in the Paf1 complex (Squazzo et al., 2002). The temperature-sensitive spt16-197 strain did not accumulate the 3′ -extended transcript at either the permissive or restrictive growth temperatures (Figure 3C). Therefore, an involvement in 3′ end formation is not a property shared by all RNA pol II elongation factors.
We also failed to observe a significant effect of 14 other RNA pol II transcription or chromatin factors on snR13 3′ end formation (Figure 3D and data not shown). These factors include those involved in histone modification (Set1, Set2, Dot1, Bre1, and Gcn5), chromatin remodeling (Isw1, Isw2, Snf2, Chd1, Swr1, and Arg82), or transcriptional activation (Swi4, Swi6, and Mbp1). The absence of the SNR13 transcriptional read-through product in set1Δ, set2Δ, and dot1Δ strains (Figure 3D) suggests that the function of the Paf1 complex in RNA 3′ end formation is independent of its role in histone H3 lysine 4, 36, and 79 methylation. Similar conclusions were drawn for Swd2, which is a member of both the Set1 histone methyltransferase complex and the APT cleavage/polyadenylation factor complex (Cheng et al., 2004; Dichtl et al., 2004).
Ctk1 and the Ser5-directed CTD phosphatase Ssu72 are required for efficient snoRNA 3′ end formation (Steinmetz and Brow, 2003; Steinmetz et al., 2001). We asked whether a mutation in FCP1, which encodes a Ser2-directed CTD phosphatase, also causes defects in snR13 synthesis. The fcp1-110 mutation was isolated in a synthetic lethal screen with an rtf1Δ mutation and is predicted to truncate the two TFIIF binding domains of Fcp1 (Costa and Arndt, 2000). The predicted fcp1-110 protein retains the phosphatase and BRCT domains, and strains expressing this protein exhibit nearly wt levels of Ser2 phosphorylation (M. Shirra and K.M.A., unpublished data). However, like defects in other CTD-modifying enzymes, the fcp1-110 mutation causes significant accumulation of the SNR13 readthrough product (Figure 3B).
Defects in the Paf1 Complex Lead to the Accumulation of Transcriptional Readthrough Products at the SNR47 Locus
To ask if there is a general requirement for the Paf1 complex in the proper 3′ end formation of snoRNAs, we assayed expression of the SNR47 gene. Northern hybridization probes were designed against SNR47 and the uncharacterized ORF YDR042c, which resides downstream of SNR47 in the genome (Figure 4A). A prominent ~1400 nt transcript was detected by the YDR042c probe in rtf1Δ, paf1Δ, ctr9Δ, and nrd1-5 strains (Figure 4A). This transcript, which is present in greatly reduced levels in wt strains, has the approximate size of the predicted SNR47-YDR042c chimeric transcript (~1150 nt + polyA tail). In addition, a transcript with the same mobility was detected by the SNR47 probe (Figure 4A). A shorter transcript (marked by asterisk in Figure 4A), which hybridized to both probes, may represent a degradation product of this readthrough transcript. Apparently, wt cells do not detectably express YDR042c under the growth conditions used in our experiments, in agreement with ChIP results that indicate a lack of RNA pol II occupancy over the 5′ end of this gene (Figures 5B and 6D and data not shown). Accumulation of 3′ -extended RNAs at both the SNR13 and SNR47 loci suggests that the Paf1 complex may be generally required for proper snoRNA synthesis.
Figure 4. Deletion of Paf1 Complex Members Impairs snR47 3′ End Formation.
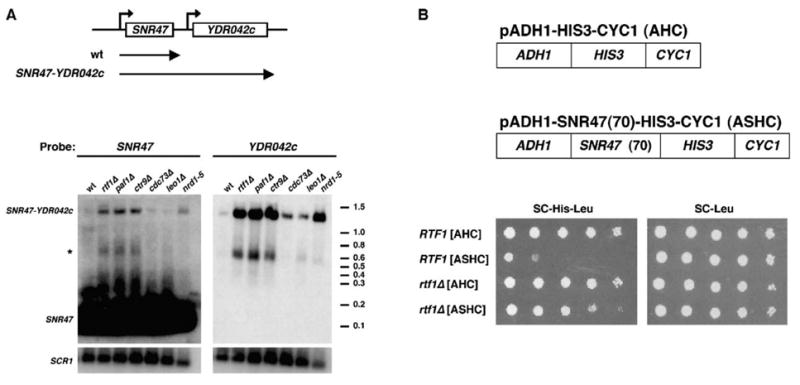
(A) Top, the SNR47 genomic locus and transcripts detected in wt cells or mutants defective in snR47 3′ end formation (SNR47-YDR042c). Transcription of YDR042c is not detected in wt cells. Bottom, Northern analysis on RNA from wt (FY118), rtf1Δ (KY656), paf1Δ (KY685), ctr9Δ (GHY1094), cdc73Δ (KY689), leo1Δ (GHY250), and nrd1-5 cells. RNA samples were loaded in duplicate on the same gel, one-half of which was subjected to Northern analysis with a YDR042c probe and the other half with an SNR47 probe. The positions of ethidium-bromide-stained RNA molecular weight markers are shown. Filters were reprobed for SCR1 transcript levels as a loading control.
(B) Top, plasmids used in the reporter assay to monitor snR47 3′ end formation. Bottom, RTF1 (KY669) and rtf1Δ (KY560) cells transformed with the LEU2-marked plasmids AHC and ASHC were plated in 10-fold serial dilutions onto SC-His-Leu and SC-Leu media (2 days incubation at 30°C).
Figure 5. RNA Pol II and Paf1 Colocalize to the SNR47 Gene.
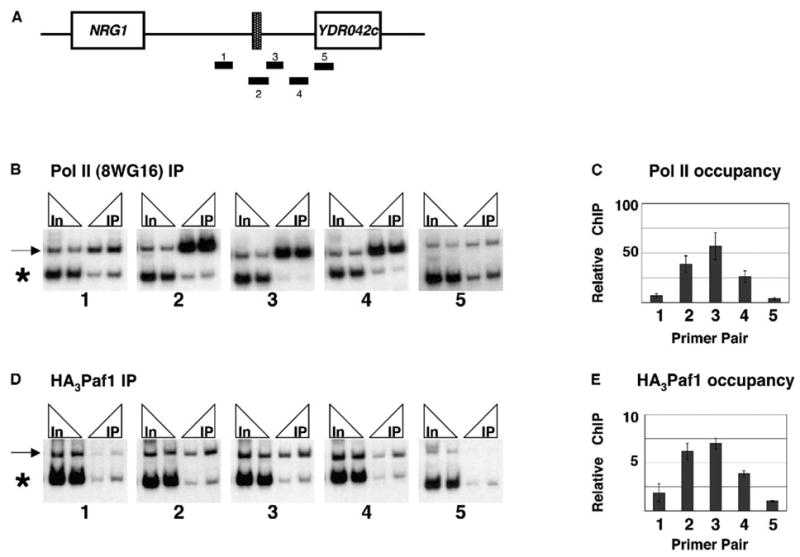
(A) SNR47 locus with PCR products amplified in ChIP experiments.
(B) Chromatin from wt (KY669) cells was immunoprecipitated with 8WG16 antibody. Two amounts of immunoprecipitated (IP) DNA (2 μl and 4 μl) and input (In) DNA (4 μl of 1:125 and 1:250 dilutions) were analyzed by PCR. The reactions included the SNR47 primer pair specified below each panel (arrow denotes PCR product) and a primer pair to an intergenic region of chromosome VIII ([Ng et al., 2003b]; asterisk denotes PCR product).
(C) SNR47 ChIP signals were normalized to chromosome VIII signals. Normalized IP values were divided by normalized input values, and the ratios from three independent ChIP experiments were averaged.
(D) ChIP was performed on chromatin prepared from HA3-PAF1 (GHY972) cells with α-HA antibody.
(E) Quantitation of three HA3-Paf1 ChIP experiments was performed as in (C).
Error bars in (C) and (E) indicate the SEM.
Figure 6. Paf1 Is Required for Normal Nrd1 Association with SNR47.
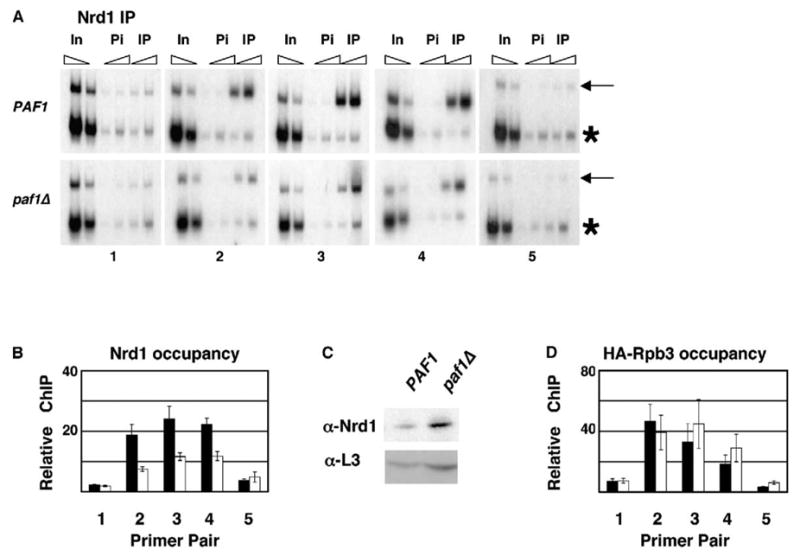
(A) ChIP was performed on chromatin prepared from PAF1 (KKY120) and paf1Δ (KKY122) cells with α-Nrd1 antibody (IP) or preimmune sera (Pi).
(B) Quantitation of three ChIP experiments as in (A). Relative ChIP signals, calculated as in Figure 5, are shown for Nrd1 in the presence (black bars) or absence (white bars) of Paf1.
(C) Protein extracts, prepared from cells grown for ChIP as in (A), were analyzed by immunoblotting with α-Nrd1 and α-L3 antibodies.
(D) ChIP analysis was performed on chromatin from PAF1 HA-RPB3 (GHY645) and paf1Δ HA-RPB3 (KY832) cells. Black and white bars represent average enrichment of HA-Rpb3 in PAF1 and paf1Δ cells, respectively. Untagged control strains (FY118 and KY685) yielded ChIP signals that were near background levels (not shown).
Error bars in (B) and (D) indicate the SEM.
To determine whether the Paf1-mediated effect on SNR47 expression maps to the known SNR47 3′ end formation element or to some other aspect of the SNR47-YDR042c transcript, we used a plasmid-based reporter assay in which the SNR47 3′ end formation element is placed in a heterologous context between a promoter and a reporter gene (Carroll et al., 2004). In the control plasmid for this assay, AHC, the ADH1 promoter drives expression of the yeast HIS3 gene and confers histidine prototrophy to his3Δ strains. The ASHC reporter plasmid contains 70 bp of SNR47 sequence required for 3′ end formation inserted between the ADH1 promoter and HIS3 (Figure 4B, top). RTF1 and rtf1Δ strains containing AHC grow in the absence of histidine, as expected (Figure 4B, bottom). However, whereas RTF1+ strains containing ASHC grow poorly in the absence of histidine, rtf1Δ strains containing this plasmid grow similarly to rtf1Δ strains containing the control plasmid that lacks the SNR47 sequence (Figure 4B, bottom). The results from this reporter assay suggest that the Paf1 complex is required for efficient recognition of the SNR47 3′ end formation signal.
The Paf1 Complex and RNA Pol II Colocalize to the SNR47 Locus
To begin to elucidate the mechanism by which the Paf1 complex affects the synthesis of snoRNA transcripts, we performed ChIP analysis. We designed five sets of primer pairs to amplify the region spanning the SNR47 gene and extending downstream into the YDR042c ORF (Figure 5A). We chose SNR47 for our ChIP assays because the adjacent YDR042c gene appears to be poorly expressed from its own promoter (Figure 4A). Therefore, the interpretation of our ChIP results should not be complicated by the association of transcription factors with the downstream gene. The occupancy of RNA pol II was mapped over the SNR47 locus by using the CTD-specific antibody 8WG16. As expected, RNA pol II associates with the SNR47 locus, including sequences, amplified by primer pair 3, known to contain Nrd1 and Nab3 binding motifs (Figures 5B and 5C) (Carroll et al., 2004). In a similar experiment, we assayed for the presence of the Paf1 complex by using strains that express a triple HA-tagged form of Paf1 (Figures 5D and 5E). The profile of HA3-Paf1 association over SNR47 closely matches that of RNA pol II. These data are consistent with a direct involvement of the Paf1 complex in the synthesis of snR47.
Nrd1 Localizes to SNR47 in a Paf1-Dependent Manner
To test whether association of Nrd1 with SNR47 requires a functional Paf1 complex, we performed ChIP analysis on Nrd1. These experiments demonstrate that Nrd1 associates with SNR47 in a pattern similar to that of RNA pol II and the Paf1 complex (Figures 6A and 6B). Importantly, Nrd1 occupancy over the SNR47 locus is significantly reduced in strains lacking PAF1 (Figures 6A and 6B). This reduction in the Nrd1 ChIP signal could be due to masked Nrd1 epitopes in chromatin prepared from paf1Δ cells, reduced levels of Nrd1 protein in paf1Δ strains, reduced RNA pol II occupancy in paf1Δ strains causing a concomitant decrease in Nrd1 occupancy, or a requirement for the Paf1 complex in the recruitment or stable association of Nrd1 with SNR47. To address the first possibility, we mapped the association of Nab3 to SNR47 by using antibodies toward an HA3-tagged form of Nab3. Our experiments revealed a reduction in HA3-Nab3 occupancy at SNR47 in paf1Δ strains similar to that observed for Nrd1, suggesting that a decrease in epitope availability is not responsible for the lower Nrd1 ChIP signal at SNR47 (data not shown). By immunoblotting, we found that Nrd1 levels are increased, not reduced, in paf1Δ extracts when compared to PAF1 extracts (Figure 6C). This finding is consistent with a role for the Paf1 complex in Nrd1-dependent 3′ end formation, because NRD1 expression is autoregulated by a termination sequence present within its 5′ untranslated region (Steinmetz et al., 2001). Finally, ChIP analysis using an epitope-tagged form of Rpb3 indicated approximately equivalent association of RNA pol II with SNR47 in PAF1 and paf1Δ strains, eliminating the possibility that reduced Nrd1 occupancy in paf1Δ strains is simply due to reduced levels of RNA pol II on the gene (Figure 6D). Of interest is the increase in HA-Rpb3 signal detected in paf1Δ cells downstream of SNR47, over the region spanned by primer pairs three to five (Figure 6D). Although small, this increased signal correlates with the production of the SNR47-YDR042c transcriptional read-through product, which accumulates over time to readily detectable levels. These results suggest that the Paf1 complex facilitates 3′ end formation of snR47, at least in part, by regulating the recruitment or stable association of Nrd1 and Nab3 with the RNA pol II elongation apparatus.
Discussion
RNA pol II molecules that are engaged in transcript elongation associate with a large number of proteins whose functions, in many cases, remain to be elucidated. A recent ChIP analysis performed in yeast suggests that very few of these factors affect the intrinsic elongation rate or processivity of RNA pol II (Mason and Struhl, 2005). A growing body of data suggests that the functions of some of these factors may be to couple RNA synthesis with RNA processing and export. However, the mechanisms by which these events are coordinated remain to be understood.
In this study, we provide evidence that the Paf1 complex plays a posttranscriptional role in the synthesis of snoRNAs, members of a group of nonpolyadenylated RNA pol II transcripts with essential functions in eukaryotic cells. We show through two different approaches that the Paf1 complex genetically interacts with the essential snRNA and snoRNA termination factors Nrd1 and Nab3. Importantly, although overexpression of Nab3 suppresses three rtf1 missense mutations, it does not suppress an rtf1Δ allele, indicating that increased Nab3 levels do not bypass the requirement for Rtf1. By using Northern analysis and in vivo reporter assays, we demonstrate that defects in the Paf1 complex cause accumulation of transcripts that read through the SNR13 and SNR47 3′ end formation sequences. Remarkably, similar defects were not observed for strains mutated in a large number of other transcription- and chromatin-related proteins. Finally, our ChIP results indicate that the Paf1 complex directly affects snR47 3′ end formation by modulating the recruitment or stable association of Nrd1 and Nab3 with RNA pol II.
The Paf1 Complex Coordinates Events during RNA Transcription and Processing
Whereas the RNA pol II CTD has well-documented roles in pre-mRNA 5′ capping, splicing, and cleavage/ polyadenylation, a functional involvement of proteins that physically associate with elongating RNA pol II, collectively referred to as elongation factors, in RNA maturation has been less clear. Our results suggest that the Paf1 complex plays important roles during both transcription elongation and RNA 3′ end formation. Previous ChIP studies demonstrated that the Paf1 complex associates with the 5′, middle, and 3′ ends of open reading frames in approximately equal levels (Simic et al., 2003). At protein-coding genes, the Paf1 complex dissociates from RNA pol II as it reaches the cleavage/ polyadenylation site (Kim et al., 2004). This ChIP pattern is clearly distinct from that of proteins with dedicated roles in 3′ end processing. Although some studies have shown that cleavage/polyadenylation factors are recruited to polymerase near the 5′ ends of genes (Licatalosi et al., 2002), their occupancy increases dramatically near the 3′ end (Kim et al., 2004). The association of the Paf1 complex with RNA pol II from the earliest to the latest stages in transcription elongation suggests that it coordinates multiple events in the synthesis of a mature RNA.
The Paf1 complex is required for the association of the histone modification enzymes Rad6, Set1, and Set2 with the open reading frames of genes (Krogan et al., 2003a, 2003b; Ng et al., 2003b; Wood et al., 2003; Xiao et al., 2005). Consequently, in strains lacking Rtf1, Paf1, or Ctr9, histone H2B K123 ubiquitylation and histone H3 K4 di- and trimethylation are absent and histone H3 K36 dimethylation is significantly reduced (Krogan et al., 2003a, 2003b; Ng et al., 2003a, 2003b; Wood et al., 2003). Deletion of these same three subunits led to the highest levels of SNR13 and SNR47 readthrough transcription in our assays. We therefore tested whether a reduction in histone H3 methylation correlated with a defect in snoRNA 3′ end formation by assaying SNR13-TRS31 expression in set1Δ, set2Δ, and dot1Δ strains. Our results suggest that the role of the Paf1 complex in mediating histone methylation is independent of its function in RNA 3′ end formation. Although we did not observe a strong effect of cdc73Δ or leo1Δ mutations on SNR13 or SNR47 transcription, the synthetic interaction between cdc73Δ and nrd1-5 mutations may suggest that Cdc73 governs posttranscriptional events at other snoRNAs or possibly mRNAs. Consistent with this idea, Nrd1 and Nab3 also associate with protein-coding genes (Nedea et al., 2003).
In addition to mediating various histone modifications, the Paf1 complex physically interacts with the chromatin remodeling factor Chd1 (Simic et al., 2003). Deletion of Rtf1 dissociates the Paf1 complex from chromatin (Mueller et al., 2004) and reduces the occupancy of Chd1 on the open reading frames of genes (Simic et al., 2003). Proudfoot and colleagues demonstrated that Chd1 is required for normal chromatin structure and transcription termination at the 3′ ends of several protein-coding genes in yeast (Alen et al., 2002). For the SNR13 gene, we have not observed 3′ -extended transcripts in strains lacking Chd1. However, it remains possible that Chd1 functions redundantly with other chromatin remodeling factors to influence snoRNA termination, as has been reported for the GAL10 gene (Alén et al., 2002).
Potential Mechanisms through which the Paf1 Complex Modulates 3′ End Formation
Collectively, our results suggest that the Paf1 complex affects utilization of snoRNA 3′ end formation signals by influencing the association of termination/RNA processing factors with elongating RNA pol II. At the 3′ ends of protein-coding genes, the recruitment of cleavage/polyadenylation factors requires Ctk1-mediated Ser2 phosphorylation of the CTD (Ahn et al., 2004). Some of these proteins participate in 3′ end formation of nonpolyadenylated RNA pol II transcripts (Cheng et al., 2004; Dheur et al., 2003; Dichtl et al., 2004; Fatica et al., 2000; Morlando et al., 2002; Nedea et al., 2003; Steinmetz and Brow, 2003). Like the CFI component Pcf11, Nrd1 contains a CTD-interacting domain, which likely mediates a direct interaction between Nrd1 and the Ser2-phosphorylated CTD (Meinhart and Cramer, 2004; Steinmetz and Brow, 1996). Interestingly, the Paf1 complex plays a role in establishing or maintaining normal levels of Ser2 phosphorylation and is required for the association of Pcf11 with a protein-coding gene (Mueller et al., 2004). Therefore, the Paf1 complex may facilitate the recruitment or binding of snoRNA termination and processing factors by influencing Ser2 phosphorylation. Consistent with this idea, mutations in the genes encoding Ctk1 (Steinmetz et al., 2001) and Fcp1 (shown here) cause readthrough transcription at snoRNA genes. However, it should be noted that our previous genetic studies also indicate a function for the Paf1 complex independent of Ctk1-mediated Ser2 phosphorylation (Costa and Arndt, 2000; Squazzo et al., 2002). ctk1 mutations are synthetically lethal with rtf1Δ and paf1Δ mutations, arguing that the Paf1 complex and Ctk1 operate, at least partly, in parallel pathways.
The transition between elongation and termination at protein-coding genes coincides with a peak in enrichment for the CFI and CPF complexes and a reduction in the association of the Paf1 complex near the polyA site (Kim et al., 2004). Whether a similar process occurs at the small snoRNA genes will be difficult to discern with the resolution of current ChIP assays. However, the observations made at protein-coding genes suggest that a crucial transition occurs in the composition of the RNA pol II elongation apparatus near the 3′ ends of genes. Therefore, an alternative function for the Paf1 complex may be to facilitate this exchange of factors, possibly through a direct physical interaction with these proteins. Although we have not detected a stable interaction between the Paf1 complex and Nrd1 by coimmunoprecipitation assays (data not shown), it remains possible that the Paf1 complex physically associates with other snoRNA processing factors. Alternatively, it may influence the activity of an elongation factor such as Spt4-Spt5, which interacts with numerous cleavage/polyadenylation factors including some involved in snoRNA 3′ end formation (Lindstrom et al., 2003).
Finally, although our collective data support a role for the Paf1 complex in facilitating snoRNA 3′ end formation, an additional and not mutually exclusive explanation for some of our results is that the Paf1 complex is required for the selective degradation of SNR read-through transcripts. The nuclear exosome complex has been implicated in both snoRNA 3′ end formation and exonucleolytic degradation of aberrant RNA pol II transcripts (Jensen et al., 2003). Therefore, it will be important to determine whether the yeast Paf1 complex, like the very recently reported human Paf1 complex (Zhu et al., 2005), interacts with exosome subunits.
Connections to Poly(A)-Dependent Transcription Termination
The sharing of factors used for poly(A)-dependent and poly(A)-independent transcription termination suggests that the Paf1 complex is likely to be required for the 3′ end processing of mRNAs. Consistent with this prediction, defects in the Paf1 complex cause a general shortening of polyA tails in vivo (Mueller et al., 2004). In addition, our discovery of NAB3 as a suppressor of the Spt− phenotype of rtf1 mutant strains, a phenotype that has no obvious connection to snoRNA processing, may reflect a requirement for the Paf1 complex in the elongation or termination of polyadenylated transcripts. Perhaps overexpression of Nab3 impairs the interaction of the Paf1 complex with factors normally involved in these stages of mRNA synthesis.
In summary, our results reveal a function for the Paf1 complex that is apparently unrelated to histone modification and support models in which this complex coordinates multiple steps in RNA synthesis. It will be interesting to learn whether defects in RNA processing account for some of the diseases associated with the newly discovered human Paf1 complex (Rozenblatt-Rosen et al., 2005; Zhu et al., 2005).
Experimental Procedures
Yeast Strains and Media
Rich (YPD), synthetic complete (SC), synthetic dextrose (SD), and 5-fluoroorotic acid (5-FOA) media were prepared as described (Rose et al., 1990). Unless otherwise indicated, the yeast strains used in this study (Table S1 available in the Supplemental Data with this article online) are isogenic with FY2, a GAL2+ derivative of S288C. Strains were constructed by tetrad dissection or transformation (Rose et al., 1990). All deletion strains were created by using a PCR-based method (Ausubel et al., 1988), with the exception of the nrd1Δ strain KKY100, which was constructed by using the disruption plasmid pRS316-nrd1Δ::HIS3 (Steinmetz and Brow, 1996) to replace the essential NRD1 gene in the diploid strain KKY97.
Plasmids
Standard techniques were used for plasmid construction, isolation, and transformation of S. cerevisiae and E. coli strains (Ausubel et al., 1988; Rose et al., 1990). Nab3 was N-terminally tagged with a triple HA1 epitope by using a PCR-based method (Stolinski et al., 1997). The NAB3 and HA3-NAB3 containing plasmids fully complemented a nab3Δ strain for viability at 30°C and 37°C.
Isolation and Characterization of rtf1 Point Mutants
Error-prone PCR was performed on plasmid pLS21-5 (HA3-RTF1 in pRS314; [Stolinski et al., 1997]) to generate mutations in RTF1, and PCR products were introduced into the pLS21-5 backbone by homologous recombination in vivo (Muhlrad et al., 1992). Mutations that did not complement the 6AUS and Spt− phenotypes of an rtf1Δ strain at 37°C were studied further. Sequence analysis of eight original candidates revealed that all contained multiple mutations. For a subset, mutations were separated by subcloning or site-directed mutagenesis (Stratagene QuikChange) to obtain single missense mutations that conferred 6AUS and/or Spt− phenotypes. During this process, the N-terminal HA epitope tag was removed from Rtf1. The rtf1-100 (Q172R), rtf1-105 (V274D), and rtf1-107 (M289K) mutations were integrated in the yeast chromosome to replace the RTF1 gene by a two-step gene replacement (Rose et al., 1990).
Identification of Dosage Suppressors of rtf1-107
An rtf1-107 strain (KKY58) was used in a dosage suppressor screen to identify genomic fragments that reverse the conditional 6AUS phenotype and/or the Spt− phenotype. ~13,000 transformants containing plasmids from a LEU2-marked, 2-micron genomic library (Yoshihisa and Anraku, 1989) were screened by replica printing to SC-Leu-His and SC-Leu-Ura + 50 μg/ml 6AU media. Library plasmids that suppressed the Spt− phenotype or the 6AUS phenotype were detected at 30°C and 37°C, respectively. Plasmids were recovered from candidates and retransformed into KKY58 to confirm that suppression was plasmid dependent.
Immunoblotting Analysis
For analysis of Rtf1 levels, whole-cell extracts (Stolinski et al., 1997) were resolved on 7.5% SDS-polyacrylamide gels and nitrocellulose filters were probed with anti-Rtf1 (1:4000 dilution) (Squazzo et al., 2002) or anti-L3 (1:5000 dilution) antisera (Vilardell and Warner, 1997). To analyze Nrd1 levels, whole-cell extracts (Squazzo et al., 2002) were resolved on 10% SDS-polyacrylamide gels and nitrocellulose filters were probed with a 1:3000 dilution of anti-Nrd1 antisera. Trimethyl H3 K4 levels (1:2000 dilution; Abcam antibody) were examined by immunoblotting analysis of whole-cell extracts as described (Ng et al., 2003a). Immunoreactive proteins were detected by chemiluminescence (Pierce) of HRP-coupled secondary antibodies (Amersham Biosciences) and visualized by using BioMax film or a 440CF digital imaging station (Kodak).
Northern Analysis and Reporter Assays for Transcriptional Readthrough
Northern analysis was performed on 20 μg of total RNA isolated from cells grown in YPD at 30°C to a density of 1–2 × 107 cells/ml (Squazzo et al., 2002). Random-prime-labeled, PCR-amplified DNA probes were used. The reporter assay for transcriptional read-through of SNR47 was performed as described (Carroll et al., 2004).
ChIP
Cells were grown in YPD to 1–2 × 107 cells/ml. ChIP assays and immunoprecipitations with 8WG16 antibody (Covance) or anti-HA antibody conjugated to agarose beads (Santa Cruz Biotechnology) were performed as described (Komarnitsky et al., 2000; Simic et al., 2003). Nrd1 ChIPs were performed by incubating chromatin extracts overnight at 4°C with a 1:100 final dilution of Nrd1 antisera, followed by incubation with protein A-coupled Sepharose beads (Amersham Biosciences). To confirm signal linearity, quantitative PCR was performed on two dilutions of input and immunoprecipitated DNA by using [α-32P]dATP, and relative ChIP signals were calculated as described in the figure legends. PCR primers amplified the following sequences relative to +1 of the SNR47 genomic sequence (per Saccharomyces Genome Database): primer pair one, −350 to −150; primer pair two, −51 to +168; primer pair three, +99 to +303; primer pair four, +298 to +484; and primer pair five, +531 to +749. Sequences are available upon request.
Supplementary Material
Acknowledgments
We are indebted to Eric Steinmetz and David Brow for Nrd1 antibody, plasmids, yeast strains, and helpful advice. We are grateful to Jeff Corden for providing plasmids and communicating unpublished results, to Steve Buratowski for many helpful discussions, to Fred Winston and Grant Hartzog for providing yeast strains, and to Rajna Simic for technical support. This work was supported by grants from the National Institutes of Health (GM52593 and AI01816) to K.M.A.
Footnotes
Supplemental Data
Supplemental Data include one table and are available with this article online at http://www.molecule.org/cgi/content/full/20/2/225/DC1/.
References
- Ahn SH, Kim M, Buratowski S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol Cell. 2004;13:67–76. doi: 10.1016/s1097-2765(03)00492-1. [DOI] [PubMed] [Google Scholar]
- Alén C, Kent NA, Jones HS, O’Sullivan J, Aranda A, Proudfoot NJ. A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol Cell. 2002;10:1441–1452. doi: 10.1016/s1097-2765(02)00778-5. [DOI] [PubMed] [Google Scholar]
- Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999;18:5399–5410. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates and Wiley-Interscience; 1988. [Google Scholar]
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol. 2005;17:251–256. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Carroll KL, Pradhan DA, Granek JA, Clarke ND, Corden JL. Identification of cis elements directing termination of yeast nonpolyadenylated snoRNA transcripts. Mol Cell Biol. 2004;24:6241–6252. doi: 10.1128/MCB.24.14.6241-6252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, He X, Moore C. The essential WD repeat protein Swd2 has dual functions in RNA polymerase II transcription termination and lysine 4 methylation of histone H3. Mol Cell Biol. 2004;24:2932–2943. doi: 10.1128/MCB.24.7.2932-2943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EJ, Takagi T, Moore CR, Buratowski S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 1997;11:3319–3326. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad NK, Wilson SM, Steinmetz EJ, Patturajan M, Brow DA, Swanson MS, Corden JL. A yeast heterogeneous nuclear ribonucleoprotein complex associated with RNA polymerase II. Genetics. 2000;154:557–571. doi: 10.1093/genetics/154.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PJ, Arndt KM. Synthetic lethal interactions suggest a role for the Saccharomyces cerevisiae Rtf1 protein in transcription elongation. Genetics. 2000;156:535–547. doi: 10.1093/genetics/156.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheur S, Vo le TA, Voisinet-Hakil F, Minet M, Schmitter JM, Lacroute F, Wyers F, Minvielle-Sebastia L. Pti1p and Ref2p found in association with the mRNA 3′ end formation complex direct snoRNA maturation. EMBO J. 2003;22:2831–2840. doi: 10.1093/emboj/cdg253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichtl B, Aasland R, Keller W. Functions for S. cerevisiae Swd2p in 3′ end formation of specific mRNAs and snoRNAs and global histone 3 lysine 4 methylation. RNA. 2004;10:965–977. doi: 10.1261/rna.7090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatica A, Morlando M, Bozzoni I. Yeast snoRNA accumulation relies on a cleavage-dependent/polyadenylation-independent 3′ -processing apparatus. EMBO J. 2000;19:6218–6229. doi: 10.1093/emboj/19.22.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong YW, Zhou Q. Stimulatory effect of splicing factors on transcriptional elongation. Nature. 2001;414:929–933. doi: 10.1038/414929a. [DOI] [PubMed] [Google Scholar]
- Hartzog GA, Speer JL, Lindstrom DL. Transcript elongation on a nucleoprotein template. Biochim Biophys Acta. 2002;1577:276–286. doi: 10.1016/s0167-4781(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Jensen TH, Dower K, Libri D, Rosbash M. Early formation of mRNP: license for export or quality control? Mol Cell. 2003;11:1129–1138. doi: 10.1016/s1097-2765(03)00191-6. [DOI] [PubMed] [Google Scholar]
- Kaplan CD, Laprade L, Winston F. Transcription elongation factors repress transcription initiation from cryptic sites. Science. 2003;301:1096–1099. doi: 10.1126/science.1087374. [DOI] [PubMed] [Google Scholar]
- Kim M, Ahn SH, Krogan NJ, Greenblatt JF, Buratowski S. Transitions in RNA polymerase II elongation complexes at the 3′ ends of genes. EMBO J. 2004;23:354–364. doi: 10.1038/sj.emboj.7600053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobor MS, Greenblatt J. Regulation of transcription elongation by phosphorylation. Biochim Biophys Acta. 2002;1577:261–275. doi: 10.1016/s0167-4781(02)00457-8. [DOI] [PubMed] [Google Scholar]
- Komarnitsky P, Cho EJ, Buratowski S. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 2000;14:2452–2460. doi: 10.1101/gad.824700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Kim M, Ahn SH, Zhong G, Kobor MS, Cagney G, Emili A, Shilatifard A, Buratowski S, Greenblatt JF. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol. 2002;22:6979–6992. doi: 10.1128/MCB.22.20.6979-6992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Dover J, Wood A, Schneider J, Heidt J, Boateng MA, Dean K, Ryan OW, Golshani A, Johnston M, et al. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol Cell. 2003a;11:721–729. doi: 10.1016/s1097-2765(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Kim M, Tong A, Golshani A, Cagney G, Canadien V, Richards DP, Beattie BK, Emili A, Boone C, et al. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol. 2003b;23:4207–4218. doi: 10.1128/MCB.23.12.4207-4218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Geiger G, Minet M, Schroeder S, Cilli K, McNeil JB, Bentley DL. Functional interaction of yeast pre-mRNA 3′ end processing factors with RNA polymerase II. Mol Cell. 2002;9:1101–1111. doi: 10.1016/s1097-2765(02)00518-x. [DOI] [PubMed] [Google Scholar]
- Lindstrom DL, Squazzo SL, Muster N, Burckin TA, Wachter KC, Emigh CA, McCleery JA, Yates JR, 3rd, Hartzog GA. Dual roles for Spt5 in pre-mRNA processing and transcription elongation revealed by identification of Spt5-associated proteins. Mol Cell Biol. 2003;23:1368–1378. doi: 10.1128/MCB.23.4.1368-1378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PB, Struhl K. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol. 2003;23:8323–8333. doi: 10.1128/MCB.23.22.8323-8333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PB, Struhl K. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol Cell. 2005;17:831–840. doi: 10.1016/j.molcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, Bentley DL. 5′ -Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997a;11:3306–3318. doi: 10.1101/gad.11.24.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson SD, Wickens M, Bentley DL. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997b;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- Meinhart A, Cramer P. Recognition of RNA polymerase II carboxy-terminal domain by 3′ -RNA-processing factors. Nature. 2004;430:223–226. doi: 10.1038/nature02679. [DOI] [PubMed] [Google Scholar]
- Morlando M, Greco P, Dichtl B, Fatica A, Keller W, Bozzoni I. Functional analysis of yeast snoRNA and snRNA 3′ -end formation mediated by uncoupling of cleavage and polyadenylation. Mol Cell Biol. 2002;22:1379–1389. doi: 10.1128/mcb.22.5.1379-1389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CL, Jaehning JA. Ctr9, Rtf1, and Leo1 are components of the Paf1/RNA polymerase II complex. Mol Cell Biol. 2002;22:1971–1980. doi: 10.1128/MCB.22.7.1971-1980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CL, Porter SE, Hoffman MG, Jaehning JA. The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol Cell. 2004;14:447–456. doi: 10.1016/s1097-2765(04)00257-6. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- Nedea E, He X, Kim M, Pootoolal J, Zhong G, Canadien V, Hughes T, Buratowski S, Moore CL, Greenblatt J. Organization and function of APT, a subcomplex of the yeast cleavage and polyadenylation factor involved in the formation of mRNA and small nucleolar RNA 3′ -ends. J Biol Chem. 2003;278:33000–33010. doi: 10.1074/jbc.M304454200. [DOI] [PubMed] [Google Scholar]
- Ng HH, Dole S, Struhl K. The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J Biol Chem. 2003a;278:33625–33628. doi: 10.1074/jbc.C300270200. [DOI] [PubMed] [Google Scholar]
- Ng HH, Robert F, Young RA, Struhl K. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol Cell. 2003b;11:709–719. doi: 10.1016/s1097-2765(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. New perspectives on connecting messenger RNA 3′ end formation to transcription. Curr Opin Cell Biol. 2004;16:272–278. doi: 10.1016/j.ceb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Riles L, Shaw RJ, Johnston M, Reines D. Large-scale screening of yeast mutants for sensitivity to the IMP dehydrogenase inhibitor 6-azauracil. Yeast. 2004;21:241–248. doi: 10.1002/yea.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon AG, Gallardo M, Garcia-Rubio M, Aguilera A. Molecular evidence indicating that the yeast PAF complex is required for transcription elongation. EMBO Rep. 2004;5:47–53. doi: 10.1038/sj.embor.7400045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, Shanmugam KS, Copeland TD, Guszczynski T, Resau JH, Meyerson M. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol. 2005;25:612–620. doi: 10.1128/MCB.25.2.612-620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SC, Schwer B, Shuman S, Bentley D. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev. 2000;14:2435–2440. doi: 10.1101/gad.836300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic R, Lindstrom DL, Tran HG, Roinick KL, Costa PJ, Johnson AD, Hartzog GA, Arndt KM. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 2003;22:1846–1856. doi: 10.1093/emboj/cdg179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squazzo SL, Costa PJ, Lindstrom DL, Kumer KE, Simic R, Jennings JL, Link AJ, Arndt KM, Hartzog GA. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J. 2002;21:1764–1774. doi: 10.1093/emboj/21.7.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz EJ, Brow DA. Repression of gene expression by an exogenous sequence element acting in concert with a heterogeneous nuclear ribonucleoprotein-like protein, Nrd1, and the putative helicase Sen1. Mol Cell Biol. 1996;16:6993–7003. doi: 10.1128/mcb.16.12.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz EJ, Brow DA. Ssu72 protein mediates both poly(A)-coupled and poly(A)-independent termination of RNA polymerase II transcription. Mol Cell Biol. 2003;23:6339–6349. doi: 10.1128/MCB.23.18.6339-6349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz EJ, Conrad NK, Brow DA, Corden JL. RNA-binding protein Nrd1 directs poly(A)-independent 3′ -end formation of RNA polymerase II transcripts. Nature. 2001;413:327–331. doi: 10.1038/35095090. [DOI] [PubMed] [Google Scholar]
- Stolinski LA, Eisenmann DM, Arndt KM. Identification of RTF1, a novel gene important for TATA site selection by TATA box-binding protein in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4490–4500. doi: 10.1128/mcb.17.8.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof A, Lennertz P, Parker R. Yeast exosome mutants accumulate 3′ -extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol Cell Biol. 2000;20:441–452. doi: 10.1128/mcb.20.2.441-452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell J, Warner JR. Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol Cell Biol. 1997;17:1959–1965. doi: 10.1128/mcb.17.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F, Sudarsanam P. The SAGA of Spt proteins and transcriptional analysis in yeast: past, present, and future. Cold Spring Harb Symp Quant Biol. 1998;63:553–561. doi: 10.1101/sqb.1998.63.553. [DOI] [PubMed] [Google Scholar]
- Wood A, Schneider J, Dover J, Johnston M, Shilatifard A. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J Biol Chem. 2003;278:34739–34742. doi: 10.1074/jbc.C300269200. [DOI] [PubMed] [Google Scholar]
- Xiao T, Kao CF, Krogan NJ, Sun ZW, Greenblatt JF, Osley MA, Strahl BD. Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol Cell Biol. 2005;25:637–651. doi: 10.1128/MCB.25.2.637-651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T, Anraku Y. Nucleotide sequence of AMS1, the structure gene of vacuolar alpha- mannosidase of Saccharo-myces cerevisiae. Biochem Biophys Res Commun. 1989;163:908–915. doi: 10.1016/0006-291x(89)92308-5. [DOI] [PubMed] [Google Scholar]
- Zhu B, Mandal SS, Pham AD, Zheng Y, Erdjument-Bromage H, Batra SK, Tempst P, Reinberg D. The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev. 2005;19:1668–1673. doi: 10.1101/gad.1292105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


