Abstract
Tendinopathy is accompanied by inflammation, tendon matrix degradation, or both. Inflammatory cytokine IL-1β, which is a potent inflammatory mediator, is likely present within the tendon. The purpose of this study was to determine the biological impact of IL-1β on tendon fibroblasts by assessing the expression of cPLA2, COX-2, PGE2 and its receptors (EP), collagen type-I, and MMPs. We also studied the role of the p38 MAPK pathway in IL-1β-induced catabolic effects. We found that IL-1β increased the intracellular levels of cPLA2 and COX-2, and also increased secretion of PGE2. Induction of MMPs, such as MMP-1 and MMP-3 at the mRNA level, was also observed after stimulation with IL-1β. Furthermore, the presence of IL-1β significantly decreased the level of collagen type-I mRNA in tendon fibroblasts. These effects were found to be mediated by selective upregulation of EP4 receptor, which is a member of G-protein-coupled receptor that transduces the PGE2 signal. Blocking EP4 receptor by a specific chemical inhibitor abolished IL-1β-induced catabolic effects. These results suggest that IL-1β-induced catabolic action on tendon fibroblasts occurs via the upregulation of two key inflammatory mediators, cPLA2 and COX-2, which are responsible for the synthesis of PGE2. IL-1β further stimulates the expression of EP4 receptor, suggesting positive feedback regulation which may lead to accelerated catabolic processes in tendon fibroblasts. Studies using pathway-specific chemical inhibitors suggest that the p38 MAPK pathway is the key signaling cascade transducing IL-1β-mediated catabolic effects. Collectively, our findings suggest that the EP4 receptor mediates the IL-1β-induced catabolic metabolism via the p38 MAPK pathway in human tendon fibroblasts and may play a major role in the tendon’s degenerative changes often seen in the later stages of tendinopathy.
Keywords: tendon fibroblasts, interleukin-1β, prostaglandin E2, collagen, MMPs, EP receptors
1. Introduction
Tendinopathy is a collective term that refers to a group of tendon disorders that commonly occur in both occupational and athletic settings (Almekinders and Temple, 1998). It is clinically associated with manifestation of pain and inflammation, greatly debilitating the workforce. Although the etiology of tendinopathy is considered multi-factorial, repetitive mechanical loading that causes tendon injuries is one of the major causative factors (Almekinders, 1998). Tendinopathy is pathologically characterized by tendon inflammation and/or degeneration (Riley, 2004). The anti-inflammatory drugs that have been prescribed over the years treat only the symptoms, not the root cause of the disease (Wang et al., 2006). Moreover, various causative factors for tendon degeneration that have not been fully characterized may precede tendon rupture (Riley, 2004). Therefore, it is essential to understand the cellular and molecular mechanisms of tendon degeneration during its early stages for the effective treatment of tendinopathy.
Tendon matrix is rich in collagens, such as collagen type-I (Wang, 2005). It is known that matrix remodeling is enabled by the balance of matrix metalloproteinases (MMPs) and their inhibitors, such as tissue inhibitors of metalloproteinases (TIMPs) (Kjaer, 2004). Imbalance in the expression of MMPs and their inhibitors can contribute to tendon matrix degradation, as collagen degradation is initiated by MMPs. It is proposed that prior to tendon matrix degradation, subtle changes involving the release of inflammatory cytokines such as IL-1 by infiltrating macrophages/monocytes may occur (Tsuzaki et al., 2003a). In addition, tendons in vivo are subjected to repetitive motion which may cause microinjuries, which is sufficient to induce endogenous IL-1β (Tsuzaki et al., 2003b). IL-1β, a potent inflammatory cytokine, has been reported to be present in the synovium of inflamed rotator cuffs, which may enhance inflammatory intensity at the site of injury (Gotoh et al., 2000; Gotoh et al., 2002). IL-1β acts through its specific receptor, which is shown to be present in tendon fibroblasts, the occupation of which can activate numerous signaling pathways, including MAPKs (Bankers-Fulbright et al., 1996; Tsuzaki et al., 2003b).
As in other connective tissue injuries, tendon injuries are likely accompanied by upregulation of IL-1β. In vitro studies have shown that IL-1β can induce inflammatory mediators such as COX-2, PGE2, and the matrix-degrading enzymes MMPs, which are all involved in tendon matrix degradation (Archambault et al., 2002; Tsuzaki et al., 2003b). Although some of these effects are suggested to be modulated through MAPK pathways in other fibroblasts (Brauchle et al., 2000; Kida et al., 2005; Saklatvala et al., 1999), the extent to which MAPKs are involved in inflammatory pathway regulation in tendon fibroblasts is not well understood. The three well-characterized subfamilies of MAPKs (ERK1/2, JNKs, and p38 MAPKs) are, however, implicated in the expression and activation of pro-inflammatory molecules such as COX-2 (Kyriakis and Avruch, 2001). Therefore, the intracellular signaling mechanisms triggered by IL-1β, as well as this pro-inflammatory cytokine-generated signaling pathway responsible for PGE2 production, need to be explored in tendon fibroblasts.
PGE2 has been identified as a central lipid mediator in inflammation and pain in many tissues, including tendons (Almekinders and Temple, 1998; Rocha et al., 2003). It is an arachidonic acid (AA) metabolite released from membrane phospholipids via activation of cPLA2 and COX-2. Both in vivo and in vitro experimental models of tendinopathy have provided evidence suggesting that production of PGE2 may be an important step in the development of tendinopathy. For example, in rabbit tendons, repeated exposure to PGE2 results in localized tendon degeneration (Khan et al., 2005); in human tendon fibroblasts, repetitive mechanical loading elevates the production of PGE2 (Khan et al., 2005; Li et al., 2004; Wang et al., 2003b; Wang et al., 2004). Furthermore, the major catabolic functions of PGE2 in connective tissues are inhibition of collagen type-I synthesis and induction of MMPs (Fall et al., 1994; Kim et al., 2005; Ruwanpura et al., 2004; Varga et al., 1987). In tendon fibroblasts, for instance, exogenous PGE2 decreases collagen type-I production and upregulates MMP-1 and -3 gene and protein expression (Cilli et al., 2004; Thampatty et al., 2006).
PGE2 acts through G-protein-coupled receptors called EP receptors, which are classified into four subtypes, EP1 through EP4 (Narumiya et al., 1999). Each of these receptors has unique signal transduction mechanisms as a result of coupling to different G proteins. EP1 receptor stimulates intracellular calcium while EP2 and EP4 receptors activate adenylate cyclase which results in elevation of intracellular cAMP levels. EP3 receptor has variants that mediate multiple signaling pathways (Negishi et al., 1995). PGE2-induced catabolic effects in fibroblasts are shown to be mediated via upregulation of one of its receptors mentioned previously. For instance, activation of EP2 receptor has been shown to reduce collagen type-I mRNA expression in lung fibroblasts (Choung et al., 1998). Also, PGE2 is unable to suppress proliferation and collagen synthesis in lung fibroblasts from EP2-deficient mice despite the expression of other EP receptors (Moore et al., 2005). Furthermore, IL-1β-induced MMP-3 production is upregulated via EP1 receptors in cells from periodontally-diseased tissue (Ruwanpura et al., 2004). However, little is presently known about the expression and regulation of EP receptors in human tendon fibroblasts.
The aims of the current study were to show that 1) exogenous IL-1β induces inflammatory mediators such as cPLA2, COX-2, and PGE2 via its specific functional receptor; 2) IL-1β-induced PGE2 (i.e. endogenous PGE2) mediates catabolic effects by modulating the target gene expression of the PGE2-mediated signaling pathway such as downregulation of collagen type-I and upregulation of MMPs; and 3) this catabolic effect of IL-1β is mediated via the p38 MAPK signaling cascade as a key mechanistic molecule in human tendon fibroblasts. To test these hypotheses, a novel cell culture model was used. In this culture model, human tendon fibroblasts were grown in microgrooved culture surfaces, as opposed to the smooth culture surface, to allow the fibroblasts to mimic the alignment and shape of these cells in vivo (Wang et al., 2003a). We report here that IL-1β increased the protein levels of cPLA2, COX-2, and PGE2 expressed by human tendon fibroblasts. The accelerated production of PGE2 appeared to occur via its functional receptor EP4, which is selectively upregulated in the presence of IL-1β playing a role as a positive feedback regulator. These upregulation of inflammatory mediators by IL-1β were, at least in part, triggered by the activation of the p38 MAPK signaling pathway. We also found significant induction of MMPs and reduction of collagen type-I mRNA after stimulation with IL-1β in human tendon fibroblasts.
2. Materials and methods
2.1. Fibroblast culture
Human patellar tendon fibroblasts (HPTFs) were isolated and maintained from tendon samples of two healthy male donors as previously described (Thampatty et al., 2006; Yang et al., 2005). The protocol for obtaining tendon samples was approved by the Institutional Review Board of the University of Pittsburgh Medical Center (IRB #0407060). Briefly, washed and minced tendon samples were transferred to 100 mm polystyrene Petri dishes and cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 50U/mL penicillin, and 50U/mL streptomycin (P/S; Life Technologies, Rockville, MD). The cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C and subcultured up to six times without change in cell morphology and doubling time.
2.2. Culture experiments
HPTFs were plated on culture surfaces in custom-made silicone dishes (culture area 3 cm x 6 cm) which were transparent, elastic, and nontoxic to cultured cells (Wang and Grood, 2000). A special feature of the dish was that the culture surface was fabricated with microgrooves of width 10 μm and depth 3 μm, with 10 μm wide ridges between the grooves (Fig. 1). To promote cell attachment, the microgrooved surfaces of the dishes were pre-coated with 10 μg/ml ProNectin-F (Sigma, St Louis, MO).
Fig 1.

Human tendon fibroblasts were grown on microgrooved culture surfaces. The cells remained aligned and the shape during cell culture experiments. The cell alignment and shape mimic the situation in vivo. (The arrow points to a fibroblast on the microgrooved substrate. Bar: 50 μm).
Next, 2 x 105 HPTFs were plated in each silicone dish and grown in DMEM supplemented with 10% FBS and 1% P/S. After 24 hrs, the growth medium was replaced by DMEM supplemented with 1% serum. The fibroblasts were then treated with human recombinant IL-1β (Chemicon International Inc., Temecula, CA) at 0.01, 0.1, and 1 ng/ml doses. Cells without IL-1β treatment served as controls. Human recombinant IL-1 receptor antagonist (IL-1ra) (R&D Systems, Minneapolis, MN) at concentrations of 10 and 100 ng/ml, p38 MAPK inhibitor, SB203580 (Calbiochem, La Jolla, CA) at concentrations of 1 and 10 μM, and EP4 receptor antagonist AH23848 (Sigma, St. Louis, MO) at concentrations of 0.1, 1 and 10 μM were added wherever appropriate 30 min prior to treating cells with IL-1β. The culture media were sampled after incubation for 24 hrs to measure PGE2 levels, and cell lysates were collected to obtain total cellular protein for Western blot analysis and total RNA for RT-PCR.
2.3. Western blot analysis
Standard Western blotting technique was used to determine cPLA2 and COX-2 protein expression levels as described previously (Yang et al., 2004). Briefly, equal amounts of the denatured cellular proteins were fractionated by electrophoresis on 10% SDS-polyacrylamide gels, and the separated proteins were then transferred to nitrocellulose membranes. After blocking for non-specific binding, the blots were incubated for 1 hr with monoclonal antibody of anti-cPLA2 (Cell Signaling, Beverly, Massachusetts) followed by 1 hr incubation with goat anti-rabbit IgG (HRP-linked Antibody, Cell Signaling, Beverly, Massachusetts). After washing, the proteins on the membranes were then detected with the ECL Plus detection system (Amersham Pharmacia Biotech, Piscataway, New Jersey) according to the manufacturer’s protocol. The membranes were stripped and probed with antibody of anti-COX-2 (Cayman Chemical, Ann Arbor, Michigan) for 1 hr followed by incubation with goat anti-mouse IgG (Jackson Immunoresearch Lab, Inc., West Grove, PA) for 1 hr and the proteins were detected as described earlier. The membranes were probed with GAPDH antibody (Biogenesis Inc, Kingston, New Hampshire) for an internal control of equal protein loading.
2.4. PGE2 Assay
PGE2 levels in the media were assayed using a commercially available ELISA kit (Assay Designs, Ann Arbor, Michigan) following the manufacturer’s protocol. This assay is based on the competitive binding technique in which PGE2 present in the sample competes with a fixed amount of alkaline phosphatase-labeled PGE2 for sites on a mouse monoclonal antibody. The amount of PGE2 in each sample was determined using a standard curve generated from known amounts of PGE2 ranging from 39–2500 pg/ml. The sensitivity of the assay was 13.4 pg/ml.
2.5. RT-PCR
Reverse transcription (RT) was carried out with 1 μg of total cellular RNA using the ThermoScript RT-PCR System (Invitrogen) for first strand cDNA synthesis in 20 μL of reaction volume. The primer sequences and optimized annealing temperature for each primer set are provided in Table 1. For all experiments, the conditions were determined to be in the linear range for the PCR amplification. Briefly, all samples were subjected to RT, and subsequent amplification of the cDNA samples was performed by PCR at the same time. The cDNA samples were then assessed for GAPDH expression. Genomic DNA was included for the PCR to ensure that there was no genomic DNA contamination in the total RNA samples. The cDNA was amplified by PCR using 28 cycles at 95°C for 30 seconds, 55–60°C for 30 seconds, and 72°C for 30 seconds in the presence of Taq polymerase (Invitrogen) and 50 pmol of sense and antisense primers. PCR products were resolved on 1.5% agarose gels by electrophoresis and visualized by staining with ethidium bromide and UV transillumination.
Table 1.
Primer sequences for RT-PCR
| Gene Annealing temperature | Primer Sequence Forward/Reverse | PCR Product Size (bp) |
|---|---|---|
| Collagen-I | 5′-GGT TAC TAC TGG ATT GAC C-3′ | 328 |
| 58°C | 5′-TTG CCA GTC TCC TCA TCC-3′ | |
| MMP-1 | 5′-CAACT CTGGAGTAAT GTCACA -3′ | 295 |
| 58°C | 5′-T ACATCAAAGC CCCGATATCA -3′ | |
| MMP-3 | 5′-TTT TGG CCA TCT CTT CCT TCA-3′ | 138 |
| 55°C | 5′-TGT GGA TGC CTC TTG GGT ATC-3′ | |
| EP-4 | 5′-TTTCCAGACTGAGCAGGACAAGGT-3′ | 525 |
| 55°C | 5′-ATAGGCATGGTTGATGGCCAGGTA-3′ | |
| GAPDH | 5′-AAATTCCATGGCACCGTCAAGGCT-3′ | 295 |
| 58°C | 5′-CTCATGGTTCACACCCATGACGAA-3′ |
3. Results
3.1. IL-1β induced cPLA2 and COX-2 protein expression
The effect of IL-1β at 0.01, 0.1, and 1 ng/ml on cPLA2 and COX-2 protein expression was determined (Fig. 2). The control shows basal expression of cPLA2 suggesting that tendon cells constitutively express cPLA2. At 0.01 ng/ml concentration of IL-1β, cPLA2 expression markedly increased, and higher dosages (0.1 and 1 ng/ml) further augmented the intracellular protein expression. This augmentation of cPLA2 by IL-1β was significantly reduced by co-incubation with IL-1ra, although IL-1ra alone did not affect cPLA2 protein expression (Fig. 2).
Fig 2.
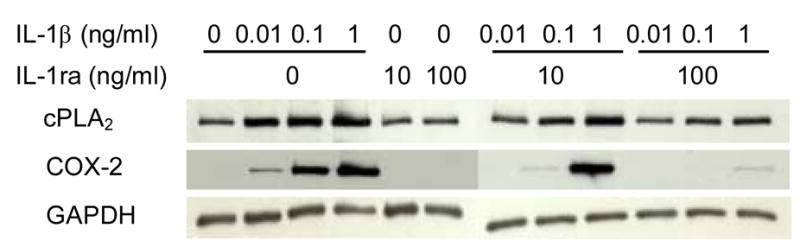
Effects of IL-1β and IL-1ra on cPLA2 and COX-2 protein expression. A representative Western blot result of three independent experiments is shown. The data show that IL-1β increased cPLA2 and COX-2 protein expressions in dose-dependent manner and co-incubation with IL-ra reduced the augmentations of protein expressions. IL-1ra by itself did not have any effect on both protein expressions.
Unlike expression of cPLA2, basal protein level of COX-2 in tendon cells was not detectable. Stimulation of cells with IL-1β increased COX-2 protein level in a dose-dependent manner. Specifically, IL-1β at 0.01 ng/ml slightly increased COX-2 protein expression, but both 0.1 ng/ml and 1 ng/ml IL-1β markedly increased it (Fig. 2). Again, co-incubation with IL-1ra completely inhibited the IL-1β-mediated stimulation of COX-2 production. Collectively, these data suggest an IL-1β-specific cellular response to regulate the expression of inflammatory mediators such as COX-2 in human tendon fibroblasts.
3.2. IL-1β induced PGE2 production
Activation cPLA2 and COX-2 can elevate PGE2 production (Funk, 2001). Therefore, we examined the effect of IL-1β on PGE2 production in tendon fibroblasts. Induction of cPLA2 and COX-2 protein expression was accompanied by an increase in PGE2 release. For example, treatment of tendon fibroblasts with 0.1 ng/ml IL-1β for 24 hrs resulted in only a slight induction of PGE2, but 1 ng/ml IL-1β induced a phenomenal increase in PGE2 production (Fig. 3). IL-1ra at 100 ng/ml completely inhibited IL-1β-induced PGE2 production, again suggesting IL-1β-specific cellular response to regulate PGE2 expression in human tendon fibroblasts.
Fig 3.
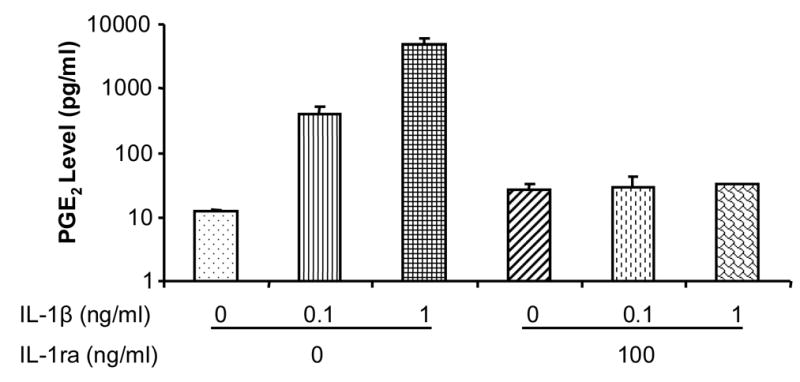
Effects of IL-1β and IL-1ra on PGE2 production. IL-1β increased PGE2 release in a dose-dependent manner and IL-1ra markedly inhibited the PGE2 production. IL-1ra by itself did not have any effect on PGE2 production. Two independent experiments were performed with a sample size of 6.
3.3. p38 MAPK inhibitor abolished IL-1β-induced cPLA2 and COX-2 protein expression and PGE2 production
Stimulation of cells with IL-1β significantly increased cPLA2 protein level (Fig. 4), and this upregulation was abrogated in the presence of a pathway-specific chemical inhibitor of p38 MAPK, SB203580 (Fig. 4). SB203580 with concentration of 1 μM was sufficient to reduce the induction of cPLA2 by IL-1β. Similarly, IL-1β-stimulation of COX-2 protein was inhibited by SB203580 (Fig. 4). In addition, PGE2 production induced by IL-1β at 0.1 and 1 ng/ml was also inhibited by 10 μM SB203580 (Fig. 5). These results suggest the involvement of the p38 MAPK signaling pathway in IL-1β-induced cPLA2 and COX-2 protein expression and PGE2 production.
Fig. 4.
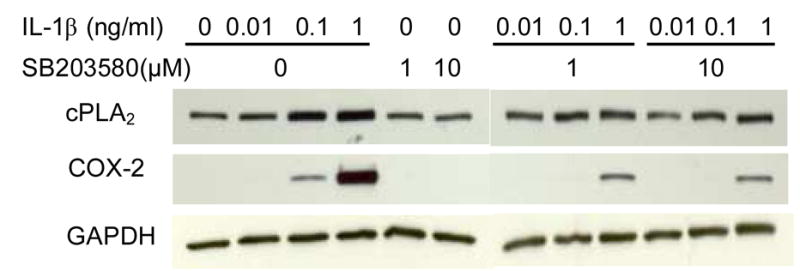
Effects of IL-1β and SB203580 on cPLA2 and COX-2 protein expression. A representative experimental result from three independent experiments is shown. The data show that IL-1β increased cPLA2 and COX-2 protein expressions in a dose-dependent manner. SB203580 reduced both protein expressions, but by itself did not affect the protein expressions.
Fig. 5.
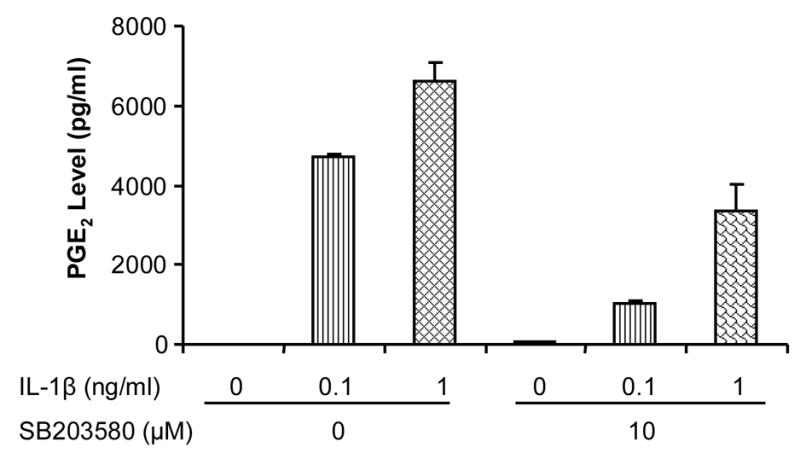
Effects of IL-1β and SB20838 on PGE2 production. IL-1β increased PGE2 levels in a dose-dependent manner and SB203580 decreased them. Two independent experiments were performed with a sample size of 6.
3.4. IL-1β modulated the expression of collagen type-I, MMP-1, and MMP-3 at the level of mRNA via specific upregulation of EP4 receptor expression
We determined the effect of treating tendon fibroblasts with 1 ng/ml IL-1β for 24 hrs on collagen type-I at the mRNA level. There was substantial downregulation of collagen type-I expression after 24 hrs of treatment (Fig. 6). On the other hand, collagen type-III, which is a minor component of tendon matrix, did not show any change in its expression after IL-1β treatment (data not shown), suggesting that the IL-1β-mediated pathway mainly decreases the expression of collagen type-I as a target gene in human tendon fibroblasts.
Fig. 6.
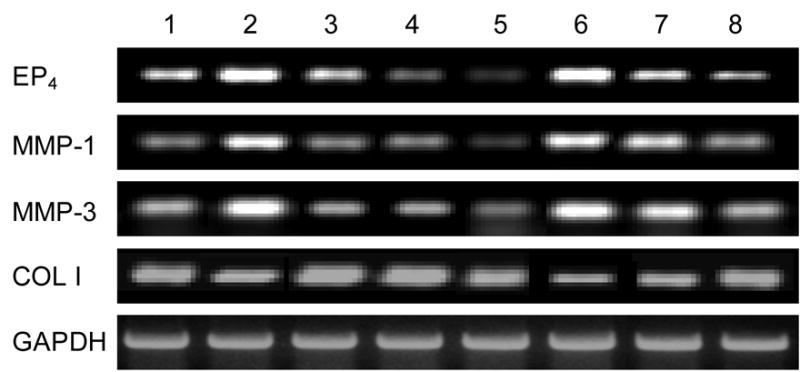
Effects of IL-1β (1 ng/ml) and AH23848 (0.1, 1, and 10 μM) on the expressions of collagen-I, MMP-1, MMP-3, and EP4 at the level of mRNA. IL-1β decreased collagen type-I whereas it increased MMP-1, MMP-3, and EP4 gene expression (lane 2) compared to the control (lane 1). A pretreatment with AH23848, a specific chemical inhibitor of EP4, inhibited the biological impact of IL-1β in a dose-dependent manner (lanes 6–8). Lanes 3–5 represent AH23848 treatment alone.
We also determined the effect of IL-1β on MMP-1 and -3 at the mRNA levels. IL-1β at 1 ng/ml significantly increased the gene expression of both MMP-1 and MMP-3 after 24 hrs of treatment (Fig. 6). Collectively, our data suggest that IL-1β exerts catabolic effects in tendon fibroblasts via upregulating MMP-1 and -3 and downregulating collagen type-I mRNA expression.
In order to determine whether IL-1β regulates EP receptor expression, we examined the expression patterns of EP receptors after IL-1β treatment. Initially, we tested if human tendon fibroblasts express EP receptors and if so, whether the presence of IL-1β affects the expression of these EP receptors (EP1 to EP4). The expression of EP3 was not detectable in human tendon fibroblasts, whereas the expression of EP1, EP2, and EP4 was observed (data not shown). The most notable change in terms of differential expression among EP receptors was EP4 receptor. After 24 hrs of treatment with IL-1β at 1 ng/ml, EP4 receptor mRNA level was considerably higher in treated cells than in control cells (Fig. 6). This stimulatory effect by IL-1β was not observed in other EP receptors, EP1 and EP2, suggesting a specific upregulation of EP4 receptor by IL-1β in human tendon fibroblasts (data not shown).
Since we observed specific upregulation of EP4 receptor expression by IL-1β treatment, we determined the effect of blocking EP4 receptor using a specific EP4 receptor antagonist, AH23848 (Coleman et al., 1994). The effect of various concentrations of the antagonist on the expression of collagen type-I, EP4, MMP-1, and MMP-3 at the mRNA level was tested with and without IL-1β treatment. The stimulatory or suppressive biological effects induced by IL-1β were inhibited by pretreatment with AH23848 in a dose-dependent manner (Fig. 6, lanes 6–8). These results suggest that the antagonist could effectively block the catabolic action induced by IL-1β.
4. Discussion
A novel culture model was used to investigate the inflammatory responses of HPTFs to IL-1β treatment. Tendon fibroblasts in this culture model aligned parallel to each other and had an elongated shape similar to that of the cells in vivo. Moreover, treatment of the tendon fibroblasts with IL-1β did not change their morphology and did not cause cell death, suggesting that IL-1β at the concentrations used in this study likely did not elicit toxic effects. Since cell organization and shape influence cell function (Chen et al., 1998; Wang et al., 2003a), this culture model system may more accurately reflect the inflammatory responses of human tendon fibroblasts to IL-1β, which is the potent inflammatory cytokine produced due to tendon microinjuries resulting from repetitive mechanical loading of tendons in vivo.
With such a novel culture model, this study showed that IL-1β induced significant, dose-dependent upregulation of cPLA2 protein expression. IL-1β also increased intracellular production levels of COX-2 and PGE2 relative to untreated cells. These IL-1β-mediated cellular responses were inhibited by co-incubation with IL-1ra, suggesting the specificity of IL-1β in its inflammatory actions mediated via its biologically functional receptor. Therefore, IL-1ra may offer protection of tendon cells against IL-1β-mediated inflammatory insult. Recently, we and others have shown that IL-1β induces dose-dependent increase in COX-2 expression at the mRNA level in human tendon fibroblasts (Tsuzaki et al., 2003b; Yang et al., 2005). Current results further confirm our hypothesis that IL-1β induces the inflammatory signaling pathway in tendon fibroblasts by upregulation of inflammatory modulators, including cPLA2, COX-2, and PGE2 at both mRNA and protein levels via specific functional IL-1β receptor. Previously, IL-1β has been shown to induce cPLA2, COX-2, and PGE2 in various other cell types such as gingival fibroblasts, neuroblastoma, and neuroglioma cells, suggesting an IL-1β-mediated inflammatory process (Fiebich et al., 2000; Kida et al., 2005; Moolwaney and Igwe, 2005). Our studies using human tendon fibroblasts show results consistent with these previous reports using other tissue/cell types. IL-1β has also been shown to enhance the expression of prostaglandin E synthase (mPGES), which catalyzes the final step in the conversion of PGH2 to PGE2 in rheumatoid synovial fibroblasts (Kojima et al., 2003).
cPLA2 is a key enzyme that plays an important role in the inflammation process, supplying AA for cellular production of inflammatory eicosanoids such as prostaglandins and leukotrienes. Furthermore, cPLA2 itself participates in tissue inflammation. For example, intratracheal, intradermal, and intra-articular injection of cPLA2 in rabbits induces profound inflammatory lesions (Gonzalez-Buritica et al., 1989). Current study in human tendon cells also shows that concomitant with the elevated expression of cPLA2, its downstream inflammatory mediators, COX-2 and PGE2 are increased in their levels. COX-2 has been recognized as playing an important role in tissue inflammation. For example, in a collagen-induced arthritis model, mice deficient in COX-2 exhibited a significant reduction in synovial inflammation and joint destruction (Loftin et al., 2002; Myers et al., 2000).
In addition to the inflammatory role of cPLA2 and COX-2, PGE2 is involved in many inflammatory conditions. Basal levels of PGE2 may be maintained by constitutively-expressed COX-1 for normal cellular function; however, elevated levels of PGE2 by IL-1β treatment can participate in the development of certain inflammatory diseases such as in neurodegenerative diseases (Angel et al., 1994; Sun et al., 2004; Xu et al., 2003) and in the formation of inflammatory lesions in gingival tissue (Yucel-Lindberg et al., 1995). Moreover, PGE2 decreases cell proliferation and collagen synthesis in lung fibroblasts (Fine and Goldstein, 1987; Fine et al., 1989; Kawamoto et al., 1995#61). Our recent studies have shown that treatment of HPTFs with exogenous PGE2 decreases cell proliferation and collagen synthesis, which may eventually lead to tendon degeneration, a hallmark of tendinopathy at later stages (Cilli et al., 2004). Thus, the expression of the inflammatory mediators, cPLA2 and COX-2 and a high level of PGE2 production by HPTFs in response to exposure of IL-1β in vivo may contribute to tendon inflammation and/or degeneration often seen in repetitive motion-induced tendon injuries.
Besides the upregulation of inflammatory mediators, we observed that IL-1β significantly downregulates the expression of collagen type-I at the mRNA level in human tendon fibroblasts. Collagen type-I is the major component of tendon matrix. Thus, IL-1β-mediated suppression of collagen type-I gene expression may lead to the reduced deposition of ECM. Consequently, it might affect normal tissue remodeling and perhaps lead to development of tendinopathy. There is experimental evidence that the downregulation of collagen type-I by IL-1β is mediated via endogenous PGE2 generated by IL-1β. For example, it has been shown that the inhibitory effect of IL-1β on lung fibroblast collagen production is partially due to the effects of newly synthesized PGE2 (Diaz et al., 1993). These results indicate that inflammatory cytokines such as IL-1β and TNF-α inhibit the expression of α1(I) procollagen gene at the transcriptional level by a PGE2-dependent pathway as well as through the effect of endogenous PGE2 released under the stimulus of inflammatory cytokines. Thus, it is reasonable to speculate that IL-1β disturbs human tendon homeostasis in part through the induction of PGE2, which initiates inhibition of collagen production.
In this study, we also showed that IL-1β upregulates both MMP-1 and MMP-3 at the level of mRNA in human tendon fibroblasts, which may accelerate tendon matrix degradation. Our results are consistent with previous observations in tendon cells (Tsuzaki et al., 2003b; Yang et al., 2005) and are also in agreement with the observation in cardiac fibroblasts that IL-1β decreases collagen synthesis and increases MMP activity (Siwik et al., 2000). Furthermore, collagen degradation by IL-1β has been accompanied by release and activation of multiple MMPs such as MMP-1, -8, and -13 in gingival fibroblasts (Cox et al., 2006). Collectively, these results suggest that IL-1β contributes to abnormal collagen matrix turn over in tendons.
The elevated expression of MMPs induced by IL-1β is also thought to be mediated via endogenous PGE2 in fibroblasts. For example, it was shown that IL-1β-induced MMP-3 production in human gingival fibroblasts was regulated by PGE2 (Ruwanpura et al., 2004). In addition, we have recently shown that exogenous PGE2 upregulates MMP-1 and -3 at the levels of mRNA and protein in tendon fibroblasts (Thampatty et al., 2006). Additional evidence for the potential role of endogenous PGE2 in mediating catabolic effects in tendon fibroblasts is provided by the current observation that IL-1β specifically upregulates the expression of EP4 receptor among other EP receptors. Blocking the EP4 receptor signaling pathway by using selective EP4 receptor antagonist switches the catabolic effects in favor of more anabolic effects. For example, we observed that IL-1β-mediated suppression of collagen type-I or stimulation of MMPs is reversed to the control level using human tendon fibroblasts. It is well established that PGE2 exerts its effects via its EP receptors (EP1–EP4). The diversity and selectivity of PGE2 effects are dependent on the expression of these four different EP subtypes of PGE2 receptors (Negishi et al., 1995). Previous observations in other fibroblasts (lung, gingival) provide evidence for the specific upregulation of EP receptor subtypes such as EP1 and EP2 in the matrix degradation process (Choung et al., 1998; Moore et al., 2005; Ruwanpura et al., 2004). Selective upregulation of EP4 receptor expression by IL-1β has been reported in PGE2-mediated cervical ripening process (Schmitz et al., 2003). Interestingly, IL-1β also selectively stimulates the expression of EP4 receptor in human tendon fibroblasts. Potentially, the specific upregulation of EP4 receptors in tendon fibroblasts by inflammatory cytokine may trigger inflammatory signaling pathways ultimately leading to tendon matrix degradation and thus tendinopathy.
This study has certain limitations. We did not determine the time course of IL-1β effect on cPLA2 and COX-2 protein expression and PGE2 production. Also, we did not investigate the possible involvement of other MAPK pathways such as ERK1/2 or JNK in IL-1β-mediated effects in tendon fibroblasts. These pathways have also been implicated in IL-1β-mediated inflammatory effects (Kida et al., 2005; Laporte et al., 1999). It is also important to investigate the potential anabolic role of EP4 receptor antagonist in models of tendinopathy in vitro and in vivo. Since repetitive mechanical loading is considered as one of the causative factors for development of tendinopathy, it would be interesting to investigate how EP4 receptor antagonist may regulate the inflammatory mediator release under mechanical loading conditions. Previously, we have shown that a small magnitude mechanical loading of tendon fibroblasts decreases COX-2 and MMP-1 gene expression and PGE2 production that were stimulated by IL-1β (Yang et al., 2005). EP4 receptor antagonist together with small magnitude loading may further augment this effect, favoring tendon inflammation reduction. Additionally, we are in the process of developing “exercise animal models” of tendinopathy to induce tendon microinjuries in mice running on treadmills with various loading intensities. In this model, administration of EP4 receptor antagonist to reduce tendon inflammation and degeneration may be a suitable clinical approach toward effective treatment of tendinopathy.
In conclusion, this study shows that treatment of HPTFs with the inflammatory cytokine IL-1β increases cPLA2 and COX-2 protein expression and PGE2 production. Furthermore, IL-1β induces catabolic effects in tendon matrix by downregulating collagen gene expression and upregulating MMP gene expression. Importantly, we found that the biological impact of IL-1β appeared to be mediated via EP4 receptor, which is specifically upregulated by IL-1β in human tendon fibroblasts. These effects are at least in part mediated via the p38 MAPK signaling pathway. IL-1β is the inflammatory cytokine produced when tendon microinjuries occur resulting from repetitive mechanical loading of tendons in vivo. Our study using a novel cell culture system that preserves tendon fibroblastic phenotype in vivo shows that the exposure of tendon fibroblasts to IL-1β induces the expression and production of inflammatory mediators and matrix degradation, which may contribute to tendon inflammation and thus participate in the pathogenesis of tendinopathy. The use of selective EP4 receptor antagonist may be an attractive therapeutic option for the treatment of tendinopathy at early stages.
Acknowledgments
This study was supported in part by NIH grant AR049921, the Arthritis Investigator Award (JHW), and the Falk Foundation (HJI). We thank Wacker Chemical (Adrian, MI) for providing us with materials for making silicone dishes used in this study.
Abbreviations
- AA
Arachidonic acid
- cAMP
Cyclic adenosine monophosphate
- COX-2
Cyclooxygenase-2
- DMEM
Dulbecco’s Modified Eagle Medium
- EP
Prostaglandin E receptor
- ELISA
Enzyme-linked immunosorbent assay
- ERK
Extracellular regulated kinase
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde phosphate dehydrogenase
- HHTF
Human patellar tendon fibroblast
- IL-1β
Interleukin-1β
- IL-1ra
Interleukin-1 receptor antagonist
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen activated protein kinase
- MMP
Matrix metalloproteinase
- PGE2
Prostaglandin E2
- cPLA2
Cytosolic phospholipase A2
- RT-PCR
Reverse transcription-polymerase chain reaction
- TIMP
Tissue inhibitors of metalloproteinases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almekinders LC. Tendinitis and other chronic tendinopathies. J Am Acad Orthop Surg. 1998;6:157–64. doi: 10.5435/00124635-199805000-00003. [DOI] [PubMed] [Google Scholar]
- Almekinders LC, Temple JD. Etiology, diagnosis, and treatment of tendonitis: an analysis of the literature. Med Sci Sports Exerc. 1998;30:1183–90. doi: 10.1097/00005768-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Angel J, Berenbaum F, Le Denmat C, Nevalainen T, Masliah J, Fournier C. Interleukin-1-induced prostaglandin E2 biosynthesis in human synovial cells involves the activation of cytosolic phospholipase A2 and cyclooxygenase-2. Eur J Biochem. 1994;226:125–31. doi: 10.1111/j.1432-1033.1994.tb20033.x. [DOI] [PubMed] [Google Scholar]
- Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36–9. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Bankers-Fulbright JL, Kalli KR, McKean DJ. Interleukin-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Gluck D, Di Padova F, Han J, Gram H. Independent role of p38 and ERK1/2 mitogen-activated kinases in the upregulation of matrix metalloproteinase-1. Exp Cell Res. 2000;258:135–44. doi: 10.1006/excr.2000.4913. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998;14:356–63. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- Choung J, Taylor L, Thomas K, Zhou X, Kagan H, Yang X, Polgar P. Role of EP2 receptors and cAMP in prostaglandin E2 regulated expression of type I collagen alpha 1, lysyl oxidase, and cyclooxygenase-1 genes in human embryo lung fibroblasts. J Cell Biochem. 1998;71:254–63. [PubMed] [Google Scholar]
- Cilli F, Khan M, Fu F, Wang JH. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med. 2004;14:232–6. doi: 10.1097/00042752-200407000-00006. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Grix SP, Head SA, Louttit JB, Mallett A, Sheldrick RL. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994;47:151–68. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Cox SW, Eley BM, Kiili M, Asikainen A, Tervahartiala T, Sorsa T. Collagen degradation by interleukin-1beta-stimulated gingival fibroblasts is accompanied by release and activation of multiple matrix metalloproteinases and cysteine proteinases. Oral Dis. 2006;12:34–40. doi: 10.1111/j.1601-0825.2005.01153.x. [DOI] [PubMed] [Google Scholar]
- Diaz A, Munoz E, Johnston R, Korn JH, Jimenez SA. Regulation of human lung fibroblast alpha 1(I) procollagen gene expression by tumor necrosis factor alpha, interleukin-1 beta, and prostaglandin E2. J Biol Chem. 1993;268:10364–71. [PubMed] [Google Scholar]
- Fall PM, Breault DT, Raisz LG. Inhibition of collagen synthesis by prostaglandins in the immortalized rat osteoblastic cell line Py1a: structure-activity relations and signal transduction mechanisms. J Bone Miner Res. 1994;9:1935–43. doi: 10.1002/jbmr.5650091213. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Mueksch B, Boehringer M, Hull M. Interleukin-1beta induces cyclooxygenase-2 and prostaglandin E(2) synthesis in human neuroblastoma cells: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. J Neurochem. 2000;75:2020–8. doi: 10.1046/j.1471-4159.2000.0752020.x. [DOI] [PubMed] [Google Scholar]
- Fine A, Goldstein RH. The effect of PGE2 on the activation of quiescent lung fibroblasts. Prostaglandins. 1987;33:903–13. doi: 10.1016/0090-6980(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Fine A, Poliks CF, Donahue LP, Smith BD, Goldstein RH. The differential effect of prostaglandin E2 on transforming growth factor-beta and insulin-induced collagen formation in lung fibroblasts. J Biol Chem. 1989;264:16988–91. [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Buritica H, Khamashita MA, Hughes GR. Synovial fluid phospholipase A2s and inflammation. Ann Rheum Dis. 1989;48:267–9. doi: 10.1136/ard.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh M, Hamada K, Yamakawa H, Nakamura M, Yamazaki H, Ueyama Y, Tamaoki N, Inoue A, Fukuda H. Perforation of rotator cuff increases interleukin 1beta production in the synovium of glenohumeral joint in rotator cuff diseases. J Rheumatol. 2000;27:2886–92. [PubMed] [Google Scholar]
- Gotoh M, Hamada K, Yamakawa H, Yanagisawa K, Nakamura M, Yamazaki H, Inoue A, Fukuda H. Interleukin-1-induced glenohumeral synovitis and shoulder pain in rotator cuff diseases. J Orthop Res. 2002;20:1365–71. doi: 10.1016/S0736-0266(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Kawamoto M, Romberger DJ, Nakamura Y, Adachi Y, Tate L, Ertl RF, Spurzem JR, Rennard SI. Modulation of fibroblast type I collagen and fibronectin production by bovine bronchial epithelial cells. Am J Respir Cell Mol Biol. 1995;12:425–33. doi: 10.1165/ajrcmb.12.4.7695922. [DOI] [PubMed] [Google Scholar]
- Khan MH, Li Z, Wang JH. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clin J Sport Med. 2005;15:27–33. doi: 10.1097/00042752-200501000-00006. [DOI] [PubMed] [Google Scholar]
- Kida Y, Kobayashi M, Suzuki T, Takeshita A, Okamatsu Y, Hanazawa S, Yasui T, Hasegawa K. Interleukin-1 stimulates cytokines, prostaglandin E2 and matrix metalloproteinase-1 production via activation of MAPK/AP-1 and NF-kappaB in human gingival fibroblasts. Cytokine. 2005;29:159–68. doi: 10.1016/j.cyto.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Kim CH, Park YG, Noh SH, Kim YK. PGE2 induces the gene expression of bone matrix metalloproteinase-1 in mouse osteoblasts by cAMP-PKA signaling pathway. Int J Biochem Cell Biol. 2005;37:375–85. doi: 10.1016/j.biocel.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kojima F, Naraba H, Sasaki Y, Beppu M, Aoki H, Kawai S. Prostaglandin E2 is an enhancer of interleukin-1beta-induced expression of membrane-associated prostaglandin E synthase in rheumatoid synovial fibroblasts. Arthritis Rheum. 2003;48:2819–28. doi: 10.1002/art.11261. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Laporte JD, Moore PE, Abraham JH, Maksym GN, Fabry B, Panettieri RA, Jr, Shore SA. Role of ERK MAP kinases in responses of cultured human airway smooth muscle cells to IL-1beta. Am J Physiol. 1999;277:L943–51. doi: 10.1152/ajplung.1999.277.5.L943. [DOI] [PubMed] [Google Scholar]
- Li Z, Yang G, Khan M, Stone D, Woo SL, Wang JH. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32:435–40. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- Loftin CD, Tiano HF, Langenbach R. Phenotypes of the COX-deficient mice indicate physiological and pathophysiological roles for COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:177–85. doi: 10.1016/s0090-6980(02)00028-x. [DOI] [PubMed] [Google Scholar]
- Moolwaney AS, Igwe OJ. Regulation of the cyclooxygenase-2 system by interleukin-1beta through mitogen-activated protein kinase signaling pathways: a comparative study of human neuroglioma and neuroblastoma cells. Brain Res Mol Brain Res. 2005;137:202–12. doi: 10.1016/j.molbrainres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, Toews GB, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174:5644–9. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov BV, Goorha S, Ballou LR. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43:2687–93. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta. 1995;1259:109–19. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–42. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- Rocha PN, Plumb TJ, Coffman TM. Eicosanoids: lipid mediators of inflammation in transplantation. Springer Semin Immunopathol. 2003;25:215–27. doi: 10.1007/s00281-003-0132-4. [DOI] [PubMed] [Google Scholar]
- Ruwanpura SM, Noguchi K, Ishikawa I. Prostaglandin E2 regulates interleukin-1beta-induced matrix metalloproteinase-3 production in human gingival fibroblasts. J Dent Res. 2004;83:260–5. doi: 10.1177/154405910408300315. [DOI] [PubMed] [Google Scholar]
- Saklatvala J, Dean J, Finch A. Protein kinase cascades in intracellular signalling by interleukin-I and tumour necrosis factor. Biochem Soc Symp. 1999;64:63–77. [PubMed] [Google Scholar]
- Schmitz T, Leroy MJ, Dallot E, Breuiller-Fouche M, Ferre F, Cabrol D. Interleukin-1beta induces glycosaminoglycan synthesis via the prostaglandin E2 pathway in cultured human cervical fibroblasts. Mol Hum Reprod. 2003;9:1–8. doi: 10.1093/molehr/gag007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–65. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–13. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- Thampatty BP, Im HJ, Wang JH. Leukotriene B4 at low dosage negates the catabolic effect of prostaglandin E2 in human patellar tendon fibroblasts. Gene. 2006;372:103–9. doi: 10.1016/j.gene.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003a;89:556–62. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003b;21:256–64. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Varga J, Diaz-Perez A, Rosenbloom J, Jimenez SA. PGE2 causes a coordinate decrease in the steady state levels of fibronectin and types I and III procollagen mRNAs in normal human dermal fibroblasts. Biochem Biophys Res Commun. 1987;147:1282–8. doi: 10.1016/s0006-291x(87)80209-7. [DOI] [PubMed] [Google Scholar]
- Wang JH. Mechanobiology of tendon. J Biomech. 2005 doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wang JH, Grood ES. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect Tissue Res. 2000;41:29–36. doi: 10.3109/03008200009005639. [DOI] [PubMed] [Google Scholar]
- Wang JH, Iosifidis MI, Fu FH. Biomechanical basis for tendinopathy. Clin Orthop Relat Res. 2006;443:320–32. doi: 10.1097/01.blo.0000195927.81845.46. [DOI] [PubMed] [Google Scholar]
- Wang JH, Jia F, Gilbert TW, Woo SL. Cell orientation determines the alignment of cell-produced collagenous matrix. J Biomech. 2003a;36:97–102. doi: 10.1016/s0021-9290(02)00233-6. [DOI] [PubMed] [Google Scholar]
- Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003b;44:128–33. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- Wang JH, Li Z, Yang G, Khan M. Repetitively stretched tendon fibroblasts produce inflammatory mediators. Clin Orthop Relat Res. 2004:243–50. doi: 10.1097/01.blo.0000126337.65685.e4. [DOI] [PubMed] [Google Scholar]
- Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukot Essent Fatty Acids. 2003;69:437–48. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37:1543–50. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE(2) production in human patellar tendon fibroblasts. Gene. 2005;363:166–72. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel-Lindberg T, Ahola H, Nilsson S, Carlstedt-Duke J, Modeer T. Interleukin-1 beta induces expression of cyclooxygenase-2 mRNA in human gingival fibroblasts. Inflammation. 1995;19:549–60. doi: 10.1007/BF01539135. [DOI] [PubMed] [Google Scholar]


