Abstract
Cytokines control cell differentiation, proliferation, and function by regulating gene expression program. Physiological roles and induction mechanisms of cytokine-inducible genes are not fully understood. Here, we identified a novel immediate-early cytokine-responsive gene, cyclon (cytokine-induced protein with coiled-coil domain), which is induced in a hematopoietic cell line by interleukin 3 (IL-3). cyclon gene encodes a phosphorylated nuclear protein consisting of repetitive sequences in the amino-terminus and a coiled-coil domain in the carboxyl-terminus. A novel transient reporter assay revealed that mouse cyclon promoter contains redundant elements for IL-3-induced gene expression.
Keywords: cytokine, IL-3, immediate-early genes, expression regulation
1. Introduction
Cytokines are a broad group of mostly soluble factors that mediate cell-to-cell communication [1]. Binding of cytokines to their specific receptors induces activation of multiple signaling pathways including Jak-Stat pathway, Src family and other protein tyrosine kinases, the Ras/MAPK pathway, the phosphatidylinositol-3 kinase/Akt pathway and others [1]. These pathways interact with one another to regulate expression of cytokine target genes. The functional redundancy of cytokine action can be mediated by the expression of the common target genes induced by various cytokines, whereas distinct cellular responses can be mediated by cytokine-specific target gene expression. Elucidation of the functional roles of cytokine target genes is critical for understanding of molecular mechanisms of cytokine action. However, the physiological roles and induction mechanisms of cytokine-inducible genes are not fully understood.
In an effort toward genome-wide search for genes induced by IL-3, we identified a novel cytokine-inducible gene, cyclon. cyclon gene encodes a nuclear phosphorylated protein consisting of amino (N)-terminal repetitive sequences and a carboxyl (C)-terminal coiled-coil domain. Analysis of cyclon promoter using a novel reporter assay revealed redundant elements required for expression by IL-3.
2. Materials and Methods
2.1. DNA Microarray Analysis
BB13 is derived from an IL-3-dependent hematopoietic cell line, Ba/F3 [2], and ectopically expresses human epidermal growth factor (EGF) receptor and Bcl-2 [3]. BB13 proliferates in response to EGF even in the absence of IL-3. Bcl-2 expression is required for EGF-induced proliferation. In the absence of EGF and IL-3, BB13 dies by apoptosis, but cell death is markedly delayed compared to its parental cell line, which does not overexpress Bcl-2. In an effort toward genome-wide search for genes differentially regulated by cytokines, BB13 cells were growth factor-starved for 5 hr and mock-stimulated or stimulated with IL-3 (10 ng/ml) for 2 hr. Total RNA was purified with RNeasy Protect Mini Kit (QIAGEN), and gene expression was analyzed using Affymetrix GeneChip Murine Genome set U74Av2.
2.2. RT-PCR
RT-PCR was performed with BD TITANIUM One-Step RT-PCR Kit (Clontech). Primers used in this study are: 5′-CGCTCCAAGAAGAGGTTCTCCCAGATGGTTCA-3′ and 5′-AAGCGGCCGCTCAGACCTTGGCTACAGGCCTC-3′ for mouse cyclon gene; 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for mouse glyceraldehyde-3-phosphate dehydrogenase gene.
2.3. Northern Blot Analysis
Northern blot analysis was performed as previously described [4] using mouse multi-tissue blot (Clontech) and cDNA probe of the C-terminal domain of mouse cyclon gene or digoxigenin-labeled actin RNA probe (Roche).
2.4. Cloning of cyclon cDNAs and Plasmid Construction
To construct retroviral vectors expressing human and mouse Cyclon fused in-frame with HA-tag at its N-terminus (HA-hCyclon and HA-mCyclon), the full-length cDNAs of human cyclon gene amplified by PCR from human leukemia TF-1 cDNA library or mouse cyclon gene were subcloned into pMXs-IG retroviral expression vector [5] together with a fragment encoding hemagglutinin (HA) epitope. pMXs-IG contains an internal ribosome entry site - green fluorescent protein (GFP) sequence, which permits simultaneous expression of a cloned gene and GFP. For construction of pEGFP-mCyclon, a vector expressing mouse Cyclon fused in-frame with GFP (GFP-mCyclon) at its N-terminus, mouse cyclon cDNA was inserted into pEGFP-C2 (Clontech). For construction of pd1EGFP/EF-hCD2, elongation factor promoter/human CD2 (hCD2) gene cleaved from pEF-hCD2 generated by inserting hCD2 cDNA [6] into pEF vector [7] was inserted into Sal I site of LexA-d1EGFP [6]. For construction of cyclon reporter plasmids, cyclon promoter sequences amplified by PCR with C57BL/6 tail DNA as template were inserted into Kpn I- and Nco I-cleaved pd1EGFP/EF-hCD2. Rat cyclon cDNA was identified from heart cDNA library of Rattus norvegicus (Norway Rat).
2.5. Flow Cytometric Sorting
After transduction of hCyclon/pMXs-IG or mCyclon/pMXs-IG, GFP (+) cells were sorted with MoFlow cell sorter (Cytomation).
2.6. Fluorescent microscopy
Ba/F3 expressing HA-hCyclon was intracellularly stained with anti-HA antibody (Ab) (12CA5, Roche) and Alexa Fluor 594-conjugated anti-mouse Immunoglobulin G (IgG) (Molecular Probes). NIH 3T3 was transfected with pEGFP-C2 or pEGFP-mCyclon using Lipofectamine 2000 (Invitrogen). Cells were also stained with 4′,6-diamidino-2-phenylindole (DAPI) to indicate the positions of nuclei. Cells were observed with a deconvolution microscope.
2.7. Production of anti-Cyclon Ab
The nucleotide sequence encoding the C-terminal 131 a.a. of human Cyclon was ligated in pGEX-4T-3 (Amersham-Pharmacia) to express glutathione S-transferase (GST)-fusion protein. Transformed DH5α was sonicated in phophate-buffered saline containing 1% Triton-X-100. Two NZW female rabbits were immunized with the GST-fusion protein purified with glutathione-Sepharose beads. IgG was purified from the resultant sera with MAb Trap Kit (Amersham-Pharmacia).
2.8. Immunoblot Analysis
Preparation of nuclear/cytoplasmic extracts and immunoblot analysis were performed as described [6]. We used anti-HA, anti-Sp1 (PEP 2, Santa Cruz), and anti-Cyclon Ab.
2.9. Transient reporter assay using pd1EGFP/EF-hCD2 plasmid
Reporter constructs were transfected into Ba/F3 by DEAE-dextran method [7]. After 24 hr culture, cells were stained with phycoerythrin (PE)-conjugated anti-hCD2 Ab (BD) and analyzed with FACS Calibur flowcytometer (BD).
3. Results and Discussion
3.1. Molecular cloning of an IL-3-inducible gene, cyclon
In an effort toward genome-wide search for genes differentially regulated by cytokines, the BB13 cell line [3] derived from IL-3-dependent murine hematopoietic Ba/F3 was growth factor-starved and mock-stimulated or stimulated with IL-3 for 2 hr. Total RNA was subjected to DNA microarray analysis. Among the top 9 genes highly upregulated by IL-3 (Supplemental Table 1), we identified a novel gene encoding a protein containing a C-terminal coiled-coil domain (Fig. 1). We named this gene as cyclon (cytokine-induced protein with coiled-coil domain). The coiled-coil domain is one of the principal subunit oligomerization motifs in proteins and involved in signal inducing events, molecular recognition, mechanical stability of cells, and movement processes [8]. Cyclon also contains repetitive sequences (SPxP/RxP), that we named the “SP-repeats”, in its N-terminus.
Figure 1.

A.a. sequence alignment of Cyclon proteins. The coiled-coil domain is boxed. The positions of exon/intron boundaries are indicated by vertical lines. The SP-repeats are shown in color. Identical and related a.a. residues are indicated as asterisks and dots, respectively.
Mouse, rat, and human cyclon gene encodes a protein with 426, 341, and 360 a.a. residues, respectively (Fig. 1). Rat and human Cyclon have shorter N-terminal domains lacking some of the SP-repeats identified in mouse Cyclon, suggesting that Cyclon is functional as long as it has certain numbers of the SP-repeats. We speculate that human and rat Cyclon proteins lost some of the SP-repeats or mouse Cyclon acquired additional SP-repeats by duplication of the gene segment encoding the SP-repeats in evolution. Mouse cyclon gene has four exons separated by introns of 2.9, 1.7, and 0.2 kbp (Fig. 2). Mouse and human cyclon genes are mapped on chromosome 19B and chromosome 11q12, respectively, and share conserved genomic structure. Potential orthologues of cyclon gene exist in fruit fly (Ensembl CG14210-PA) and nematode (Ensembl Y40B1B.7). Interestingly, fly and nematode orthologues lack the N-terminal domain.
Figure 2.
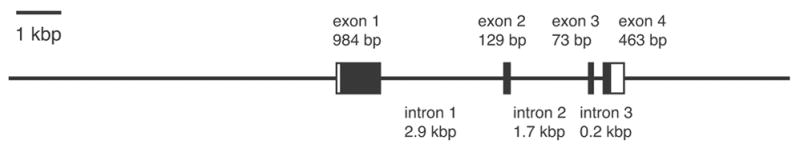
Genomic structure of mouse cyclon gene.
3.2. Expression of cyclon mRNA
cyclon mRNA was induced by IL-3 as early as 30 min and reached its peak 6 hr after stimulation (Fig. 3A). The expression of cyclon mRNA by IL-3 was not inhibited by an inhibitor of protein synthesis, cycloheximide (data not shown), indicating that cyclon is an immediate early gene.
Figure 3.
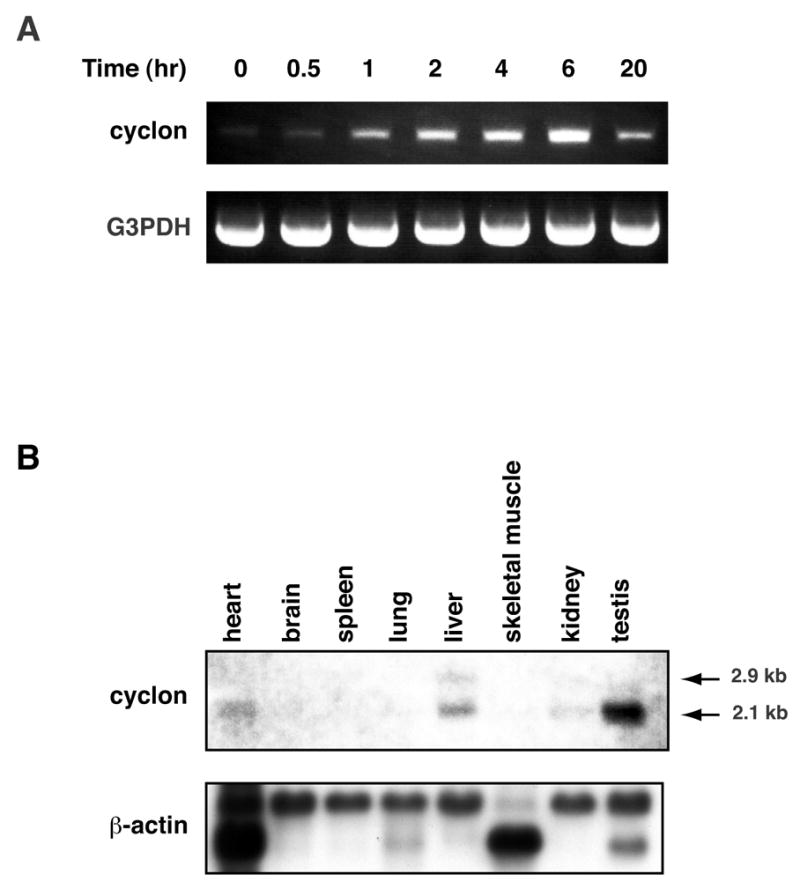
Expression of cyclon mRNA. (A) Induction of cyclon by IL-3 in Ba/F3. Total RNA purified at indicated time after IL-3 stimulation was analyzed by RT-PCR. G3PDH: glyceraldehyde-3-phosphate dehydrogenase. (B) Tissue distribution of cyclon mRNA. Northern blot analysis was performed using mouse multi-tissue blot.
Expression of cyclon mRNA was detected in heart, liver, kidney, and highest in testis (Fig. 3B). Two transcript sizes of 2.1 kb and 2.9 kb were detected. We also detected cyclon mRNA expression in activated mouse peripheral CD4 (+) and CD8 (+) T cells but not in naïve T cells (unpublished observation).
3.3. Cyclon is a phosphorylated nuclear protein
Nuclear extracts and cytoplasmic extracts prepared from Ba/F3 expressing HA-tagged human or mouse Cyclon (HA-hCyclon and HA-mCyclon) were subjected to immunoblot analysis with anti-HA Ab. HA-hCyclon was detected in the nuclear extracts but not in the cytoplasmic extracts (Fig. 4A). HA-mCyclon was also detected in the nuclear extracts (data not shown). Fluorescent microscopy using HA-hCyclon and GFP-mCyclon fusion protein further confirmed nuclear localization of Cyclon in Ba/F3 and NIH 3T3 (Fig. 4B and C). In this regard, motif analysis with PSORT (http://psort.ims.u-tokyo.ac.jp/) identified three potential bipartite nuclear localization signals (KKRVIASPQAPASKKLKE, RRRQEKKERRAENLRRRL and RKAEIVQVIRNPAKLKK).
Figure 4.
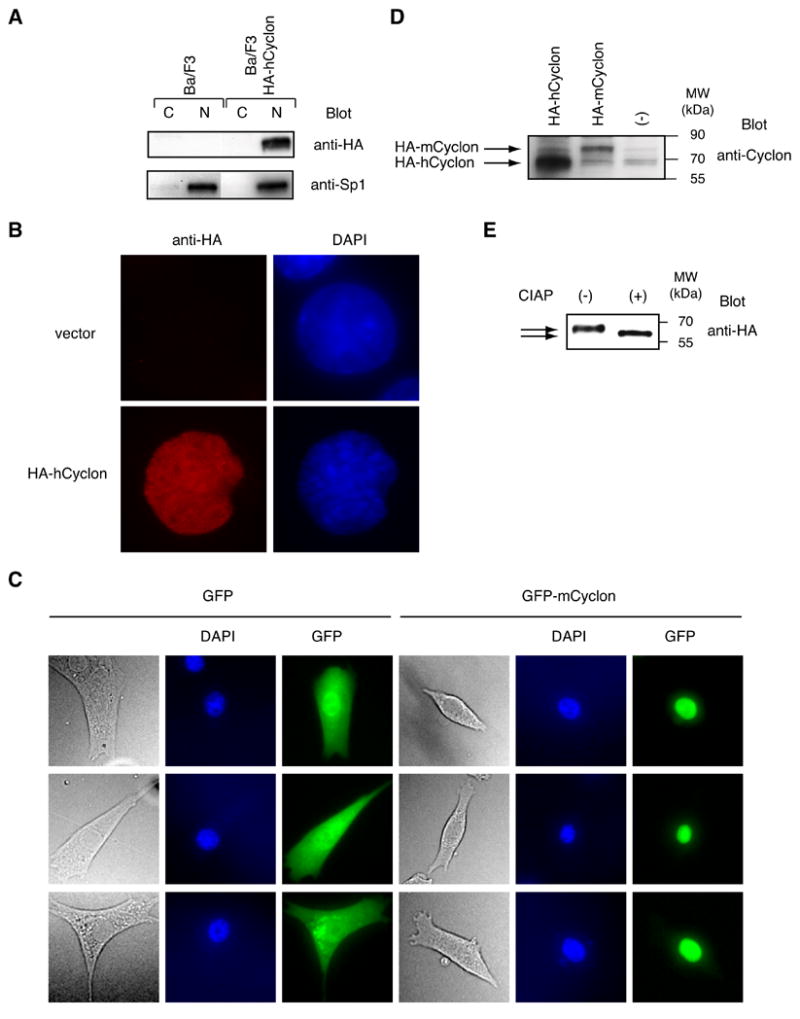
Cyclon is a nuclear phosphorylated protein. (A) The nuclear extracts (N) and the cytoplasmic extracts (C) prepared from Ba/F3 expressing HA-hCyclon were subjected to immunoblot analysis with anti-HA or anti-Sp1 Ab. (B) Ba/F3 transduced with HA-hCyclon or empty vector was stained with anti-HA Ab and Alexa Fluor 594-conjugated anti-mouse IgG. Cells were counter-stained with DAPI to indicate the positions of the nuclei. (C) NIH 3T3 was transfected with pEGFP-C2 or pEGFP-mCyclon. Cells were counter-stained with DAPI to indicate the positions of the nuclei. (D) Nuclear extracts from Ba/F3 expressing HA-hCyclon or HA-mCyclon were subjected to immunoblot analysis with anti-Cyclon Ab. (E) Phosphorylation of Cyclon. Nuclear extracts prepared from Ba/F3 expressing HA-hCyclon were treated with 5 units of CIAP for 30 min at 37°C and analyzed by immunoblot analysis using anti-HA Ab.
The molecular weights (MWs) of Cyclon on sodium dodecyl sulfate-polyacrylamide gel electrophoresis detected with anti-Cyclon Ab (human Cyclon: 65 kDa; mouse Cyclon: 75 kDa) are higher than calculated MWs (human Cyclon: 40.2; mouse Cyclon: 46.5 kDa) (Fig. 4D), suggesting possible modifications of Cyclon. To examine phosphorylation of Cyclon, nuclear extracts prepared from Ba/F3 expressing HA-hCyclon were treated with calf intestinal alkaline phosphatase (CIAP). CIAP-treated HA-hCyclon migrated faster than non-treated HA-hCyclon (Fig. 4E), indicating phosphorylation of Cyclon. Multiple serine residues in the N-terminal SP-repeats are potential phosphorylation sites. The MW of CIAP-treated Cyclon was still larger than expected MW, suggesting possible modifications other than phosphorylation.
3.4. Identification of critical elements of cyclon promoter for IL-3-induced expression
To delineated a critical region(s) of cyclon promoter for IL-3-induced expression, the region between 1061-bp upstream of the initiation methionine (iMet) and iMet of mouse cyclon gene was cloned into pd1EGFP/EF-hCD2 (Fig. 5A) (the 1061-bp cyclon reporter). The pd1EGFP/EF-hCD2 has a destablized GFP (Clontech) driven by promoters cloned in the multi-cloning site. It also has hCD2 gene under the control of constitutive EF promoter [9]. Since GFP and hCD2 are expressed from a single plasmid, hCD2 expression can be used as a strict internal control for transfection efficiency. In fact, percentages of GFP (+) cells in hCD2 (+) populations are highly consistent in several independent experiments, although percentages of hCD2 cells (i.e., percentages of transfected cells) can be considerably different in different experiments. After incubation with IL-3 for 24 hr or growth-factor-starved for the last 8 hr period in 24 hr incubation, Ba/F3 transfected with pd1EGFP/EF-hCD2 or the 1061-bp cyclon reporter were stained with PE-conjugated anti-hCD2 Ab, and GFP expression was analyzed for live cells that showed equivalent expression levels of hCD2. In this starvation condition, viability of IL-3-starved cells was still high (> 98%), judged by forward scatter (reflecting cell size) and side scatter (reflecting granularity) distribution of flowcytometric analysis and Trypan blue exclusion assay. Cells transfected with control pd1EGFP/EF-hCD2 did not express GFP either in the presence or absence of IL-3 (see below). In contrast, IL-3-dependent GFP expression was observed in cells transfected with the 1061-bp cyclon reporter (Fig. 5B), indicating that the 1061-bp fragment contains an IL-3-responsive element(s) for its transcriptional activation. Because viability of IL-3-starved cells was high and GFP expression was compared in live cells expressing equivalent levels of hCD2, we do not believe that the decrease of GFP reporter expression by IL-3-starvation may merely reflect aberrant gene expression by apoptosis.
Figure 5.
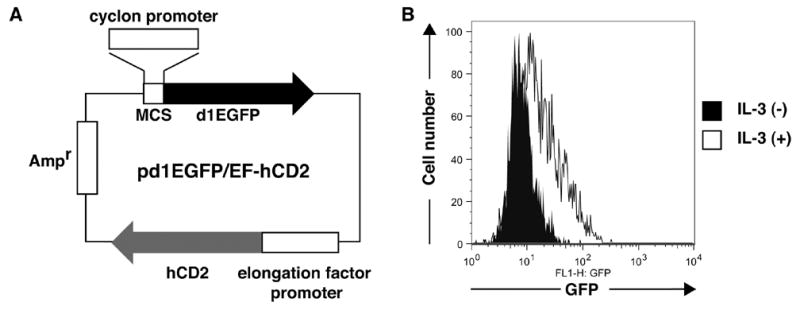
The pd1EGFP/EF-hCD2 system used for promoter analysis of cyclon gene. (A) pd1EGFP/EF-hCD2 contains a multi-cloning site (MCS) to clone promoter sequences and the destablized EGFP reporter gene. It also contains hCD2 gene driven by the constitutive elongation factor promoter. Ampr: ampicillin resistance gene. (B) IL-3-dependent expression of cyclon reporter. Ba/F3 cells were transfected with the 1061-bp cyclon reporter and cultured with IL-3 (1 nM) for 24 hr or IL-3-starved for the last 8 hr period in 24 hr incubation. GFP expression was compared between cells with comparable hCD2 expression levels.
Next, we examined promoter activities of various constructs of mouse cyclon promoter. The 4139-, 586- and 142-bp fragements upstream of iMet supported GFP expression comparable to the 1061-bp fragment (Fig. 6A). However, the 100-bp fragment upstream of iMet showed decreased expression of GFP reporter compared to the 142-bp fragment. Additional deletion of 40-bp further decreased but did not abolish reporter expression. In contrast, the 48-bp fragment upstream of iMet did not support GFP expression. Differences among the 142-, 100-, 60-, and 48-bp fragments were statistically significant. Interestingly, a construct lacking the region between −142 and −49 bp upstream of iMet in the 1061-bp reporter [the Δ(−142/−49)] reporter] was still capable of inducing GFP expression. In contrast, a construct lacking the region between −185 and −49 bp upstream of iMet [the Δ(−185/−49) reporter] failed to support GFP expression (Fig. 6A). Expression of GFP from reporter constructs was IL-3-dependent (data not shown). These results suggest that (i) the region between −185 and −49 bp upstream of iMet is essential for IL-3-induced cyclon transcription and (ii) this region contains at least four discrete and redundant elements: E(−185/−143), E(−142/−101), E(−100/−61), and E(−60/−49), that cooperatively support IL-3-induced cyclon expression.
Figure 6.
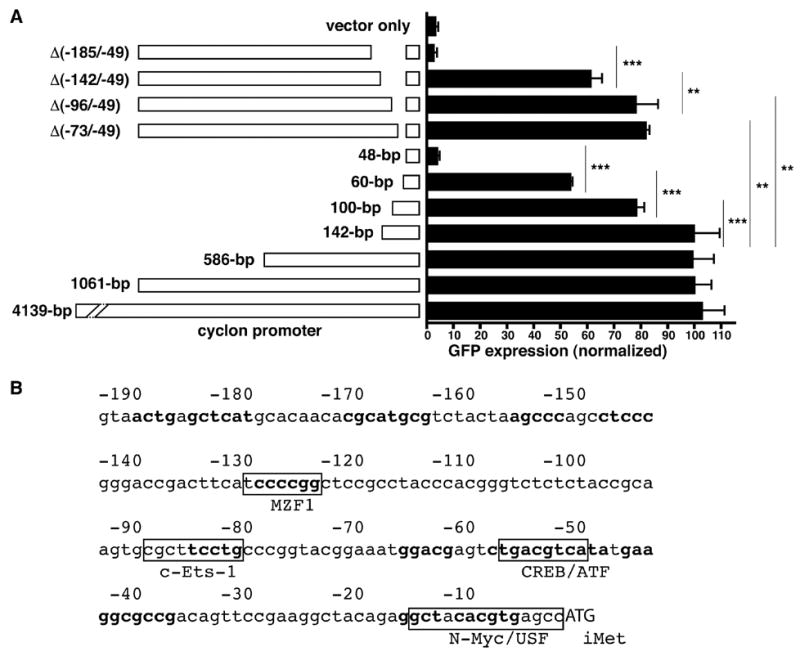
cyclon promoter contains redundant IL-3-responsive elements. (A) Ba/F3 transfected with various reporter constructs was cultured in the presence of IL-3 (1 nM) for 24 hr. Cells were stained with PE-conjugated anti-hCD2 Ab, and GFP expression was analyzed for hCD2 (+) cells. The bp represents the length of the cyclon promoter from the initiation methionine (iMet) of the cyclon gene. Since both d1EGFP and hCD2 are expressed from a single plasmid, hCD2 expression can be used as a strict internal control for transfection efficiency. hCD2 (+) cells, which represent transfected cells, were gated and percentages of GFP (+) cells in hCD2 (+) populations were calculated. Normalized GFP expression = [Percentage of GFP (+) cells in hCD2 (+) cells of a reporter construct] / [Percentage of GFP (+) cells in hCD2 (+) cells of the 1061-bp reporter] x 100. **: P < 0.01; ***: P < 0.001. Expression of GFP from reporter constructs was IL-3-dependent (data not shown). (B) The nucleotide sequence of 190-bp upstream of iMet of mouse cyclon gene. Conserved sequences among human and mouse genes are in bold. Conserved consensus sequences of transcription factors are boxed.
Analysis using TFSearch (http://www.cbrc.jp/research/db/TFSEARCH.html) revealed conserved binding elements of myeloid zinc finger protein 1 (MZF-1), c-Ets-1, and cAMP-responsive element binding protein (CREB)/activating transcription factor within E(−142/−101), E(−100/−61), and E(−60/−49), respectively. IL-3-induced activation of CREB and Elk-1, a member of c-Ets family transcription factors, have been reported [10,11]. Involvement of MZF-1, a transcription factor of the C2H2 zinc finger gene family [12], in growth-promoting cytokine signaling has not been documented. It is an important future issue whether these transcription factors bind to cyclon promoter and how they are regulated by IL-3. Interestingly, E(−185/−143) does not contain consensus sequences of any known transcription factors, suggesting a possibility of a novel DNA-binding protein(s) recognizing this element. The essential region of the cyclon promoter for IL-3-induced expression does not contain any binding sites of Stat5, which is activated by IL-3 [1], suggesting Stat5-independent induction of cyclon.
In our preliminary experiments, transgenic expression of Cyclon in T cells of several autoimmune mouse strains normalized symptoms of autoimmunity, indicating its important role in the regulation of immune homeostasis (unpublished observation). Our results in this study may provide clues to understand mechanism of Cyclon action.
Supplementary Material
Acknowledgments
We thank Dr. T. Kitamura for pMXs-IG vector. This work was supported by NIH grant AI059315 (H.F.). The GenBank accession numbers are as follows: human Cyclon, DQ501251; mouse Cyclon, DQ501252; rat Cyclon, DQ501253.
Abbreviations
- IL
interleukin
- EGF
epidermal growth factor
- a.a
amino acid
- HA
hemagglutinin
- hCD2
human CD2
- GFP
green fluorescent protein
- DAPI
4′,6-diamidino-2-phenylindole
- GST
glutathione S-transferase
- Ab
antibody
- MW
molecular weight
- CIAP
calf intestinal alkaline phosphatase
- iMet
initiation methionine
- PE
phycoerythrin
- MZF-1
myeloid zinc finger protein 1
- CREB
cAMP responsive element binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leonard WJ. Type I Cytokines and Interferons and their receptors. In: Paul WE, editor. Fundamental Immunology. Lippincott Williams & Wilkins; Philadelphia, PA: 2003. pp. 701–747. [Google Scholar]
- 2.Palacios R, Steinmetz M. IL3-dependent mouse clones that express B-220 surface-antigen, contain Ig genes in germ-line configuration, and generate lymphocytes-B in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki T, Liu ZJ, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian EL, Perlmutter RM, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino A, Saint Fleur S, Fujii H. Regulation of Stat1 protein expression by phenylalanine 172 in the coiled-coil domain. Biochem Biophys Res Commun. 2006;346:1062–1066. doi: 10.1016/j.bbrc.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui AL, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino A, Matsumura S, Kondo K, Hirst JA, Fujii H. Inducible translocation trap: a system for detecting inducible nuclear translocation. Mol Cell. 2004;15:153–159. doi: 10.1016/j.molcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Kawahara A, Minami Y, Miyazaki T, Ihle JN, Taniguchi T. Critical role of the interleukin 2 (IL-2) receptor γ-chain-associated Jak3 in the IL-2-induced c-fos and c-myc, but not bcl-2, gene induction. Proc Natl Acad Sci USA. 1995;92:8724–8728. doi: 10.1073/pnas.92.19.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhard P, Stetefeld J, Strelkov SV. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 2001;11:82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- 9.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto KM, Fraser J, Lee HJ, Lehman E, Gasson JC. Granulocyte-macrophage colony-stimulating factor and interleukin-3 signaling pathways converge on the CREB-binding site in the human egr-1 promoter. Mol Cell Biol. 1994;14:5975–5985. doi: 10.1128/mcb.14.9.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosaka Y, Arai A, Miyasaka N, Miura O. CrkL mediates Ras-dependent activation of the Raf/ERK pathway through the guanine nucleotide exchange factor C3G in hematopoietic cells stimulated with erythropoietin or interleukin-3. J Biol Chem. 1999;274:30154–30162. doi: 10.1074/jbc.274.42.30154. [DOI] [PubMed] [Google Scholar]
- 12.Hromas R, Collins SJ, Hickstein D, Raskind W, Deaven LL, O’Hara P, Hagen FS, Kaushansky K. A retinoic acid-responsive human zinc finger gene, MZF-1, preferentially expressed in myeloid cells. J Biol Chem. 1991;266:14183–14187. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


