Abstract
An understanding of the relationship between the type of analgesic prescription and the prevalence and severity of side effects is crucial in making appropriate treatment decisions. The purposes of this study were: to determine if there were differences in the prevalence of side effects among four different types of analgesic prescriptions (i.e., no opioid, only an as needed (PRN) opioid, only an around-the-clock (ATC) opioid, or an ATC + PRN opioid); to determine if there were differences in the severity of side effects among the four prescriptions groups; and to determine the relationships between the total dose of opioid analgesic medication prescribed and taken and the severity of side effects. As part of a larger study, 174 cancer patients with bone metastasis reported their analgesic use and the prevalence and severity of eleven side effects. Significant differences (P < 0.05) were found in prevalence rates for seven of the side effects among the four prescription groups. The highest prevalence rates were found in the only ATC and ATC + PRN groups. Significant differences were found in the severity scores for five of the side effects, with the highest severity scores reported by patients in the only ATC and ATC + PRN groups. Significant positive correlations were found between the severity of six of the side effects and the total dose of opioid prescribed and taken. Risk factors for analgesic-induced side effects are ATC and ATC + PRN prescription types and higher doses of opioid analgesics.
Keywords: Opioid-induced side effects, analgesic side effects, cancer pain, constipation, chronic pain, sedation, nausea, sleep disturbance, fatigue
Introduction
Side effects of analgesic medications are a well documented barrier to successful pain management. These side effects limit the titration of analgesics to achieve optimal pain control and decrease the patient's quality of life (1,2). Side effects commonly associated with chronic administration of various classes of analgesic medications include gastrointestinal (e.g., nausea, vomiting, indigestion, constipation), central nervous system (e.g., drowsiness, difficulty concentrating, hallucinations/nightmares, lightheadedness, poor coordination, lack of energy), and autonomic nervous system (e.g., urinary retention, xerostomia) effects. The recently revised clinical practice guideline for cancer pain management (3) noted that analgesic medications should be titrated to achieve effective analgesia with tolerable side effects.
An understanding of the relationships between the type of analgesic prescription and the prevalence and severity of side effects is crucial in making appropriate treatment decisions for both pain control and side effect management. However, very little data are available on the differences in either the prevalence or the severity of side effects associated with different types of analgesic prescriptions. In addition, no data exist on the relationships between the severity of side effects and the total dose of opioid analgesics prescribed or taken. Therefore, the purposes of this study, in a sample of oncology outpatients with pain from bone metastasis, were: to determine if there were differences in the prevalence of side effects among four different types of analgesic prescriptions (i.e., no opioid, only an as needed (PRN) opioid, only an around-the-clock (ATC) opioid, or an ATC + PRN opioid); to determine if there were differences in the severity of side effects among the four different types of analgesic prescriptions; and to determine the relationships between the total dose of opioid analgesic prescribed and taken and the severity of side effects.
Literature Review
A number of systematic reviews have evaluated the prevalence of analgesic side effects associated with the treatment of cancer (1,4) and chronic noncancer (5-9) pain. Most prevalence rates were derived from adverse event data reported as a part of studies of new analgesics. In these reviews, constipation with opioid use ranged from 27% to 70%; nausea and vomiting from 10% to 30%; sedation from 20% to 70%; and poor sleep or difficulty sleeping from 19% to 31%. However, the majority of the studies included in these systematic reviews were short-term trials (i.e., less than 28 days) with opioid analgesics. Therefore, little is known about the prevalence and severity of side effects in patients who are taking opioids for longer than one month.
A few studies have provided limited data on the relationship between opioid-induced side effects and total opioid dose (10,11). While the primary purpose of these studies was not to evaluate the prevalence and severity of analgesic side effects or the relationship between total dose and severity of side effects, some information can be extrapolated from this work. Boureau et al. (10) compared the efficacy and adverse effects of controlled-release morphine suspension and controlled-release morphine tablets for chronic cancer pain in a crossover study of 44 patients. The prevalence rates for those side effects that persisted throughout the two-week study ranged from 75.8% to 78.8% for constipation, 57.1% to 75.0% for nausea, 50.0% for vomiting, and 69.0% to 86.2% for daytime drowsiness. While the mean daily dose of oral morphine was reported to be 108 mg ± 57, no data were reported on the relationship between opioid dose and severity of these side effects.
Comparing the safety and efficacy of morphine immediate-release tablets and sustained-release morphine tablets, Walsh et al. (11) found that 9% to 10% of study participants experienced nausea and 35% experienced sedation. The mean daily dose of opioid ranged from 108 mg ± 11.7 to 120 mg ± 13.0. Again, no data were provided on the relationship between the total opioid dose and the severity of side effects.
Two recent evidence-based reviews on cancer pain summarized symptom management strategies for the most common side effects of analgesic medications (1,4). Cherny et al. (1) identified six side effects that adversely impact oncology patients use of opioid medications, namely: nausea/vomiting, constipation, sedation, cognitive failure, myoclonus, and pruritis. McNicol et al. (4) conducted a systematic review of 67 studies on the management of side effects associated with opioids. The side effects evaluated in this review were sedation, nausea/vomiting, delirium, myoclonus, pruritis, respiratory depression, and constipation. Additional work is warranted to determine whether differences exist in the prevalence and severity of side effects associated with analgesic medications in oncology outpatients who are taking these drugs on an as needed or routine basis for the management of cancer pain.
Methods
Sample and Settings
This descriptive correlational study is part of a large randomized clinical trial (RCT) that evaluated the effectiveness of the PRO-SELF© Pain Control Program compared to standard care in improving cancer pain management (12-14). Two hundred and twelve oncology outpatients were recruited from seven outpatient settings in Northern California including a university-based cancer center, two community-based oncology practices, one outpatient radiation therapy center, one health maintenance organization, one Veterans Administration facility, and one military hospital. Only those patients (n = 174) who completed the study were included in this analysis. Some patients (n = 38) did not complete the entire study for a variety of reasons, including increased severity of illness, intervening cancer treatments that required hospitalization, and death. No differences were found in any of the demographic, disease, or baseline pain characteristics between patients who did and did not complete the study.
The participants were adult oncology outpatients (> 18 years old) who were able to read, write, and understand English. All participants had a Karnofsky Performance Status (KPS) score of ≥ 50; had an average pain intensity score of ≥ 2.5 on a 0 to 10 numeric rating scale (based on an average of seven days of baseline ratings of pain intensity); and had radiographic evidence of bone metastasis.
Instruments
Patients completed a demographic questionnaire, the KPS rating scale (15), a pain management diary, and a side effects checklist. In addition, the patient's medical record was reviewed for disease and treatment information. The demographic questionnaire obtained information on age, gender, marital status, living arrangements, education level, and ethnicity. Baseline information about the patient's pain problem was obtained using 0 (no pain) to 10 (excruciating pain) numeric rating scales for pain now, as well as average, worst, and least pain.
Patients were asked to rate the number of hours per day and the number of days per week they experienced pain that interfered with their mood or activities. Interference times were taken from the Brief Pain Inventory (16) and determined how cancer-related pain interfered with the person's ability to perform eight activities. For each of the interference items, patients circled a number between 0 (“does not interfere”) and 10 (“completely interferes”) that indicated the degree to which pain interfered with different activities. A total interference score was calculated as the sum of the responses to the eight items. In addition, patients were asked to indicate the amount of relief they received from their pain medicine in the last week (i.e., 0% = no relief to 100% = complete relief).
The KPS scale measures the patients' ability to accomplish normal activities of daily living and their need for caregivers' assistance (15). The KPS scale used in this study consisted of eight items that patients used to rank their functional status. The items ranged from 30 (i.e., disability requiring hospitalization) to 100 (i.e., adequate health status with no complaints and no evidence of disease). Reliability and validity of the KPS have been established previously.
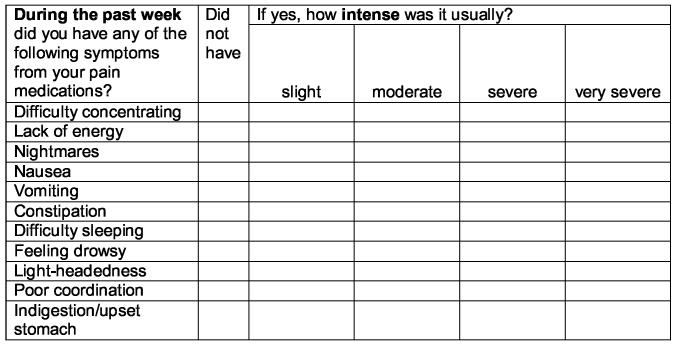
Detailed data were collected on all analgesics prescribed and taken on a PRN and ATC basis using a pain management diary (17). The bottom portion of the diary obtained the information on pain medication. Patients recorded both routine (i.e., ATC) and extra (i.e., PRN) analgesic medications and the times they were taken. Side effects of analgesic medications were evaluated on a weekly basis using the checklist shown in Figure 1. Intensity of side effects was rated using a 0 to 4 scale (i.e., 0 = did not have, 1 = slight, 2 = moderate, 3 = severe, and 4 = very severe) (14). Side effect data from the end of the first week of the RCT are reported in this paper.
Figure 1.
Side effects checklist from the PRO-SELF© Pain Management Diary.
Directions: below is a list of symptoms that can sometimes occur when taking pain medicine. If you have had any of these symptoms during the past week, indicate how intense or bad it usually was by marking the appropriate box with an X. If you did not have the symptom, mark an X in the box marked “did not have”.
Data Collection Procedures
This study was approved by the Committee on Human Research at the University of California, San Francisco and at each of the study sites. Patients were approached in an outpatient setting by a recruitment nurse who explained the study procedures and obtained written informed consent. Patients completed the demographic questionnaire and KPS rating at the time of enrollment into the study. Patients were taught during the first home visit how to complete the pain management diary and side effect checklist. Analgesic prescriptions were verified by the research nurses at the time of the home visit.
Data Analysis
Descriptive statistics and frequency distributions were generated for the demographic and disease-related characteristics. Each patient's analgesic prescription was categorized on the day of enrollment into one of four categories (i.e., no opioid, only PRN opioid, only ATC opioid, or ATC + PRN opioid). All of the opioid doses were converted to morphine equivalents. One-way analyses of variance (ANOVAs) or Chi-square analyses were performed to evaluate for differences in demographic, treatment, and baseline pain characteristics among the patients in the four analgesic prescription groups. Chi-square tests were done to evaluate for differences in the prevalence of each of the side effects among the four analgesic prescription groups. Kruskall-Wallis tests were done to evaluate for differences in the severity of each of the side effects among the four analgesic prescription groups. The relationships between the total dose of opioid analgesic prescribed and taken on the day of enrollment and the severity of each of the side effects were evaluated using Spearman correlations.
All calculations used actual values. Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest set of complete data across groups. For all primary statistical tests, a P-value of <0.05 was considered statistically significant. Pairwise contrasts were done to determine precisely where the differences were among the four prescription groups. With four opioid prescription groups, six pairwise comparisons were possible (i.e., none versus only PRN, none versus only ATC, none versus ATC + PRN, only PRN versus only ATC, only PRN versus ATC + PRN, and only ATC versus ATC + PRN). For each dependent variable, the family of six contrasts was given an alpha of .05. Using the Bonferroni method to keep the family alpha level at 0.05, each pairwise contrast was considered statistically significant if its P-value was <0.008 (.05/6). The P-values presented for each of the pairwise contrasts have been adjusted so that values of less than 0.05 indicate statistical significance.
Results
Distribution of Types of Analgesic Prescriptions
The distribution of patients' analgesic prescriptions was as follows: 11% no opioids, 42% only PRN opioids, 18% only ATC opioids, and 29% ATC + PRN opioids.
Demographic Characteristics
The demographic characteristics of patients in the four prescription groups are summarized in Table 1. No differences were found among the four prescription groups for the majority of demographic characteristics except age, KPS score, and living arrangements. Significant differences in age and living arrangements were found among the four prescription groups. However, none of the pairwise contrasts demonstrated significant differences for either age or living arrangements. KPS scores for patients in the ATC + PRN group were significantly lower than for patients in either the no opioid or the only PRN opioid groups.
Table 1.
Demographic Characteristics of the Patients in the Four Prescription Groups
| Characteristic | No Opioid n = 19 |
Only PRN Opioid n = 72 |
Only ATC Opioid n = 32 |
ATC + PRN Opioid n = 50 |
Statistic |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 55.7 (11.3) | 62.5 (12.2) | 57.5 (11.8) | 57.6 (12.2) | F(3,169)=2.8, P=0.04 |
| Education (years) | 16.3 (3.3) | 14.3 (3.2) | 15.3 (3.3) | 14.6 (3.1) | F(3,167)=2.4, P=0.07 |
| KPS score | 76.0 (12.4) | 74.0 (9.3) | 68.0 (11.3) | 63.9 (12.6) | F(3,153)=9.4, P<0.0001 |
| % | % | % | % | ||
| Gender | |||||
| Female | 84.2 | 70.8 | 68.7 | 66.0 | X2=2.3, P=0.52 |
| Male | 15.8 | 29.2 | 31.3 | 34.0 | |
| Lives alone | |||||
| Yes | 10.5 | 39.4 | 15.6 | 24.5 | X2=10.3, P=0.02 |
| No | 89.5 | 60.6 | 84.4 | 75.5 | |
| Marital status | |||||
| Married | 78.9 | 40.8 | 53.1 | 64.0 | X2=15.9, P=0.39 |
| Other | 21.1 | 59.2 | 46.9 | 36.0 | |
| Ethnicity | |||||
| White | 26.3 | 16.7 | 16.1 | 10.0 | X2=12.5, P=0.64 |
| Non-white | 73.7 | 83.3 | 83.9 | 90.0 | |
ATC = around-the-clock; KPS = Karnofsky Performance Status; PRN = pro re nata; SD = standard deviation.
Post hoc contrasts:
Age – no significant pair wise differences were found in the post hoc contrasts using the Bonferroni criteria.
KPS score – ATC + PRN < only PRN (P<0.0001) and no opioid (P=0.002).
Lives alone - no significant pair wise differences were found in the post hoc contrasts using the Bonferroni criteria.
Disease and Treatment Characteristics by Type of Analgesic Prescription
As shown in Table 2, no differences in disease or treatment characteristics were found among patients in the four prescription groups.
Table 2.
Disease and Treatment Characteristics of the Patients in the Four Prescription Groups
| Characteristic | No Opioid n = 19 |
Only PRN Opioid n = 72 |
Only ATC Opioid n = 32 |
ATC + PRN Opioid n = 50 |
Statistic |
|---|---|---|---|---|---|
| % | % | % | % | ||
| Diagnosis | |||||
| Breast | 78.9 | 53.5 | 46.9 | 44.0 | |
| Prostate | 5.3 | 15.5 | 9.4 | 14.0 | X2=26.2, P=0.20 |
| Lung | 5.3 | 9.9 | 9.4 | 22.0 | |
| Other | 10.5 | 21.1 | 34.3 | 20.0 | |
| Current Treatmentsa | |||||
| Radiation | 15.8 | 16.9 | 18.8 | 16.0 | X2=.12, P =0.99 |
| Chemotherapy | 47.4 | 46.5 | 43.8 | 43.8 | X2=.15, P =0.99 |
| Hormonal therapy | 36.8 | 33.8 | 25.0 | 30.0 | X2=1.1, P =0.78 |
| Biotherapy | 0.0 | 1.4 | 0.0 | 2.0 | X2=.94, P =0.82 |
| No treatment | 15.8 | 5.9 | 12.5 | 20.4 | X2=5.7, P =0.13 |
ATC = around-the-clock; PRN = pro re nata
Because patients could have been receiving one or more cancer treatments, separate Chi-square analyses were done.
Baseline Pain Characteristics and Analgesic Doses
No significant differences were found in pain now, average pain, worst pain, or length of time in pain among patients in the four prescription groups. As shown in Table 3, significant differences were found among the four groups in total pain interference score, percentage of pain relief in the past week, total dose of opioid prescribed, and total dose of opioid taken. Total pain interference scores were significantly higher in the ATC + PRN opioid group than in the no opioid group (P = 0.002). Percentage of pain relief was significantly lower in the no opioid group compared to the only PRN opioid group (P = 0.008).
Table 3.
Baseline Pain Characteristics of the Patients in the Four Prescription Groups
| Characteristic | No Opioid n = 19 |
Only PRN Opioid n = 72 |
Only ATC Opioid n = 32 |
ATC + PRN Opioid n = 50 |
Statistic |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Pain now | 2.9 (2.0) | 3.5 (21.) | 4.1 (2.3) | 3.6 (2.3) | F(3,158)=1.3, P=0.29 |
| Average pain | 3.7 (1.6) | 4.1 (1.7) | 4.5 (1.8) | 4.2 (1.8) | F(3,159)=0.8, P =0.50 |
| Worst pain | 5.9 (2.2) | 6.4 (2.3) | 7.2 (2.2) | 7.1 (2.1) | F(3,160)=2.0, P =0.12 |
| Total pain interference score | 3.3 (2.2) | 4.4 (2.0) | 4.9 (2.4) | 5.4 (2.1) | F(3,168)=5.1, P =0.002 |
| % pain relief in the past week | 50.0 (30.1) | 71.5 (19.2) | 67.2 (20.0) | 63.5 (25.0) | F(3,157)=4.0, P =0.009 |
| Total dose of opioid prescribed (mg/day in morphine equivalents) |
59.7 (51.6) | 99.5 (83.8) | 443.9 (453.3) | F(2,150)=33.6, P <0.0001 |
|
| Total dose of opioid taken (mg/day in morphine equivalents) |
15.4 (20.7) | 75.8 (74.2) | 264.3 (358.8) | F(2,150)=21.4, P <0.0001 |
|
| % | % | % | % | ||
| Length of time in pain | |||||
| < 1 week | 5.3 | 2.9 | 0.0 | 2.0 | |
| 1 to 2 weeks | 5.3 | 1.4 | 3.3 | 6.0 | |
| About 1 month | 5.3 | 2.9 | 6.7 | 4.0 | X2=7.3, P =0.95 |
| 2 to 6 months | 31.6 | 33.3 | 40.0 | 26.0 | |
| 7 months to 1 year | 26.3 | 20.3 | 13.3 | 24.0 | |
| > 1 year | 26.3 | 39.1 | 36.7 | 38.0 | |
| Patients on NSAIDs | 18.5 | 50.6 | 13.6 | 17.3 | X2=19.9, P <0.0001 |
| Patients on co-analgesics | 10.0 | 33.3 | 13.3 | 43.3 | X2=3.8, P =0.29 |
ATC = around-the-clock; mg = milligrams; NSAIDs = nonsteroidal anti-inflammatory drugs; PRN = pro re nata; SD = standard deviation.
Post hoc contrasts:
Total pain interference score – no opioid < ATC + PRN (P =0.002).
% pain relief in the past week – no opioid < only PRN (P =0.008).
Total dose of opioid prescribed – only PRN and only ATC < ATC + PRN (both P <0.0001).
Total dose of opioid taken – only PRN and only ATC < ATC + PRN (both P <0.0001).
Patient on NSAIDs – only ATC < no opioid (P =0.002); ATC + PRN < only PRN and no opioid (both P ≤ 0.002).
The most common short-acting opioids that were prescribed and taken were acetaminophen with codeine and acetaminophen with hydrocodone. The most common controlled release opioids that were prescribed and taken were controlled release morphine and transdermal fentanyl. As shown in Table 3, significant differences in the total dose of opioid prescribed and taken (both P < 0.0001) were found among the three opioid prescription groups. Patients in the ATC + PRN group had significantly higher doses of opioids prescribed and taken than either the PRN or ATC opioid groups. No differences were found among the groups in the percentage of patients who had a co-analgesic prescribed. However, significant differences were found in the percentage of patients with a prescription for an NSAID (P < 0.0001), with the highest percentage found in the only PRN opioid group.
Prevalence of Analgesic Side Effects by Type of Analgesic Prescription
Table 4 provides data on the prevalence of each of the eleven side effects by type of analgesic prescription. No significant differences in prevalence rates were found among the four prescription groups for difficulty sleeping and indigestion/upset stomach. Significant differences in prevalence rates were found for the remaining nine side effects. An examination of the post hoc contrasts provides more detailed information on the effects of a specific analgesic prescription on the prevalence of each side effect. For example, the prevalence of constipation was significantly less in the no opioid group compared to the only ATC and the ATC + PRN opioid groups. A similar pattern was observed for feeling drowsy and poor coordination.
Table 4.
Prevalence of Analgesic Side Effects by Type of Analgesic Prescription
| Side effect | No Opioid n = 19 |
Only PRN Opioid n = 72 |
Only ATC Opioid n = 32 |
ATC + PRN Opioid N = 50 |
Statistic |
|---|---|---|---|---|---|
| % | % | % | % | ||
| Difficulty concentrating | 10.5 | 26.8 | 41.9 | 52.1 | X2=14.0, P =0.003 |
| Lack of energy | 52.6 | 57.7 | 71.0 | 79.2 | X2=7.7, P =0.05 |
| Nightmares | 0.0 | 8.5 | 6.7 | 24.5 | X2=11.6, P =0.009 |
| Nausea | 31.6 | 28.2 | 41.9 | 53.1 | X2=8.1, P =0.04 |
| Vomiting | 5.3 | 7.1 | 32.3 | 18.4 | X2=12.6, P =0.006 |
| Constipation | 15.8 | 44.3 | 54.8 | 63.3 | X2=13.4, P =0.004 |
| Difficulty sleeping | 56.2 | 47.9 | 45.2 | 47.9 | X2=.26, P =0.97 |
| Feeling drowsy | 31.6 | 57.1 | 71.0 | 83.3 | X2=18.6, P <0.0001 |
| Light headedness | 10.5 | 23.9 | 48.4 | 26.5 | X2=10.0, P =0.02 |
| Poor coordination | 5.3 | 26.8 | 38.7 | 39.6 | X2=9.1, P =0.03 |
| Indigestion/upset stomach | 31.6 | 38.0 | 51.6 | 47.9 | X2=3.1, P =0.37 |
ATC = around-the-clock; PRN = pro re nata.
Post hoc contrasts:
Difficulty concentrating – only PRN < ATC + PRN (P =0.007).
Lack of energy – no opioid and only PRN < ATC + PRN (both P ≤ 0.004).
Nightmares - no significant pair wise differences were found in the post hoc contrasts using the Bonferroni criteria.
Nausea – only PRN < ATC + PRN (P =0.008).
Vomiting - no significant pair wise differences were found in the post hoc contrasts using the Bonferroni criteria.
Constipation – no opioid < only ATC and ATC + PRN (both P ≤ 0.008).
Feeling drowsy – no opioid < only ATC and ATC + PRN (both P ≤ 0.009); only PRN < ATC + PRN (P =0.003).
Light headedness – no opioid < only ATC (P = 0.007).
Poor coordination – no opioid < only ATC and ATC + PRN (both P ≤ 0.009).
Severity of Analgesic Side Effects
Table 5 provides data on the severity of each of the eleven side effects by type of analgesic prescription. Side effect severity scores could range from 0 (did not have) to 4 (very severe). No significant differences in the severity scores for lack of energy, nausea, difficulty sleeping, or indigestion/upset stomach were found among the four prescription groups. Again, an examination of the post hoc contrasts provides more detailed information on the effects of analgesic prescriptions on the severity of side effects. For example, the severity of difficulty concentrating, constipation, feeling drowsy, and poor coordination were significantly less in the no opioid group compared to the ATC + PRN opioid group.
Table 5.
Differences in the Severity of Analgesic Side Effects by Type of Analgesic Prescription
| Side effect | No Opioid n = 19 |
Only PRN Opioid n = 72 |
Only ATC Opioid n = 32 |
ATC + PRN Opioid n = 50 |
Statistic |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Difficulty concentrating | 0.11 (0.32) | 0.38 (0.68) | 0.58 (0.77) | 0.85 (0.95) | KW=15.5, P =0.001 |
| Lack of energy | 1.05 (1.13) | 1.11 (1.08) | 1.29 (1.10) | 1.56 (1.03) | KW=5.9, P =0.12 |
| Nightmares | 0.00 (0.00) | 0.13 (0.45) | 0.71 (1.04) | 0.39 (0.80) | KW=11.3, P =0.01 |
| Nausea | 0.58 (0.96) | 0.45 (0.84) | 0.71 (1.04) | 0.78 (0.87) | KW=6.7, P =0.08 |
| Vomiting | 0.11 (0.46) | 0.11 (0.44) | 0.48 (0.85) | 0.35 (0.83) | KW=11.9, P =0.008 |
| Constipation | 0.37 (0.90) | 0.74 (1.02) | 1.10 (1.19) | 1.37 (1.32) | KW=14.1, P =0.003 |
| Difficulty sleeping | 0.89 (1.05) | 0.77 (0.93) | 0.71 (0.97) | 0.77 (0.95) | KW=0.4, P =0.93 |
| Feeling drowsy | 0.63 (1.01) | 0.91 (0.97) | 1.16 (0.90) | 1.46 (0.85) | KW=15.9, P =0.001 |
| Light headedness | 0.16 (0.50) | 0.34 (0.65) | 0.61 (0.72) | 0.33 (0.69) | KW=9.3, P =0.03 |
| Poor coordination | 0.05 (0.23) | 0.31 (0.55) | 0.48 (0.68) | 0.54 (0.82) | KW=9.5, P =0.02 |
| Indigestion/upset stomach | 0.42 (0.69) | 0.61 (0.92) | 0.84 (1.00) | 0.67 (0.86) | KW=3.0, P =0.40 |
ATC = around-the-clock; PRN = pro re nata; SD = standard deviation.
Post hoc contrasts:
Difficulty concentrating – no opioid and only PRN < ATC + PRN (both P ≤ 0.003).
Nightmares - no significant pair wise differences were found in the post hoc contrasts using the Bonferroni criteria.
Vomiting – only PRN < only ATC (P =0.001).
Constipation – no opioid and only PRN < only ATC + PRN (both P ≤ 0.008).
Feeling drowsy – no opioid and only PRN < ATC + PRN (both P ≤ 0.002).
Light headedness – no significant pair wise differences were found in the post hoc contrasts using the Bonferroni criteria.
Poor coordination – no opioid < only ATC and ATC + PRN (both P ≤ 0.009).
Relationships Between Severity of Analgesic Side Effects and Opioid Dose
The relationships between the severity of the eleven side effects and the total dose of opioid prescribed and taken are listed in Table 6. Significant correlations were found between both the total dose of opioid prescribed and the total dose of opioid taken and the severity scores for difficulty concentrating, nausea, vomiting, constipation, feeling drowsy, and poor coordination. No significant correlations were found between either the total dose of opioid prescribed or the total dose of opioid taken and the severity scores for lack of energy, nightmares, difficulty sleeping, light headedness, or indigestion/upset stomach.
Table 6.
Spearman Correlations Between the Severity of Side Effects and Total Dose of Opioid Prescribed and Taken
| Side Effect | Total Dose of Opioid Prescribed | Total Dose of Opioid Taken |
|---|---|---|
| Difficulty concentrating | 0.29; P <0.0001 | 0.36; P <0.0001 |
| Lack of energy | 0.15; NS | 0.13; NS |
| Nightmares | 0.15; NS | 0.16; NS |
| Nausea | 0.23; P =0.006 | 0.25; P =0.003 |
| Vomiting | 0.19; P =0.019 | 0.22; P =0.007 |
| Constipation | 0.23; P =0.005 | 0.30; P <0.0001 |
| Difficulty sleeping | −0.01; NS | −0.11; NS |
| Feeling drowsy | 0.32; P <0.0001 | 0.37; P <0.0001 |
| Light headedness | 0.05; NS | 0.12; NS |
| Poor coordination | 0.18; P =0.03 | 0.23; P =0.005 |
| Indigestion/upset stomach | 0.16; NS | 0.13; NS |
NS = not significant
Discussion
This study is the first to describe the prevalence and severity of side effects by the type of analgesic prescription as well as the relationships between the severity of opioid-induced side effects and the total dose of opioids prescribed and taken in a sample of oncology outpatients with chronic cancer pain. Previous studies have reported limited information on the prevalence and severity of analgesic side effects usually as part of adverse event reporting in the context of an analgesic trial.
The prevalence rates for the various side effects found in this study are similar to those reported in previously published systematic reviews of cancer pain management (1,4). As shown in previous studies, the prevalence rates for the majority of analgesic side effects ranged between 25% and 80% despite recommendations in clinical practice guidelines to treat these side effects aggressively (3). Of note, the highest prevalence rates and severity ratings for the majority of the side effects were found in the only ATC and ATC + PRN groups. Patients in these two prescription groups reported prevalence rates between 24.5% and 83.3% for all eleven side effects. These data suggest that patients with ATC + PRN or only ATC opioid prescriptions are at the greatest risk for analgesic side effects. In addition, findings from this study suggest that patients with a poor functional status, who in this patient sample with bone metastasis may have had more extensive disease and pain severity scores that warranted the use of higher doses of opioid analgesics, may be at greater risk for more severe side effects. The associations between functional status, opioid analgesic intake, and side effects warrant additional research.
Significant positive correlations were found between the total dose of opioid prescribed and taken for six of the eleven side effects, suggesting that a higher dose of an opioid medication is also a risk factor for many side effects. In this study, the mean total opioid dose (mg/day in morphine equivalents) prescribed and taken for the ATC + PRN analgesic group was 443.9 mg (± 453.3) and 264.3 mg (± 358.8), respectively. The dose of opioids taken by patients in the ATC + PRN group was three and a half times higher than that in the only ATC group and seventeen times higher than that in the only PRN group. While this study demonstrates that both prescription type (i.e., only ATC and ATC + PRN) and total opioid dose are associated with more severe analgesic-induced side effects, additional research is needed to determine the relative contribution of these two factors to the prevalence and severity of each side effect.
The possible range for the severity ratings of each of the analgesic side effects was 0 (did not have) to 4 (very severe). As shown in Table 5, the majority of the side effects, while prevalent, had extremely low mean severity ratings. The low severity ratings may be explained in part because the rating of “did not have” was included in the calculation of the mean severity ratings. When mean severity ratings were calculated for only those patients that actually had the side effect, the mean severity ratings ranged between 1.25 (i.e., poor coordination) and 1.95 (i.e., constipation), indicating that on average these side effects were all in the slight to moderate range.
Another possible explanation for the low severity ratings is that these patients may have become tolerant to some of the side effects or were using strategies to overcome these side effects. A limitation of this study is that detailed information was not collected in a systematic fashion on the duration of the analgesic prescription prior to enrollment into this study or on specific side effect management strategies. However, based on qualitative analyses of data from this study (2,18), one of the many obstacles to adequate pain management that patients reported was unrelieved analgesic side effects, particularly constipation. Another limitation of this study is that while patients were asked to attribute their side effects to their analgesic medications, we cannot guarantee that other factors (e.g., disease progression, co-morbidities, or side effects of cancer treatment or other medications) did not contribute to these reports. However, the differences in the prevalence and severity of many of these side effects found in this study suggest that these side effects were associated with analgesic intake.
It should be noted that findings from several studies suggest that sex (19-24) and ethnic (25-28) differences exist in the prevalence and severity of side effects. For example, women were found to be more sensitive to the respiratory effects of morphine (22). Since the majority of the patients in this study were female and Caucasian, gender and ethnic differences in the side effect of analgesics were not evaluated but do warrant investigation in future studies.
In conclusion, as noted in previous reviews (1,4) side effects of analgesic medications used to control cancer pain are a barrier to adequate pain control and have a negative impact on patients' quality of life. Despite practice guidelines that recommend early and aggressive treatment of analgesic side effects (3), side effects continue to occur in the majority of oncology patients. Regardless of the type of analgesic prescription, prevalence rates were high for most of the side effects, with the highest rates and severity in the only ATC and ATC + PRN opioid groups. Severity ratings for these side effects remained in the mild to moderate range regardless of the opioid prescription group and related treatments. In addition, the severity of many of the side effects was strongly related to higher total opioid dose prescribed and taken.
Findings from this study suggest that significant risk factors for both prevalence and severity of analgesic side effects are ATC and ATC + PRN prescription types and total opioid dose. An understanding of the risk factors for analgesic side effects must play a role in the ongoing management of cancer pain. Future research on analgesic side effects needs to obtain more detailed information on the severity of these side effects (e.g., have patients rate the severity of the side effects using a 0 to 10 numeric rating scale) and on effective and ineffective management strategies.
Acknowledgments
The authors would like to acknowledge the support and assistance of all of the physicians and nurses at our study sites as well as all of our project staff. We are especially grateful to all of the patients and family caregivers who participated in this study.
Footnotes
This study was supported by a grant (CA64734) from the National Cancer Institute. Additional support for the corresponding author's program of research was provided through unrestricted grants from Janssen Pharmaceutica and Purdue Pharma LP.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cherny N, Ripamonti C, Pereira J, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–2554. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher KL, Koresawa S, West C, et al. Putting cancer pain management regimens into practice at home. J Pain Symptom Manage. 2002;23:369–382. doi: 10.1016/s0885-3924(02)00385-8. [DOI] [PubMed] [Google Scholar]
- 3.Miaskowski C, Cleary J, Burney R, et al. Guideline for the management of cancer pain in adults and children. Vol. 3. American Pain Society; Glenview, IL: 2005. [Google Scholar]
- 4.McNicol E, Horowicz-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4:231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 5.Clark AJ, Ahmedzai SH, Allan LG, et al. Efficacy and safety of transdermal fentanyl and sustained-release oral morphine in patients with cancer and chronic non-cancer pain. Curr Med Res Opin. 2004;20:1419–1428. doi: 10.1185/030079904X2114. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA. 2005;293:3043–3052. doi: 10.1001/jama.293.24.3043. [DOI] [PubMed] [Google Scholar]
- 7.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–1051. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palos GR, Mendoza TR, Cantor SB, Aday LA, Cleeland CS. Perceptions of analgesic use and side effects: what the public values in pain management. J Pain Symptom Manage. 2004;28:460–473. doi: 10.1016/j.jpainsymman.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Boureau F, Saudubray F, d'Arnoux C, et al. A comparative study of controlled-release morphine (CRM) suspension and CRM tablets in chronic cancer pain. J Pain Symptom Manage. 1992;7:393–399. doi: 10.1016/0885-3924(92)90018-d. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TD, MacDonald N, Bruera E, et al. A controlled study of sustained-release morphine sulfate tablets in chronic pain from advanced cancer. Am J Clin Oncol. 1992;15:268–272. doi: 10.1097/00000421-199206000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Miaskowski C, Dodd M, West C, et al. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22:1713–1720. doi: 10.1200/JCO.2004.06.140. [DOI] [PubMed] [Google Scholar]
- 13.Rustoen T, Moum T, Padilla G, Paul S, Miaskowski C. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manage. 2005;30:234–242. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.West CM, Dodd MJ, Paul SM, et al. The PRO-SELF(c): Pain Control Program--an effective approach for cancer pain management. Oncol Nurs Forum. 2003;30:65–73. doi: 10.1188/03.ONF.65-73. [DOI] [PubMed] [Google Scholar]
- 15.Karnofsky D. Performance scale. In: Kennealey GT, Mitchell MS, editors. Factors that influence the therapeutic response in cancer. Plenum Press; New York, NY: 1977. [Google Scholar]
- 16.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 17.Schumacher KL, Koresawa S, West C, et al. The usefulness of a daily pain management diary for outpatients with cancer-related pain. Oncol Nurs Forum. 2002;29:1304–1313. doi: 10.1188/02.ONF.1304-1313. [DOI] [PubMed] [Google Scholar]
- 18.Schumacher KL, West C, Dodd M, et al. Pain management autobiographies and reluctance to use opioids for cancer pain management. Cancer Nurs. 2002;25:125–133. doi: 10.1097/00002820-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Zun LS, Downey LV, Gossman W, Rosenbaumdagger J, Sussman G. Gender differences in narcotic-induced emesis in the ED. Am J Emerg Med. 2002;20:151–154. doi: 10.1053/ajem.2002.32631. [DOI] [PubMed] [Google Scholar]
- 20.Fillingim RB, Ness TJ, Glover TL, et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Cepeda MS, Farrar JT, Baumgarten M, et al. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74:102–112. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 22.Zacny JP. Morphine responses in humans: a retrospective analysis of sex differences. Drug Alcohol Depend. 2001;63:23–28. doi: 10.1016/s0376-8716(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 23.Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–547. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Sarton E, Olofsen E, Romberg R, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93:1245–1254. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Cepeda MS, Farrar JT, Roa JH, et al. Ethnicity influences morphine pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2001;70:351–361. [PubMed] [Google Scholar]
- 26.Lee A, Gin T, Oh TE. Opioid requirements and responses in Asians. Anaesth Intensive Care. 1997;25:665–670. doi: 10.1177/0310057X9702500613. [DOI] [PubMed] [Google Scholar]
- 27.Zhou HH, Sheller JR, Nu H, Wood M, Wood AJ. Ethnic differences in response to morphine. Clin Pharmacol Ther. 1993;54:507–513. doi: 10.1038/clpt.1993.182. [DOI] [PubMed] [Google Scholar]
- 28.Yue QY, Svensson JO, Alm C, Sjoqvist F, Sawe J. Interindividual and interethnic differences in the demethylation and glucuronidation of codeine. Br J Clin Pharmacol. 1989;28:629–637. doi: 10.1111/j.1365-2125.1989.tb03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]