Abstract
Glycinergic synaptic inhibition is part of acoustic information processing in brain stem auditory pathways and contributes to the regulation of neuronal excitation. We found previously that unilateral cochlear ablation (UCA) in young adult guinea pigs decreased [3H]strychnine binding activity in several brain stem auditory nuclei. This study determined if the UCA-induced deficit could be regulated by protein kinase C (PKC), protein kinase A (PKA) or Ca2+/calmodulin-dependent protein kinase II (CaMKII). The specific binding of [3H]strychnine was measured in slices of the dorsal (DCN), posteroventral (PVCN) and anteroventral (AVCN) cochlear nucleus (CN), the lateral (LSO) and medial (MSO) superior olive, and the inferior colliculus (IC) 145 days after UCA. Tissues from age-matched unlesioned animals served as controls. UCA induced deficits in specific binding in the AVCN, PVCN and LSO on the ablated side and in the MSO bilaterally. These deficits were reversed by 3 μM phorbol 1,2-dibutyrate, a PKC activator, or 0.2 mM dibutyryl-cAMP, a PKA activator. However, 50 nM Ro31-8220, a PKC inhibitor, and 2 μM H-89, a PKA inhibitor, had no effect in unlesioned controls and after UCA. In contrast, 4 μM KN-93, a CaMKII inhibitor, relieved or reversed the UCA-induced binding deficits and elevated binding in the IC. These findings suggest a UCA-induced down regulation of glycine receptor synthesis may have occurred via reduced phosphorylation of proteins that control receptor synthesis; this effect was reversed by diminishing CaMKII activity or increasing PKC and PKA activity.
Keywords: [3H]strychnine binding, phorbol ester, dibutytyl-cyclic-AMP, H-89, KN-93, Ro31-8220
Introduction
Glycinergic synaptic inhibition in brain stem auditory pathways is an important component of acoustic information processing and, together with GABAergic inhibition, contributes to the regulation of neuronal excitation (Caspary et al., 1994; Faingold et al., 1991; Grothe and Sanes, 1994; Backoff et al., 1999; Davis and Young, 2000). Glycinergic inhibition, however, may become impaired after sensorineural hearing loss, which usually involves damage to the cochlea and degeneration of the cochlear nerve. Destruction of the cochlear nerve in young adult guinea pigs reduces [3H]strychnine binding in several auditory brain stem nuclei on the ablated side without affecting glycine transmitter release (Suneja et al., 1998a,b; Potashner et al., 2000). This implies a decline in glycine receptor activity, which may contribute to additional pathological symptoms that often accompany sensorineural hearing loss, such as tinnitus, loudness misperceptions and poor isolation of important sounds in a background of noise (Olson et al., 1975; Jastreboff, 1990; Salvi et al., 2000). Several protein kinases are associated with postsynaptic sites (Liu and Jones, 1996; Ramakers et al., 1997; Colbran, 2004) and phosphorylation of the glycine receptor alters its properties (Smart, 1997; Gentet and Clements, 2002). It is conceivable, therefore, that the actions of protein kinases might be involved in the change of glycinergic receptor activity after UCA. To assess this possibility, we quantified the specific binding of [3H]strychnine, using microdissected slices of brain stem auditory nuclei 145 days after UCA, when the deficit in glycine receptor activity is fully developed (Suneja et al., 1998a; Potashner et al., 2000). We compared this binding activity to that in tissues of age-matched, unlesioned control animals. A proportion of the tissues from both the controls and the ablated animals were treated with activators or inhibitors of PKC, PKA or CaMKII to determine their effects on [3H]strychnine binding. Some of the findings have been reported in an abstract (Yan et al., 2004).
Materials and Methods
Procedures involving animals were approved by the University Animal Care Committee in accordance with federal and state policies. Hartley albino guinea pigs of either sex were 50 days of age when they received a UCA and then survived an additional 145 days. Unlesioned age-matched animals served as intact controls. Animals receiving the UCA were anesthetized with pentobarbital (Nembutal, 32 mg/kg, i.p.; Abbott Laboratories, North Chicago, IL ) before the left cochlea was ablated mechanically, as described previously (Potashner, 1983). Postoperatively, animals received subcutaneous injections of 0.9% saline (10 ml), to prevent dehydration, and buprenorphine (0.05mg/kg,), as an analgesic. After 145 days, and an equivalent period for unlesioned controls, the brain stem was removed from the anesthetized animal and immersed in ice-cold high-Na+-Ringer, containing 122 mM NaCl, 3.1 mM KCl, 1.2 mM MgSO4, 1.3 mMCaCl2, 0.4 mM KH2PO4, 25 mM NaHCO3, 10 mM D-glucose (pH 7.4), and bubbled with 95% O2 - 5% CO2. The brain stem was cut transversely into 400 μm thick slices, using a tissue chopper. As described previously (Potashner, 1983; Suneja et al., 1995), microdissection and micropunching of the slices provided samples from both sides of the brain stem of the DCN, PVCN and AVCN; the LSO and MSO; and the central nucleus of the inferior colliculus (ICc).
To assess the actions of protein kinase reagents on strychnine binding, some of the tissue samples were treated with one of these compounds before binding was assessed. Treated tissues were incubated for 30 min in high-Na+-Ringer (0.5 ml, 37 °C) containing one of the following: 3μM phorbol 1,2-dibutyrate (PDBu), a PKC activator; 50 nM Ro31-8220, a PKC inhibitor, 0.2 mM dibutyryl-cAMP (DBcAMP), a PKA activator; 2 μM H-89, a PKA inhibitor; or 4μM KN-93, a CaMKII inhibitor. These compounds were dissolved in DMSO, which exposed tissues to 1.4 mM DMSO (PDBu and Ro31-8220) or 4.3 mM DMSO (H-89 and KN-93). Untreated controls received the same incubation as above, with the appropriate concentration of DMSO.
After the incubations above, tissues were rinsed twice with ice-cold 0.9% saline, buffered with 20mM sodium phosphate, pH 7.4, (PBS: 0.5 ml, 5 min each) and weighed. To measure total strychnine binding, tissue samples were incubated for 90 min at 0 °C in 0.75 ml PBS containing 10 nM [3H]strychnine (23.7Ci or 8.7 × 108 Bq/mmol). Nonspecific binding was measured by adding 20 mM glycine to the radioligand. After incubation, the tissues were washed and decanted onto glass microfiber filter paper under a vacuum. Tissues and filters were washed once and transferred into scintillation vials. The tissues were solubilized in 1 ml of 3.5% KOH at 50 °C for 1 h, cooled to room temperature and neutralized with HCl. Radioactivity was measured by liquid scintillation counting and converted to fmoles of strychnine bound per mg of tissue protein. The specific binding, expressed as fmoles bound per mg protein, was computed as the total binding minus the mean non-specific binding. The specific binding after UCA or treatment with a protein kinase reagent was expressed as a percentage of that from the unlesioned, untreated control. These percentage data were finally expressed as means ± the standard error of the mean.
Control groups consisted of tissues from unlesioned age-matched controls that were untreated with protein kinase reagents (8 animals), and tissues from unlesioned controls treated with PDBu, Ro31-8220, DBcAMP, H-89 or KN-93 (4 animals per treatment group). UCA groups consisted of UCA animal tissues that were untreated with protein kinase reagents (4 animals), and UCA animal tissues treated with PDBu, Ro31-8220, DBcAMP, H-89 or KN-93 (4 animals per treatment group). Each animal usually provided a single sample of an auditory nucleus from the left and right sides of the brain stem. Changes in specific binding relative to the appropriate control were assessed using analyses of variance (ANOVA) (SPSS, Chicago, IL). Significant main effects and interactions were analyzed further with Duncan's multiple comparison test. Significant differences were those for which P ≤ 0.05.
Results
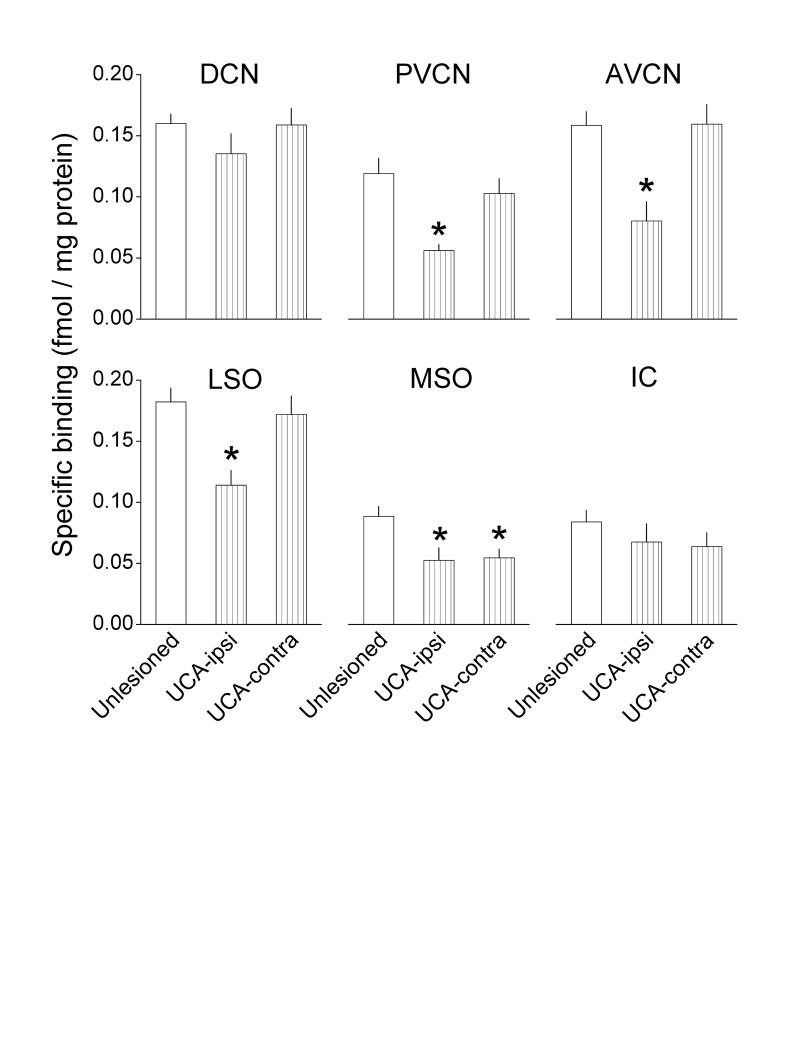
The auditory nuclei from unlesioned controls exhibited two levels of binding activity. The CN subdivisions and the LSO bound 0.12 - 0.18 fmols of strychnine per mg of tissue protein, while the MSO and IC bound approximately 0.08 fmols per mg protein (Fig. 1, white bars).The relative levels of binding activity determined in the present 400 μm-thick samples of the auditory nuclei, quantified with liquid scintillation spectrometry, were similar to those observed in 15 μm-thick samples, quantified autoradiographically (Suneja et al., 1998a). The autoradiographic study indicated that the distribution of binding activity was similar to that of high glycine concentrations, suggesting a corresponding distribution of receptors and glycine presumed to be in presynaptic endings.
Fig. 1.
Effects of UCA on specific binding of [3H]strychnine in several of the brain stem auditory nuclei. [3H]strychnine binding was measured 145 days after UCA, as described in the Methods; the number of animals in each group is provided in the Statistics subsection. Asterisks denote significant differences from unlesioned controls (Duncan's multiple comparison test; P≤ 0.05).
One hundred and forty five days after UCA, binding declined in the PVCN, AVCN and LSO on the ablated side by 53%, 49% and 37%, respectively (Fig. 1, vertically hatched bars). Binding also declined bilaterally in the MSO by 40%. Apparent declines of 15-24% in the DCN and IC did not achieve statistical significance. The magnitudes of these postablation deficits resembled those observed in the autoradiographic material.
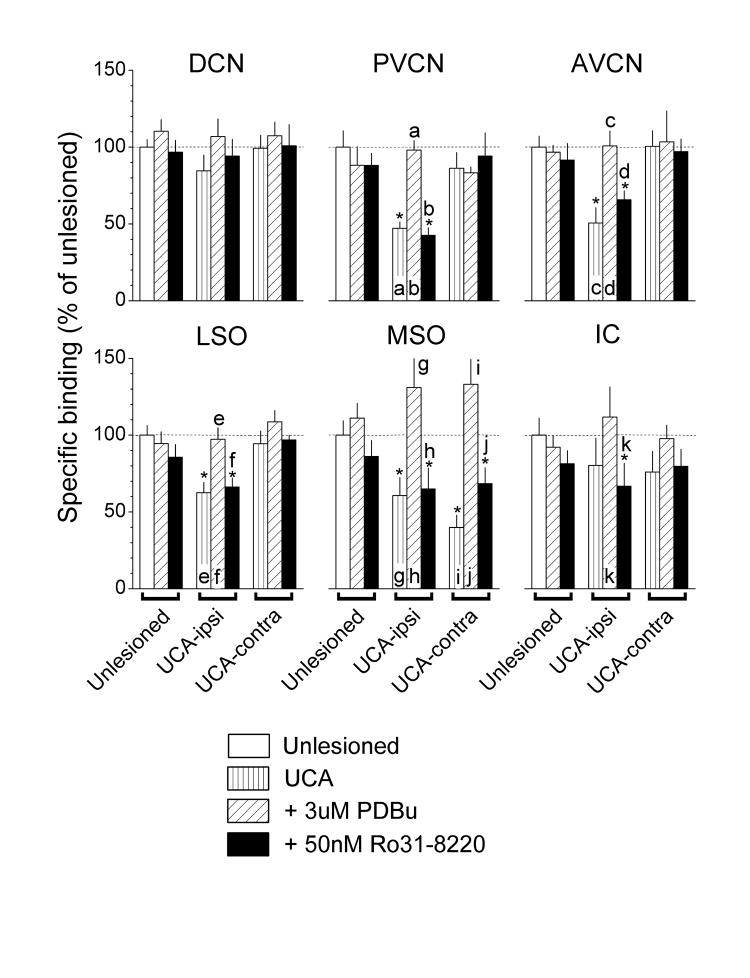
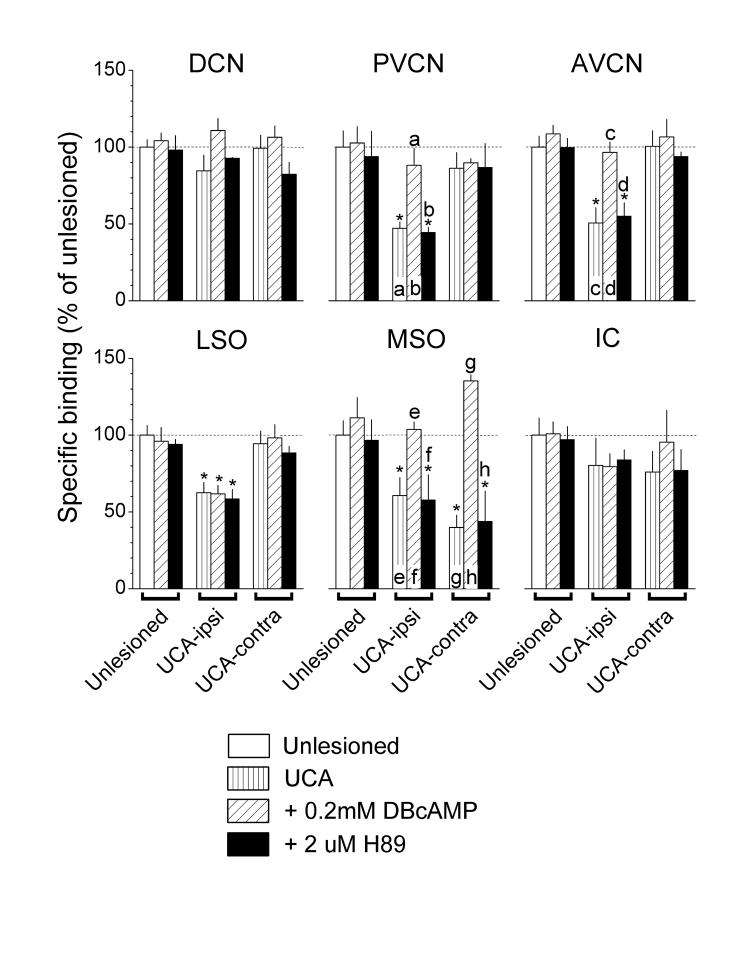
Treatment with activators or inhibitors of PKC (Fig. 2), PKA (Fig. 3) and CaMKII (Fig. 4) did not alter strychnine binding in tissues taken from unlesioned controls (see Unlesioned, compare white bars to diagonally hatched and black bars). By contrast, in tissues taken from UCA animals, both the PKC activator, PDBu (Fig. 2), and the PKA activator, DBcAMP (Fig. 3), reversed nearly all the deficits in strychnine binding (see UCA-ipsi and -contra, compare vertically to diagonally hatched bars) but had no significant effects where binding levels were normal. A unique exception to this pattern was the failure of DBcAMP to elevate the deficient binding in the LSO on the ablated side (Fig. 3, LSO, UCA-ipsi, compare vertically to diagonally hatched bars).
Fig. 2.
Actions of PDBu, a PKC activator, and Ro31-8220, a PKC inhibitor, on the specific binding of [3H]strychnine. Values from Fig. 1 for binding in unlesioned controls and animals that received the UCA are reproduced here for convenience. PDBu elevated binding that was deficient after UCA but had little or no effect where binding was normal. Ro31-8220 did not alter binding. Asterisks denote significant differences from unlesioned controls (Duncan; P ≤ 0.05). A lower case letter above a bar denotes a significant difference from the bar containing the same letter (Duncan; P ≤ 0.05).
Fig. 3.
Actions of DBcAMP, a PKA activator, and H-89, a PKA inhibitor, on the specific binding of [3H]strychnine. Values from Fig. 1 for binding in unlesioned controls and animals that received the UCA are reproduced here for convenience. DBcAMP elevated binding that was deficient after UCA but had little or no effect where binding was normal. H-89 did not alter binding. Asterisks and lower case letters above bars were defined in the legend to Fig. 2.
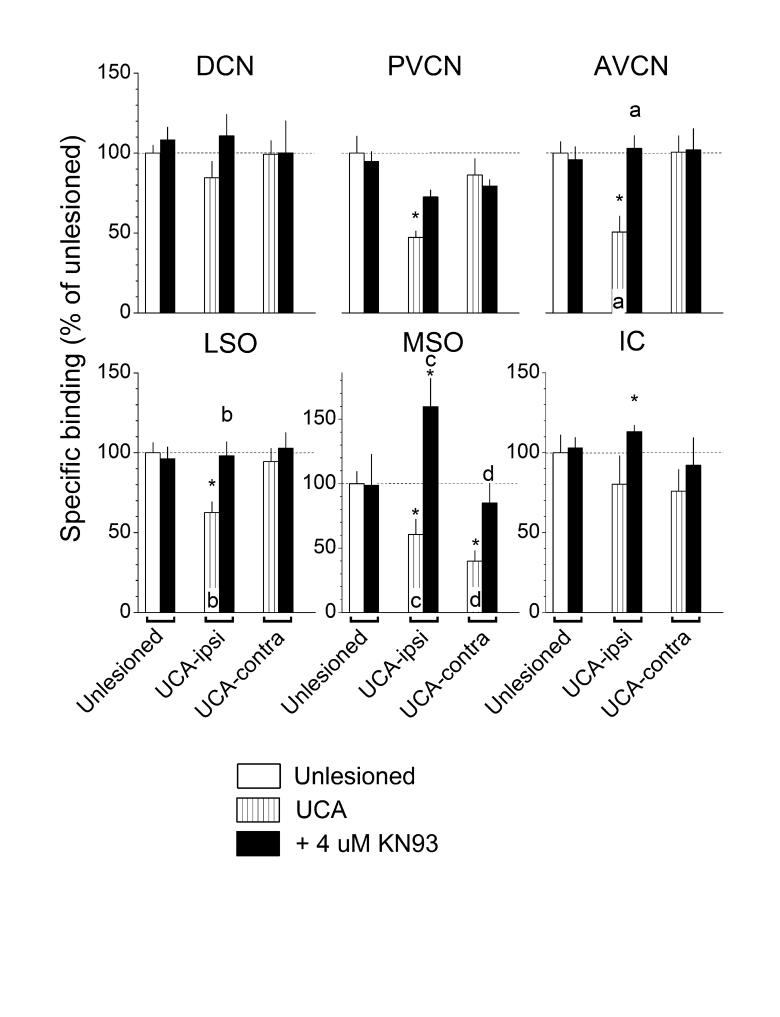
Fig. 4.
Actions of KN-93, a CaMKII inhibitor, on the specific binding of [3H]strychnine. Values from Fig. 1 for binding in unlesioned controls and animals that received the UCA are reproduced here for convenience. KN-93 usually elevated binding that was deficient after UCA but had little or no effect where binding was normal. Asterisks and lower case letters above bars were defined in the legend to Fig. 2.
In tissues taken from UCA animals, both the PKC inhibitor, Ro31-8220 (Fig. 2), and the PKA inhibitor, H-89 (Fig. 3), did not alter the deficient strychnine binding (see UCA-ipsi and -contra, compare vertically hatched to black bars). In contrast, the CaMKII inhibitor, KN-93 (Fig. 4), reversed most of the deficits in strychnine binding (see UCA-ipsi and -contra, compare vertically hatched to black bars). The exception was the failure of KN-93 to elevate significantly the deficient binding in the PVCN on the ablated side (Fig. 4, PVCN, UCA-ipsi, compare vertically hatched to black bars). KN-93 usually had no significant effects where binding levels were normal, except in the IC, where it elevated binding activity.
Discussion
The findings indicate that UCA reduced [3H]strychnine binding in tissue samples of several of the auditory nuclei (Fig. 1). The glycine receptor of mature animals is thought to consist of two α and three β subunits (Grudzinska et al., 2005). Since glycine and strychnine bind to the interface between these subunits (Grudzinska et al., 2005), [3H]strychnine binding depends on the presence of both these proteins in the receptor. In addition, the receptor of mature animals typically contains α1, α3 and β subunits (Sato et al., 2000; Legendre, 2001). Receptor subunit composition, however, may change with age (Milbrandt and Caspary 1995; Krenning et al., 1998) and after cochlear lesions (Holt et al., 2005; Sassu et al., 2005). Since receptors containing α1, α2 and α3 subunits exhibit similar sensitivities to strychnine (Kuhse et al., 1990; Legendre, 2001; Gisselmann et al., 2002; Han et al., 2003), it is unlikely that changes in receptor subunit composition, if they occurred, directly reduced the [3H]strychnine binding activity after UCA in the present study. It is more likely that the present declines in binding reflect a net loss of whole receptors or a reduced affinity for strychnine caused by a mechanism other than a change in subunit composition.
As proposed previously (Suneja et al., 1997), a decline of excitatory synaptic input may induce the down regulation of glycine receptor binding after UCA. The degeneration of the cochlear nerve after UCA (Benson et al., 1997) ends cochlear excitation of the ipsilateral CN and produced [3H]strychnine binding deficits in the CN subdivisions (Fig. 1; Suneja et al., 1997). The deafferented CN, in turn, presumably no longer excites the ipsilateral LSO and both the ipsi- and contralateral MSO; binding deficits also appeared in these nuclei (Fig. 1; Suneja et al., 1997). Thus, the loss of excitatory transmission after UCA may be responsible for the induction of mechanisms that regulate glycine receptors.
In most auditory nuclei that exhibited post-UCA deficits in [3H]strychnine binding, binding was restored by a PKC or PKA activator or a CaMKII inhibitor (Figs. 2 - 4). Since these compounds did not affect binding in tissues taken from unlesioned animals, the findings suggest that regulation of ligand binding was induced or increased after UCA, presumably through changes in the phosphorylation level of proteins that may include the glycine receptor itself (Smart, 1997; Legendre, 2001; Caraiscos et al., 2002). With this regulation in place, it is possible that the elevation of PKA and PKC activity by exogenous activators and the inhibition of CaMKII increased receptor synthesis. For example, receptor protein synthesis might be up regulated by the phosphorylation of several mRNA translation initiation factors by PKC and PKA (Mathews at al., 2000; Raught et al., 2000). In addition, a phosphorylated binding protein, which may be phosphorylated by CaMKII, blocks the action of one of the initiation factors (eIF4E), and requires either dephosphorylation or a block of its phosphorylation to release the initiation factor and allow it to function. In addition, glycine receptor subunit phosphorylation levels may have altered glycine receptor clustering and trafficking (Meier et al., 2001). Thus, one explanation of the present deficits after UCA would include a diminished expression of glycine receptor subunit expression and trafficking through an induced down regulation of protein phosphorylations (proposed in Suneja et al., 1997). If so, the recovery of ligand binding activity after reducing CaMKII activity or increasing PKC and PKA activity implies that exogenous control of protein phosphorylations may alleviate these deficits. However, not every auditory nucleus conformed to this presumed regulatory scheme. For example, PKA-dependent regulation of glycine receptors probably did not develop in the LSO after UCA, as deficient binding in that nucleus was not elevated by the PKA activator (Fig. 3). Thus, other mechanisms may contribute to the regulation of glycine receptors in the LSO.
Acknowledgments
This work was supported by NIH grant R01-DC00199 from the National Institute on Deafness And Other Communicative Disorders.
Abbreviations
- ANOVA
analyses of variance
- AVCN
anteroventral cochlear nucleus
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CN
cochlear nucleus
- DBcAMP
dibutyryl-cAMP
- DCN
dorsal cochlear nucleus
- DMSO
dimethyl sulfoxide
- FMRP
fragile X mental retardation protein
- GABA
gamma-amino buytric acid
- IC
inferior colliculus
- ICc
central nucleus of the inferior colliculus
- i.p.
intraperitoneal
- LSO
lateral superior olive
- MSO
medial superior olive
- PBS
phosphate buffer saline
- PDBU
phorbol 1,2-dibutyrate
- PKA
protein kinase A
- PKC
protein kinase C
- PVCN
posteroventral cochlear nucleus
- s.c.
subcutaneous
- UCA
unilateral cochlear ablation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backoff PM, Shadduck Palombi P, Caspary DM. Gamma-aminobutyric acidergic and glycinergic inputs shape coding of amplitude modulation in the chinchilla cochlear nucleus. Hear Res. 1999;134:77–88. doi: 10.1016/s0378-5955(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 2.Benson CG, Gross JS, Suneja SK, Potashner SJ. Synaptophysin immunoreactivity in the cochlear nucleus after unilateral cochlear or ossicular removal. Synapse. 1997;25:243–257. doi: 10.1002/(SICI)1098-2396(199703)25:3<243::AID-SYN3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Caraiscos VB, Mihic SJ, MacDonald JF, Orser BA. Tyrosine kinases enhance the function of glycine receptors in rat hippocampal nucleus and human α1β glycine receptors. J Physiol. 2002;539:495–502. doi: 10.1113/jphysiol.2001.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspary DM, Backoff PM, Finlayson PG, Palombi PS. Inhibitory inputs modulate discharge rate within frequency receptive fields of anteroventral cochlear nucleus neurons. J. Neurophysiol. 1994;72:2124–2133. doi: 10.1152/jn.1994.72.5.2124. [DOI] [PubMed] [Google Scholar]
- 5.Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol. 2000;83:926–940. doi: 10.1152/jn.2000.83.2.926. [DOI] [PubMed] [Google Scholar]
- 7.Faingold CL, Gehlbach G, Caspary DM. Functional pharmacology of inferior colliculus neurons. In: Altschuler RA, Bobbin RP, Clopton BM, Hoffman DW, editors. Neurobiology of Hearing: The Central Auditory System. Raven; New York: 1991. pp. 223–251. [Google Scholar]
- 8.Gentet LJ, Clements JD. Binding site stoichiometry and the effects of phosphorylation on human α1 homomeric glycine receptors. J Physiol. 2002;544:97–106. doi: 10.1113/jphysiol.2001.015321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisselmann G, Galler A, Friedrich F, Hatt H, Bormann J. Cloning and functional characterization of two glycine receptor α-subunits from perch retina. Eur J Neurosci. 2002;16:69–80. doi: 10.1046/j.1460-9568.2002.02070.x. [DOI] [PubMed] [Google Scholar]
- 10.Grothe B, Sanes DH. Synaptic inhibition influences the temporal coding properties of medial superior olivary neurons: an in vitro study. J. Neurosci. 1994;14:1701–1709. doi: 10.1523/JNEUROSCI.14-03-01701.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Han Y, Li P, Slaughter MM. Selective antagonism of rat inhibitory glycine receptor subunits. J Physiol. 2003;554:649–658. doi: 10.1113/jphysiol.2003.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- 14.Jastreboff PJ. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci. Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 15.Kuhse J, Schmieden V, Betz H. A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990;5:867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- 16.Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XB, Jones EG. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Nat Acad Sci USA. 1996;93:7332–7336. doi: 10.1073/pnas.93.14.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathews MB, Sonenberg N, Hershey JWB. Origins and principles of translational control. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Press; Cold Spring Harbor, NY: 2000. pp. 1–31. [Google Scholar]
- 19.Meier J, Vannier C, Serge A, Triller A, Choquet D. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci. 2001;4:253–260. doi: 10.1038/85099. [DOI] [PubMed] [Google Scholar]
- 20.Olson WO, Noffsinger D, Kurdziel S. Speech discrimination in quiet and in white noise by patients with peripheral and central lesions. Acta Otolaryngol. 1975;80:375–382. doi: 10.3109/00016487509121339. [DOI] [PubMed] [Google Scholar]
- 21.Potashner SJ. Uptake and release of D-aspartate in the guinea pig cochlear nucleus. J. Neurochem. 1983;41:1094–1101. doi: 10.1111/j.1471-4159.1983.tb09057.x. [DOI] [PubMed] [Google Scholar]
- 22.Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Hear Res. 2000;147:125–136. doi: 10.1016/s0378-5955(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 23.Ramakers GM, Pasinelli P, Hens JJ, Gispen WH, De Graan PN. Protein kinase C in synaptic plasticity: changes in the in situ phosphorylation state of identified pre- and postsynaptic substrates. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:455–486. doi: 10.1016/s0278-5846(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 24.Raught B, Gingras AC, Sonenberg N. Regulation of ribosomal recruitment in eukaryotes. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Press; Cold Spring Harbor, NY: 2000. pp. 245–293. [Google Scholar]
- 25.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 26.Sassu R, Argence M, Kerhuel L, Vidal P, de Wale C. Abstract Viewer and Itinerary Planner. Soc Neurosci Online; Washington, DC: 2005. Auditory system plasticity after unilateral cochleotomy in rats: An in situ hybridization and immunofluorescence study. Program No. 436.13. [Google Scholar]
- 27.Sato K, Kuriyama H, Altschuler RA. Expression of glycine receptor subunit mRNA in the rat cochlear nucleus. Hearing Res. 2000;144:47–52. doi: 10.1016/s0378-5955(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 28.Smart TG. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Cur Opin Neurobiol. 1997;7:358–367. doi: 10.1016/s0959-4388(97)80063-3. [DOI] [PubMed] [Google Scholar]
- 29.Suneja SK, Benson CG, Gross J, Potashner SJ. Uptake and release of D-aspartate, GABA and glycine in guinea pig brain stem auditory nuclei. J Neurochem. 1995;64:147–160. doi: 10.1046/j.1471-4159.1995.64010147.x. [DOI] [PubMed] [Google Scholar]
- 30.Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol. 1998a;154:473–488. doi: 10.1006/exnr.1998.6946. [DOI] [PubMed] [Google Scholar]
- 31.Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp. Neurol. 1998b;151:273–288. doi: 10.1006/exnr.1998.6812. J Neurosci. 20: 8551-8565. [DOI] [PubMed] [Google Scholar]
- 32.Yan L, Suneja SK, Potashner SJ. Protein kinases regulate glycine receptor activity in the brainstem auditory nuclei after unilateral cochlear ablation Assoc. Res. Otolaryngol. Abstr. 2004;27:299. doi: 10.1016/j.brainres.2006.12.013. (#885) [DOI] [PMC free article] [PubMed] [Google Scholar]