Abstract
The β-chemokines RANTES, macrophage inflammatory protein (MIP)-1α, and MIP-1β suppress infection by macrophage-tropic strains of HIV and simian immunodeficiency virus (SIV) by binding and down-regulating the viral coreceptor, CCR5. Accordingly, we have examined whether higher levels of CCR5 ligands are associated with a more favorable clinical status in AIDS. A cross-sectional study of 100 subjects enrolled in the Multicenter AIDS Cohort Study at the Baltimore site was conducted to measure chemokine production and lymphocyte proliferation by peripheral blood mononuclear cells (PBMC). Statistical analyses of the data revealed that the production of HIV-suppressive β-chemokines by HIV antigen-stimulated PBMC was significantly higher in HIV-positive subjects without AIDS compared with subjects with clinical AIDS. Increased chemokine production was also correlated with higher proliferative responses to HIV antigens. Both parameters were significantly lower in the AIDS versus non-AIDS group. Notably, significantly higher levels of MIP-1α were also observed with unstimulated PBMC from seronegative subjects at risk for HIV infection released as compared with seropositive and non-Multicenter AIDS Cohort Study seronegative subjects. The association of chemokine production with antigen-induced proliferative responses, more favorable clinical status in HIV infection, as well as with an uninfected status in subjects at risk for infection suggests a positive role for these molecules in controlling the natural course of HIV infection.
Keywords: lymphocyte proliferation
A group of chemokines produced by activated primary T cells potently suppress infection by HIV (1–5). These molecules act through a common mechanism involving interactions with chemokine receptors that also serve as coreceptors for HIV entry (4–11). These interactions block and down-regulate coreceptors and, thereby, effectively inhibit the HIV life cycle at the cell surface (12–14). The importance of coreceptor down-regulation in natural HIV infection has been demonstrated clearly by clinical studies of coreceptor gene alleles. In particular, certain mutations in the CCR5 gene confer resistance to primary HIV infection and/or AIDS progression by reducing or eliminating cell surface CCR5 expression, respectively (15–24). Extrapolating from these findings, it is reasonable to expect that ligand-induced receptor down-regulation also may prevent or slow HIV infection in vivo (25).
RANTES, macrophage inflammatory protein (MIP)-1α, and MIP-1β are the natural ligands for CCR5 and are major suppressors of macrophage-tropic strains of HIV-1 (1, 6–10). Accordingly, there has been considerable interest in whether these chemokines impact natural HIV infection as a result of their ability to reduce cell surface CCR5 expression. Several studies have shown that activated peripheral blood mononuclear cells (PBMC) from HIV-exposed but uninfected individuals (26–28) and nonhuman primates protected from simian immunodeficiency virus challenge by certain vaccines (29–32) produced high levels of RANTES, MIP-1α, and MIP-1β. Other studies show an association between higher-production HIV-suppressive chemokines from activated PBMC and a more favorable clinical status in HIV+ individuals (27, 33, 34). Taken together, these results merely could reflect the more robust immune responses found in clinically healthy subjects and implicate elevated chemokine levels as surrogate markers for disease status. However, these results might also reflect a functional role for lymphocyte proliferation and elevated levels of RANTES, MIP-1α, and MIP-1β in controlling the natural course of HIV infection. Further study therefore is warranted to evaluate whether the previously observed correlates between chemokine levels and clinical status are reiterated or strengthened.
A key question concerns whether chemokines are released during responses that occur at local sites of infection. Support for this possibility was provided by studies showing that proliferative responses against HIV antigens involve the release of HIV-suppressive chemokines (34, 35). However, relationships between chemokine release in HIV+ individuals and HIV antigen-specific responses have not been clearly defined. To address this issue, we analyzed β-chemokine production in response to HIV vs. non-HIV antigens in homosexual men who were enrolled in the Baltimore center of the Multicenter AIDS Cohort Study (MACS). Antigen-induced proliferative responses also were measured to evaluate whether chemokine production and T cell proliferation are related. We find that antigen-induced chemokine production and anti-HIV proliferative responses are significantly higher in PBMC from HIV-positive subjects without AIDS compared with subjects with clinically defined AIDS. To our surprise, unstimulated cells from HIV seronegative subjects in the cohort released higher levels of HIV-suppressive chemokines, compared with unstimulated cells from non-MACS controls or HIV-positive subjects. These results further support the possibility that antigen-induced production of certain chemokines and lymphocyte proliferation play a role in controlling natural HIV infection.
Methods
Study Population.
Subjects were selected from the Baltimore site of the MACS, a longitudinal study of the natural history of HIV-1 infection in homosexual men whose design has been described previously (36). Briefly, 1,253 men were recruited in 1983–84 and in 1987–91 and have been followed at 6-month intervals with clinical and laboratory testing as well as storage of repository specimens. For the present study, blood was obtained from 77 HIV-1 seropositive MACS participants, randomly selected from those attending study visits 27 and 28 up to a total of 3 per clinic night. Twenty-three HIV-1 seronegative MACS participants were included as control subjects. Demographics of these subjects are reported in Table 1. HIV-positive subjects were categorized in two groups, “AIDS” (12 subjects) and “Non-AIDS” (65 subjects), according to the 1993 definition of AIDS by the Centers for Disease Control and Prevention, except that subjects with <200 CD4+ lymphocytes per μl but no AIDS-defining illnesses were classified as “non-AIDS.” Ten seronegative subjects with no history of exposure to HIV infection were recruited from the laboratory staff at the Institute of Human Virology and included as controls in this study.
Table 1.
Demographic parameters of the subject population
| Range (population) N = 100 | Median (±SD) (population) | Range (seronegatives) N = 23 | Median (±SD) (seronegatives) | Range (non-AIDS) HIV+ | Median (±SD) (non-AIDS) N = 65 | Range (AIDS) N = 12 | Median (±SD) (AIDS) | |
|---|---|---|---|---|---|---|---|---|
| Age | 33–64.1 | 45.8 (6.4) | 41.15–64.10 | 48.9 (5.7) | 33–62.6 | 43.96 (6.2) | 35.6–55.8 | 43.7 (7.2) |
| CD4 counts | 35–2059 | 629 (435.2) | 872–2059 | 1085 (296.6) | 38–1992 | 513 (356.9) | 35–260 | 138.5 (75.4) |
| Viral load (log10) | 1.9–6.2 | 3.3 (1.2) | — | — | 2–5.99 | 2.9 (1.1) | 1.93–6.2 | 4.3 (1.3) |
N = number of subjects.
Laboratory Studies.
The lymphocyte proliferation assay was modified from AIDS Clinical Trial Group protocol 209. At each time point, fresh PBMC, obtained by centrifugation of whole blood from HIV-1 seropositive and seronegative subjects in CPT tubes (Becton Dickinson), were cultured in round-bottomed, 96-well plates (Falcon) in RPMI 1640 medium (GIBCO) with 10% human AB serum and antibiotics (100 units/ml penicillin, 100 mg/ml streptomycin) (GIBCO) at a concentration of 2 × 105 cells per well. Cells were incubated with media alone or with 10 μg/ml gp120-depleted, inactivated HIV-1, 10 μg/ml purified p24 antigen (Immune Response Corporation), 10 μg/ml Candida albicans (Greer Laboratories), or 20 μg/ml phytohemagglutinin (PHA) (Sigma). The HIV-1 antigen preparation consisted of density gradient-purified, inactivated, gp120-depleted HZ321 virus. All lymphocyte proliferation assays were done in triplicate. After 3 days (for PHA-stimulated cells) or 6 days (for antigen-stimulated cells) of incubation at 37°C, the cells were labeled with 1 μCi of 3[H]thymidine in complete RPMI. Cells were harvested and incorporated label was determined by scintillation counting. Geometric mean cpm were calculated from the triplicate wells with and without antigen. Results were expressed as a “lymphocyte stimulation index” (LSI), which is the geometric mean cpm of the cells plus antigen divided by the geometric mean cpm of the cells without antigen (medium alone). Supernatants were collected on day 6 and frozen at −70°C for RANTES, MIP-1α, and MIP-1β measurements. All β-chemokine assays were performed by commercial ELISA from R & D Systems. CD4+ and CD8+ lymphocyte counts were determined as described (37), and plasma HIV RNA concentrations were measured by the AMPLICOR HIV-1 Monitor Test (Roche Diagnostics) according to the manufacturer’s instructions.
Antigens.
HIV-1 HZ321 immunogen was obtained by concentration and purification from the supernatant fluid of HZ321-infected HUT-78 cells. In the preparation of the immunogen, envelope gp120 was depleted during freezing and thawing and later, during the purification process (38).
Native p24 was preferentially lysed from purified inactivated HIV-1 (HZ321) with 2% Triton X-100 and then purified by using Pharmacia Sepharose Fast Flow S resin. Chromatography was carried out at pH 5.0, and p24 was eluted by using a linear salt gradient. Purity of the final product was estimated by both SDS/PAGE and reverse-phase HPLC to be >99% (38).
Data Analysis.
Data were abstracted from clinical records, maintained in an Excel database, and imported into spss 7.5 for statistical analysis. Frequency distributions and Kurtosis plots were examined to determine the modality of each of the chemokine production distributions, for subsequent stratification, and were found to be nonnormal. Group differences in chemokine production were assessed by using the Mann–Whitney (nonparametric) test (in the case of spontaneous production of chemokines) or the unpaired Student’s t test (parametric; used for antigen-activated chemokine production). RNA copies/ml values were log-transformed to base 10 before being analyzed. Correlations between chemokine production and CD4+ or CD8+ T cell counts were analyzed with Pearson’s correlation, which assumes that the two variables are measured on at least interval scales and determines the extent to which values of the two variables are related to each other (see Table 2 legend). Chemokine production data, CD4+ and CD8+ T cell count distributions, and log10 viral load distributions were examined for modality in the entire cohort and in each of the following groupings: AIDS/No AIDS/CD4+ T cell count <200/CD4+, T cell count >200, and AIDS/No AIDS/Seronegative. To determine the association of chemokine production and clinical status, distributions were examined before dichotomizing the values into high vs. low production grouping before χ2 analysis by disease status. The distributions were found to be positively skewed toward the lower values.
Table 2.
Pearson’s correlation between antigen-induced chemokine production and CD4+ T cell counts
| MIP-1α PHA | MIP-1β PHA | RANTES PHA | MIP-1α Candida | MIP-1β Candida | RANTES Candida | MIP-1α p24 | MIP-1β p24 | RANTES p24 | MIP-1α HIV | MIP-1β HIV | RANTES HIV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s correlation, CD4 counts (r) | .124 | .125 | .062 | .259 | .260 | .159 | .224 | .224 | .128 | .154 | .248 | .078 |
| Significance, two-tailed (P) | .252 | .253 | .570 | .016 | .016 | .141 | .037 | .037 | .238 | .154 | .021 | .473 |
| N | 87 | 86 | 87 | 87 | 86 | 87 | 87 | 87 | 87 | 87 | 87 | 87 |
Pearson’s correlation analyses were used to establish the correlation between CD4+ T cell counts and chemokine production. The analyses assume that the two variables are measured on at least interval scales and determine the extent to which values of the two variables are related to each other. The value of correlation (i.e., correlation coefficient, or r) indicates the extent to which values of the two variables are proportional (i.e., linearly related) to each other, independently of the specific measurement units used. An r value of 1 indicates that the two variables are perfectly linearly related. For biological data, values below 0.4 are arbitrarily considered “weak.” N = number of subjects. Columns in bold face type are statistically significant (i.e., P ≤ 0.05).
Results
Antigen-Induced Proliferation.
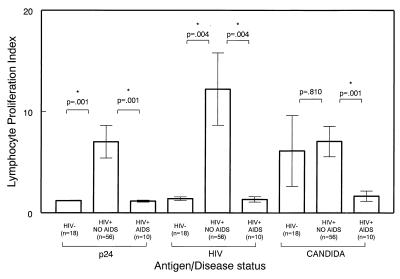
As shown in Fig. 1, cells obtained from seropositive subjects who did not have AIDS were significantly more responsive to stimulation with select antigens as compared with subjects with AIDS. The envelope-depleted HIV antigen induced the highest lymphocyte proliferative responses in subjects belonging to the “non-AIDS” group (P = 0. 004) (mean LSI ± SE = 12.22 ± 3.57). In comparison, such responses among subjects from the AIDS group were nearly identical to those observed with seronegative subjects (mean LSI ± SE = 1.33 ± 0.27). This result is close to the average index for seronegatives (mean LSI ± SE = 1.35 ± 0.16). Similarly, significant differences in proliferative responses were observed by using p24, the core antigen of HIV-1 (P = 0.001), and Candida antigen (P = 0.001) (Fig. 1) between patients with AIDS and asymptomatic subjects. Proliferation upon PHA stimulation was not significantly different between the disease groups (not shown). PBMC from seronegative subjects did not proliferate significantly in response to HIV antigens.
Figure 1.
Antigen-induced proliferation indices and disease status. Cells (2 × 105) per well of fresh PBMC were cultured in round-bottomed, 96-well plates, as described in Methods, in medium alone or with 10 μg/ml gp120-depleted, inactivated HIV-1 (HIV), 10 μg/ml purified p24 antigen (p24), 10 μg/ml C. albicans (candida), or 20 μg/ml PHA (not shown). After 3 days (for PHA) or 6 days of incubation at 37°C, the cells were labeled with 1 μCi of 3[H]thymidine and harvested, and incorporated label was determined by scintillation counting. Geometric mean cpm were calculated from the triplicate wells with and without antigen. Results were calculated as an LSI, which is the geometric mean cpm of the cells plus antigen divided by the geometric mean cpm of the cells without antigen (medium alone). (Bars = SEM.)
Correlation of CD4+ and CD8+ T Cell Counts and Antigen-Induced Chemokine Production and T Cell Proliferation.
Pearson’s correlation was used to establish whether a correlation existed between CD4+ and CD8+ T cell counts and lymphocyte proliferation indices or chemokine production. Some of the chemokines levels were significantly correlated with CD4 counts (Table 2), whereas none of the chemokine levels or lymphocyte proliferation values were significantly correlated with CD8 counts (not shown). Significant but weak correlations were found between CD4+ T cell counts and the levels of production of MIP-1α (r = 0.259, P = 0.016) and MIP-1β (r = 0.260, P = 0.016) in response to C. albicans and with the levels of MIP-1α (r = 0.224, P = 0.037) and MIP-1β (r = 0.224, P = 0.037) produced in response to p24 simulation. MIP-1β production induced by HIV-1 also significantly correlated with CD4+ T cell counts (r = 0.248, P = 0.021). RANTES production was not associated with CD4+ T cell counts after any stimulus. No significant correlation was found between CD4+ T cell counts and antigen-induced lymphocyte proliferation (not shown).
Antigen-Induced Chemokine Production.
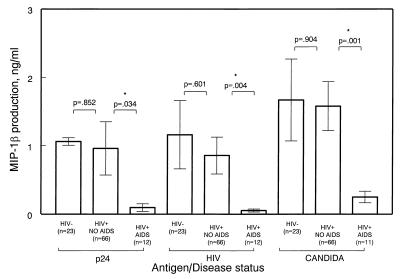
Similar to the results of lymphocyte antigen-induced proliferation, the production of select chemokines upon antigen stimulation was significantly different in subjects diagnosed with AIDS when compared with subjects without AIDS or non-AIDS (Fig. 2). Cells obtained from subjects belonging to the non-AIDS group produced significantly higher levels of MIP-1α (not shown) and MIP-1β, but not RANTES, upon antigen stimulation, as compared with cells obtained from subjects with AIDS. As illustrated in Fig. 2, HIV-1 antigen-stimulated production of MIP-1β was increased significantly (P = 0.004; mean ± SE = 857 ± 27 pg/ml) in non-AIDS subjects compared with AIDS subjects (mean ± SE = 53 ± 20 pg/ml) (Fig. 2). Similarly, MIP-1α production upon HIV antigen stimulation was significantly higher (P = 0.038) in asymptomatic subjects (mean ± SE = 1,131 ± 520 pg/ml) compared with subjects with AIDS (mean ± SE = 26 ± 8 pg/ml). Similar trends were observed by using p24 and C. albicans antigens (Fig. 2). Interestingly, significant differences in chemokine production were not observed between the two disease status groups when PHA was used as the stimulus (not shown). The difference in RANTES production from HIV-stimulated (envelope-depleted) cells obtained from patients belonging to the two disease status groups became more significant when corrected for CD8+ T cell counts (P = 0.037).
Figure 2.
Antigen-induced chemokine production and disease status. Fresh PBMC (1.6 × 106) were cultured as described in Methods in medium alone or with 10 μg/ml gp120-depleted, inactivated HIV-1 (HIV), 10 μg/ml purified p24 antigen (p24), 10 μg/ml C. albicans (candida), or 20 μg/ml PHA (not shown). After 3 days (for PHA) or 6 days of incubation at 37°C, supernatants were collected and assayed for RANTES, MIP-1α, and MIP-1β by ELISA. (Bars = SEM.)
A correlation existed between antigen-induced MIP-1α and MIP-1β, but not RANTES, levels and proliferation. In the case of HIV (envelope-stripped) antigen-stimulated cells, MIP-1β production correlated with the proliferation index (r = 0.475, P < 0.001 by Pearson correlation).
Comparison between above and below median MIP-1β production by those with AIDS and non-AIDS is presented in Table 3 (median chemokine production in response to HIV, 100 pg/ml; p24, 120 pg/ml; C. albicans, 430 pg/ml; PHA, 55,900 pg/ml). Unlike what was observed in non-AIDS cases, almost all of the subjects who had AIDS produced levels of chemokines that were below the median level upon antigen stimulation (Table 3). Analyses of the odds ratio indicated that the below median chemokine production in response to stimulation with C. albicans, HIV p24, and envelope-depleted HIV was 6.16, 5.5, and 5.6 times more likely to occur in the AIDS group. Similar outcomes were observed with MIP-1α levels, but not RANTES. Again, PHA-stimulated chemokine levels were not significantly different between the two disease status groups. In addition, similar trends were observed when χ2 analyses were performed on groups stratified according to chemokine production quartiles (not shown).
Table 3.
χ2 analysis of chemokine production upon antigen stimulation
| Chemokine production | Above or below median | N No AIDS | N AIDS | Total | χ2(P) | Odds ratio |
|---|---|---|---|---|---|---|
| MIP-1β | Below | 30 | 8 | 38 | 2.66 | 3.12 |
| PHA-stimulated | Above | 35 | 3 | 38 | (0.191) | |
| MIP-1β | Below | 29 | 9 | 38 | 5.41 | 6.16 |
| Candida antigen-stimulated | Above | 37 | 2 | 39 | (0.025)* | |
| MIP-1β | Below | 33 | 10 | 43 | 4.56 | 5.5 |
| p24 antigen-stimulated | Above | 33 | 2 | 35 | (0.056) | |
| MIP-1β | Below | 31 | 10 | 43 | 5.38 | 5.6 |
| HIV antigen-stimulated | Above | 35 | 2 | 35 | (0.027)* |
N = number of subjects;
P ≤ 0.05.
Spontaneous Production of Chemokines.
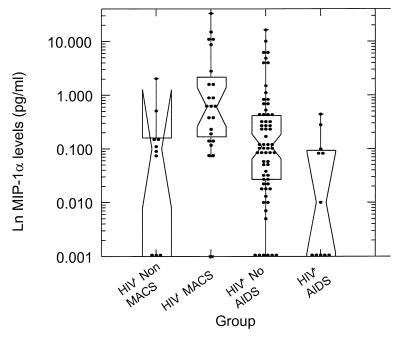
Twenty-three of the study subjects studied were seronegative. When compared with non-MACS controls and with seropositive subjects without AIDS, unstimulated cells from seronegative subjects from the MACS cohort produced high levels of chemokines. We defined this production as “spontaneous.” In the case of MIP-1α (Fig. 3), the difference between the non-MACS and the MACS seronegatives was statistically significant. The average spontaneous production of MIP-1α in non-MACS control was 312 ± 560 pg/ml, compared with 4,666 ± 1,735 in MACS seronegative subjects (P = 0.009). Spontaneous levels of MIP-1β production did not differ significantly among these two groups. When compared with MACS seronegatives, seropositive subjects without AIDS were found to produce significantly lower levels of MIP-1α (977 ± 323 pg/ml compared with 4,666 ± 1,735 pg/ml; P = 0.001), MIP-1β (681 ± 178 pg/ml in asymptomatic seropositives, compared with 2,423 ± 1,025 pg/ml in MACS seronegatives; P = 0.022), but not RANTES. Subjects with AIDS were found to spontaneously release significantly lower levels of chemokines when compared with both MACS [MIP-1α, 90 ± 40 vs. 4,666 ± 1,735 pg/ml (P = 0.001); MIP-1β, 123 ± 45 vs. 2,423 ± 1,025 pg/ml (P < 0.001)] and non-MACS seronegatives [MIP-1β, 123 ± 45 vs. 956 ± 938 pg/ml (P = 0.011); RANTES, 1,810 ± 658 vs. 5,305 ± 4,876 pg/ml (P = 0.030)].
Figure 3.
Spontaneous MIP-1α production. Fresh PBMC (1.6 × 106) were cultured as described in Methods in medium alone. After 3 days of incubation at 37°C, supernatants were collected and assayed for MIP-1α by ELISA. On the abscissa, population groups include: Controls, healthy laboratory control; seronegative, MACS non-HIV-1-infected; HIV+ No AIDS, MACS HIV-1-infected, AIDS not diagnosed; HIV+ AIDS, MACS HIV-1-infected with AIDS. The ordinate shows natural logarithm of chemokine production. The difference in MIP-1α production between the non-MACS and the MACS seronegatives is statistically significant. When compared with MACS seronegatives, seropositive subjects without AIDS were found to produce significantly lower levels of MIP-1α. Subjects with AIDS were found to spontaneously release significantly lower levels of chemokines when compared with both MACS and non-MACS seronegatives. Box plots are interpreted as follows: the waist is the median, diagonal lines indicate 95% confidence intervals about the median, lower and upper horizontal lines indicate 25th and 75th percentiles of the distribution, and upper and lower bars indicate the range of the distribution excluding outliers. One subject of the seronegative group, producing outstanding levels of MIP-1α, was not used to compose this figure.
Discussion
The discovery that chemokines block HIV infection in vitro (1–5) prompted us and others to ask the question of whether these proteins may have similar effects in vivo (1, 25–28, 33, 34). HIV-inhibitory chemokines, blocking and/or down-regulating CCR-5 in vivo, might be correlates of better disease outcome and protection from HIV infection, similar to CCR5 genetic defects (15–24). Because chemokines are involved in the regulation of immune response, we studied chemokine production in the context of antigen stimulation. To investigate whether antigen stimulation influences chemokine production because of the expansion of antigen-specific subset, or independently of it, we also measured proliferation indices, a parameter known to correlate with long-term nonprogression and better prognosis (34, 39). Our results show that antigen-induced chemokine production is decreased significantly in HIV-positive subjects with AIDS as compared with asymptomatic HIV-positive subjects. The magnitude of this effect is not yet known but is sufficient to be detected in a cross-sectional analysis. Of importance is the result that antigen stimulation was more informative in this respect than PHA stimulation. This might indicate that the response to a “maximal” and nonspecific stimulus, like PHA, is not as significantly impaired in subjects with AIDS, whereas the loss of response to antigen stimulation occurs earlier on in the course of AIDS. This is consistent with the finding that, as AIDS progresses, a loss of T helper response to antigen stimulation is observed (40–42) and that, chronologically, loss of response to PHA is the last to occur, being associated with a severe immune dysfunction involving both CD4+ and CD8+ T cells (42). The lack of significance in differences in levels of chemokine production upon PHA stimulation might help to explain why studies by Clerici et al. (43) and Mackewicz et al. (44) failed to show a beneficial effect of chemokines in AIDS, because their studies used PHA as a stimulus.
Interestingly, we observed a parallel decrease in antigen-induced MIP-1α and MIP-1β from PBMC, whereas the production of RANTES did not seem to differ significantly between subjects with and without AIDS; however, this could be due to partial platelet contamination. Activated platelets produce high quantities of chemokines, especially RANTES (45–49). Other studies performed by analyzing plasma/serum levels of β-chemokines that did not show a positive effect of chemokines in HIV infection (50–56) probably were affected by this unspecific release. Similar to the results of Ullum et al. (33), we have found a more significant role of MIP-1α and MIP-1β in disease progression; this is biologically important because MIP-1β is a specific ligand for CCR-5, and CCR-5 mutations have been associated with protection from HIV-infection (in the homozygous state) and slower progression to AIDS (in the heterozygous state). In contrast, RANTES and MIP-1α also bind to other receptors (57). Because MIP-1β does not bind to any other known chemokine receptor besides CCR-5, it might have a more important role in this respect, specifically inducing CCR-5 blockage and/or down-regulation.
Interestingly, we found that in some cases chemokine levels correlated with CD4, but not CD8 counts. This may be because of greater contribution of chemokines by CD4+ T cells in the tests employed here. The correlation with CD4 T cell counts is particularly surprising in light of reports documenting high chemokine release by CD8+ T cells (58, 59). It is possible that the stimuli used in our study were less capable of inducing CD8 activation and/or that CD8 activation depended on prior activation of CD4+ (see below).
Lymphocyte proliferation, measured as stimulation indices, also was decreased in subjects with AIDS, and the differences were significant when cells were antigen-stimulated as opposed to PHA-stimulated. Notably, T cell proliferation is considered to be driven predominantly by CD4+ T cells. However, recent observations suggest that after immunization with the HIV envelope-depleted antigen that we used here, the phenotype of cells proliferating in response to the HIV antigens include not only CD4+ cells, but also CD8 and natural killer (NK) cells (60). The decrease in both cell proliferation and chemokine production, which we found to correlate, could reflect deterioration of the immune system. However, these results also may suggest that CD4+ T cells may orchestrate the activation of CD8 and NK cells that may be the effective source of HIV-suppressive chemokines (58, 59, 61). Similar associations between HIV-suppressive chemokines and lymphocyte proliferation have been reported by Rosenberg et al. (34), who described an association between the lymphocyte proliferative response to HIV-1 core proteins (i.e., p24) and control of plasma viremia in subjects not taking antiviral drug therapy. The same authors observed relatively increased levels of p24-induced, HIV-suppressive chemokines in nonprogressors. In addition, another study has shown that T cells from subjects who were exposed to HIV, but remained uninfected, produced high levels of chemokines in response to HIV antigens (26). Antigen-specific chemokine production was also found to correlate with protection from infection in a recent study of perinatal HIV transmission (28).
An unexpected result came from the observation that spontaneous chemokine production (i.e., in the absence of stimulation) was significantly higher in PBMC obtained from exposed but seronegative subjects as compared with the non-MACS seronegatives. The results suggest that some individuals may spontaneously produce high levels of chemokines and be relatively resistant to HIV infection, analogous to what has been observed for individuals who are homozygous for CCR-5 mutations (15–24). Others have reported similar observations in documented cases of subjects who remained uninfected despite repeated exposure to HIV (27, 62, 63). Whether the high chemokine production is due to intrinsic differences in chemokine gene expression or to environmental factors such as the presence of or exposure to infectious agents, sexual behavior, differences in sample collection, or other factors, is not known.
Our results do not address whether the observed impairments of chemokine production and proliferation are the reflection of a general failure of the immune response or the product of an altered ratio of specific cell subsets. The mechanism(s) underlying this observation will be addressed in future studies that will define which cells are responsible for chemokine production in response to antigen. Nonetheless, it is clear that more studies on the regulation of chemokine production in health and disease and in specific subsets of lymphocytes upon antigen stimulation are necessary to understand further the role of these proteins in the pathogenesis and treatment of AIDS.
Acknowledgments
We thank Ellen Taylor for recruitment coordination; Elvia Ramirez, Stacey Meyerer, Karen Eckert-Kohl, Sanjay Pancholi, and Amy Evangelista for technical assistance; D. Katherine Conant for valuable suggestions; and Anna Mazzuca for editorial assistance. This work was partially supported by National Institutes of Health Grants AI 35042 and 5M01RR00052.
Abbreviations
- LSI
lymphocyte stimulation index
- MACS
Multicenter AIDS Cohort Study
- MIP
macrophage inflammatory protein
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 2.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 3.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, et al. Nature (London) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 4.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, et al. Nature (London) 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 5.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, et al. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 6.Alkhatib G, Combardiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 7.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P, Lijun W, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.Doranz B J, Rucker J, Yi Y J, Smith R J, Samson M, Peiper S C, Parmentier M, Cullman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 12.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhatib G, Locati M, Kennedy P E, Murphy P M, Berger E A. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 14.Mack M, Luckow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, et al. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 16.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. Nature (London) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, et al. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman P A, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J A, Combardiere C, Weissman D, Cohen O, Rubbert A, Lam G, et al. Mol Med. 1997;3:23–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 21.Eugen-Olsen J, Iversen A K N, Garred P, Koppelhus U, Pedersen C, Benfield T L, Sorensen A M, Katzenstein T, Dickmeiss E, Gerstoft J, et al. AIDS. 1997;11:305–310. doi: 10.1097/00002030-199703110-00007. [DOI] [PubMed] [Google Scholar]
- 22.Katzenstein T L, Eugen-Olsen J, Hofmann B, Benfield T, Pedersen C, Iversen A K, Sorensen A M, Garred P, Koppelhus U, Svejgaard A, Gerstoft J. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;16:10–14. doi: 10.1097/00042560-199709010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Meyer L, Magierowska M, Hubert J B, Rouzioux C, Deveau C, Sanson F, Debre P, Delfraissy J F, Theodorou I. AIDS. 1997;11:F73–F78. doi: 10.1097/00002030-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 24.de Roda Husman A M, Koot M, Cornelissen M, Keet I P, Brouwer M, Broersen S M, Bakker M, Roos M T, Prins M, de Wolf F, et al. Ann Intern Med. 1997;127:882–890. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Garzino-Demo A, DeVico A L, Cocchi F, Gallo R C. AIDS Res Hum Retroviruses. 1998;14:S177–S184. [PubMed] [Google Scholar]
- 26.Furci L, Scarlatti G, Burastero S, Tambussi G, Colognesi C, Quillent C, Longhi R, Loverro P, Borgonovo B, Gaffi D, et al. J Exp Med. 1997;186:455–460. doi: 10.1084/jem.186.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J-F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasik T J, Bratosiewicz J, Wierzbicki A, Whiteman V E, Rutstein R R, Starr S E, Douglas S D, Kaufman D, Sison A V, Polansky M, et al. J Immunol. 1999;162:4355–4364. [PubMed] [Google Scholar]
- 29.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, et al. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Tao L, Mitchell E, Bogers W M, Doyle C, Bravery C A, Bergmeier L A, Kelly C G, Heeney J L, Lehner T. Proc Natl Acad Sci USA. 1998;95:5223–5228. doi: 10.1073/pnas.95.9.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Jones I, Doyle C. Dev Biol Stand. 1998;92:225–235. [PubMed] [Google Scholar]
- 32.Ahmed, R. K. S., Nilsson, C., Wang, Y., Lehner, T., Biberfeld, G. & Thorstensson, R. (1999) J. Gen. Virol., in press. [DOI] [PubMed]
- 33.Ullum H, Cozzi A, Victor J, Aladdin A, Phillips A N, Gerstoft J, Skinhej P, Pedersen B K. J Infect Dis. 1998;133:331–336. doi: 10.1086/514192. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1998;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 35.Moss R B, Trauger R J, Giermakowska W K, Turner J L, Wallace M R, Jensen F C, Richieri S P, Ferre F, Daigle A E, Duffy C, et al. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;14:343–350. doi: 10.1097/00042560-199704010-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kaslow R A, Ostrow D G, Detels R, Phair J P, Polk B F. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 37.Schenker E L, Hultin L E, Bauer K D, Ferbas J, Margolick J B, Giorgi J V. Cytometry. 1993;14:307–317. doi: 10.1002/cyto.990140311. [DOI] [PubMed] [Google Scholar]
- 38.Richieri S P, Bartholomew R, Aloia R C, Savary J, Gore R, Holt J, Ferre F, Musil R, Tian H R, Trauger R, et al. Vaccine. 1998;16:119–129. doi: 10.1016/s0264-410x(97)00196-5. [DOI] [PubMed] [Google Scholar]
- 39.Valentine F T, Paolino A, Saito A, Holzman R S. AIDS Res Hum Retroviruses. 1998;14:S161–S166. [PubMed] [Google Scholar]
- 40.Clerici M, Stocks N I, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. J Clin Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clerici M, Via C S, Lucey D R, Roilides E, Pizzo P A, Shearer G M. Eur J Immunol. 1991;21:665–670. doi: 10.1002/eji.1830210319. [DOI] [PubMed] [Google Scholar]
- 42.Shearer G M, Clerici M. Curr Opin Immunol. 1992;4:463–465. doi: 10.1016/s0952-7915(06)80040-3. [DOI] [PubMed] [Google Scholar]
- 43.Clerici M, Balotta C, Trabattoni D, Papagno L, Ruzzante S, Rusconi S, Fusi M L, Colombo M C, Galli M. AIDS. 1996;10:1432–1433. doi: 10.1097/00002030-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Mackewicz C E, Barker E, Greco G, Reyes-Teran G, Levy J A. J Clin Invest. 1997;100:921–930. doi: 10.1172/JCI119608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kameyoshi Y, Dorschner A, Mallet A I, Christophers E, Schroder J M. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Power C A, Clemetson J M, Clemetson K J, Wells T N. Cytokine. 1995;7:479–482. doi: 10.1006/cyto.1995.0065. [DOI] [PubMed] [Google Scholar]
- 47.Klinger M H, Wilhelm D, Bubel S, Sticherling M, Schroder J M, Kuhnel W. Int Arch Allergy Immunol. 1995;107:541–546. doi: 10.1159/000237097. [DOI] [PubMed] [Google Scholar]
- 48.Bubel S, Wilhelm D, Entelmann M, Kirchner H, Kluter H. Transfusion. 1996;36:445–449. doi: 10.1046/j.1537-2995.1996.36596282589.x. [DOI] [PubMed] [Google Scholar]
- 49.Holme P A, Muller F, Solum N O, Brosstad F, Froland S S, Aukrust P. FASEB J. 1998;12:79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 50.Zanussi S, D’Andrea M, Simonelli C, Tirelli U, De Paoli P. AIDS. 1996;12:1431–1432. doi: 10.1097/00002030-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 51.Weiss L, Si-Mohamed A, Giral P, Castiel P, Ledur A, Blondin C, Kazatchkine M D, Haeffner-Cavaillon N. J Infect Dis. 1997;176:1621–1624. doi: 10.1086/517341. [DOI] [PubMed] [Google Scholar]
- 52.McKenzie S W, Dallalio G, North M, Frame P, Means R T. AIDS. 1996;10:F29–F33. doi: 10.1097/00002030-199610090-00001. [DOI] [PubMed] [Google Scholar]
- 53.Krowka J F, Gesner M L, Ascher M S, Sheppard H W. Clin Immunol Immunopathol. 1997;85:21–27. doi: 10.1006/clin.1997.4411. [DOI] [PubMed] [Google Scholar]
- 54.Kakkanaiah V N, Ojo-Amaize E A, Peter J B. Clin Diag Lab Immunol. 1998;5:499–502. doi: 10.1128/cdli.5.4.499-502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hittinger G, Poggi C, Delbeke E, Profizi N, Lafeuillade A. Infection. 1988;26:100–103. doi: 10.1007/BF02767768. [DOI] [PubMed] [Google Scholar]
- 56.Polo S, Veglia F, Malnati M S, Gobbi C, Farci P, Raiteri R, Sinicco A, Lusso P. AIDS. 1999;13:447–454. doi: 10.1097/00002030-199903110-00002. [DOI] [PubMed] [Google Scholar]
- 57.Murphy P M. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 58.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternack M S, Luster A D. Nature (London) 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 59.Price D A, Sewell A K, Dong T, Tan R, Goulder P J, Rowland-Jones S L, Phillips R E. Curr Biol. 1998;8:355–358. doi: 10.1016/s0960-9822(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 60.Moss, R. B., Wallace, M. R., Giermakowska, W. K., Webb B., Savary, J., Chamberlin-Brandt, C., Theofan, G., Musil, R., Richieri, S., Jensen, F. C., et al. (1999) J. Infect. Diseases, in press. [DOI] [PubMed]
- 61.Oliva A, Kinter A L, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, et al. J Clin Invest. 1998;102:223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, et al. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 63.Paxton W A, Liu R, Kang S, Wu L, Gingeras T R, Landau N R, Mackay C R, Koup R A. Virology. 1998;244:66–73. doi: 10.1006/viro.1998.9082. [DOI] [PubMed] [Google Scholar]