Abstract
Background
Selenium is a potential chemopreventive agent against prostate cancer, whose chemoprotective effects are possibly mediated through the antioxidative properties of selenoenzymes. Interrelations with other antioxidative agents and oxidative stressors, such as smoking, are poorly understood.
Objectives
The aims were to investigate the association between serum selenium and prostate cancer risk and to examine interactions with other antioxidants and tobacco use.
Design
A nested case-control study was performed within the screening arm of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Serum selenium in prospectively collected samples was compared between 724 incident prostate cancer case subjects and 879 control subjects, frequency-matched for age, time since initial screen, and year of blood draw. The men were followed for up to 8 y.
Results
Overall, serum selenium was not associated with prostate cancer risk (P for trend = 0.70); however, higher serum selenium was associated with lower risks in men reporting a high (more than the median: 28.0 IU/d) vitamin E intake [odds ratio (OR) for the highest compared with the lowest quartile of selenium: 0.58; 95% CI: 0.37, 0.91; P for trend = 0.05; P for interaction = 0.01] and in multivitamin users (OR for highest compared with the lowest quartile of selenium: 0.61; 95% CI: 0.36, 1.04; P for trend = 0.06; P for interaction = 0.05). Furthermore, among smokers, high serum selenium concentrations were related to reduced prostate cancer risk (OR for the highest compared with the lowest quartile of selenium: 0.65; 95% CI: 0.44, 0.97; P for trend = 0.09; P for interaction = 0.007).
Conclusion
Greater prediagnostic serum selenium concentrations were not associated with prostate cancer risk in this large cohort, although greater concentrations were associated with reduced prostate cancer risks in men who reported a high intake of vitamin E, in multivitamin users, and in smokers.
Keywords: Selenium, prostate cancer, vitamin E, smoking, serum, nested case-control study
INTRODUCTION
Interest in selenium as a nutrient with potential preventive effects against prostate cancer was heightened in the mid-1990s, after reports from the Nutritional Prevention of Cancer Trial showed that men who received 200 μg selenium/d had a significantly reduced risk of this disease (1–3). This trial was conducted in areas of the southeast United States notable for low soil content of selenium. Lower risks were found only among participants with low baseline concentrations of serum selenium (1, 3, 4). Further epidemiologic evidence for the preventive role of selenium in selenium-poor populations comes from studies conducted in malnourished populations in Linxian, China (5), where combined intervention with selenium, vitamin E, and β -carotene was related to reduced incidence and mortality of gastric cancer and total cancer.
Results from the Linxian trial suggesting an anticarcinogenic activity of selenium, perhaps in combination with vitamin E or other antioxidants (5), was supported by data from non-prostate cancer animal models that showed reduced tumor development related to treatment with the combination of selenium and vitamin E (6–8). Although these and recent studies in prostate cancer cell lines (9–11) point to synergistic effects of selenium and other antioxidants, specifically vitamin E, support from observational studies is limited (12, 13).
The chemopreventive effects of selenium may be due to its roles in cell cycle arrest, decreasing proliferation, inducing apoptosis, facilitating DNA repair by activation of p53, disruption of androgen receptor signaling, and being a key component of selenoenzymes (14–23), which incorporate selenium as selenocysteine, an infrequently occurring amino acid, into their active center (24–26). The unique redox characteristics of selenocysteine confer important antioxidant properties to these selenoenzymes, such as glutathione peroxidases, selenoprotein P, and thioredoxin reductase, which are all expressed in the prostate (26–31).
Because oxidative stress increases with androgen exposure (32–34), a putative risk factor for prostate cancer, the antioxidative activity of selenoenzymes may be particularly relevant for prevention of this disease. Also, the preventive effect of selenium could be modified by exposure to oxidative stress, eg, by smoking, or to intake of other antioxidative nutrients such as vitamin E (35–38).
SUBJECTS AND METHODS
Study setting
This nested case-control study was conducted within the screening arm of the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Trial, a randomized trial to evaluate the effectiveness of prostate, lung, colorectal, and ovarian cancer screening and to investigate etiologic factors and early markers of cancer (39). Participants in the PLCO Trial, aged 55–74 y, were recruited at 10 centers in the United States (Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St Louis, MO; and Washington, DC) between September 1993 and June 2001.
Men who were randomly assigned to the screening arm of the trial were offered prostate cancer screening by serum prostate-specific antigen (PSA) at entry and annually for 5 y and digital rectal examination (DRE) at entry and annually for 3 y. If the PSA test result was ≥ 4 ng/mL or the DRE was suspicious for prostate cancer, the men were referred to their medical care providers for prostate cancer diagnostic evaluation. In addition, follow-up for recent diagnosis of cancer was carried out by annual mailed questionnaires and through periodic searches of the National Death Index. All medical and pathologic records related to the diagnosis were obtained for the participants suspected of having prostate cancer by either screening examination or annual questionnaire. Furthermore, death certificates and supporting medical or pathologic records were collected. Data related to the diagnosis of prostate cancer were abstracted by trained medical record specialists. All trial participants are followed for incidence of cancer and all causes of mortality for ≥ 13 y from the randomization date. The screening arm participants were asked to provide a blood sample at each screening visit. The institutional review boards of the US National Cancer Institute and the 10 study centers approved the trial and the participants provided written informed consent.
Study population
Of the 38 352 men randomly assigned to the screening arm of the trial, we excluded men reporting a history of prostate cancer (other than nonmelanoma skin cancer), men whose first valid screen (PSA test or DRE) was after 1 October 2001 (the censor date for the present analysis), men who received a screening exam but for whom there was no subsequent contact, men who did not complete a baseline risk factor questionnaire, men with an ethnic or racial background other than non-Hispanic white or non-Hispanic black, men without a signed informed consent for etiologic studies on cancer, and men without any blood collections for etiological studies at any of the screening visits. After exclusions, the analytic cohort comprised 26 975 men. All men were followed from their initial valid prostate cancer screen (PSA, DRE, or both), to first occurrence of prostate cancer, loss-to-follow-up, death, or censor date (1 October 2001), whichever came first. Case subjects are men diagnosed with adenocarcinoma of the prostate. Staging procedures corresponded to the Tumor, Nodes, and Metastases stage of disease classification (40). Cases were defined as advanced prostate cancer if they were stages III or IV (regional or distant) or Gleason Score ≥ 7.
The eligible 26 975 men included 1320 prostate cancer cases. For the present study, we included non-Hispanic white prostate cancer cases diagnosed ≥ 1 y after baseline blood draw (n = 803). For comparison, we selected control subjects by incidence-density sampling (41) with a case-control ratio of 1:1.2, frequency-matched by age (5-y intervals), time since initial screening (1-y time windows), race, and year of blood draw (n = 949).
Serum selenium analysis
Serum selenium concentrations were determined by using an inductively coupled plasma mass spectrometry method [for details, see Stürup et al (42)]. Serum for selenium analysis was available for 724 (90.2%) cases and 879 (92.6%) controls. Cases and their matched controls were analyzed in the same batch to minimize interassay variability. Blinded quality control samples (15%) were randomly inserted within each batch and monitored throughout the analysis. The CV, estimated from 181 blinded duplicates, was 9.4%.
Assessment of questionnaire-based covariates
At enrollment, all participants were asked to complete a questionnaire to obtain information on age, ethnicity, education, occupation, current and past smoking behavior, history of cancer and other diseases, use of selected drugs, recent history of screening exams, and prostate related health factors. Usual dietary intake over the 12 mo before enrollment was assessed with a 137-item food-frequency questionnaire, which included 14 additional questions about intake of vitamin and mineral supplements and 10 additional questions on meat cooking practice (43). Daily dietary nutrient intake was calculated by multiplying the daily frequency of each consumed food item by the nutrient value of the sex-specific portion size (44) with the use of the nutrient database from the US Department of Agriculture (45). Total vitamin and mineral intake was calculated by adding dietary and supplemental intake. Multivitamin (and mineral) users were defined as men taking a one-a-day type vitamin, therapeutic type vitamin, high-dose type vitamin, stresstabs, or B-complex in the last 2 y before enrollment (yes or no). Within a subset of controls, the partial Spearman correlation between intake of β-carotene, lycopene, and α -tocopherol and serum concentrations was 0.44, 0.31, and 0.58, respectively (coefficients were adjusted for months of blood draw, serum cholesterol concentrations, smoking, body mass index (BMI), age, and energy intake).
Statistical analysis
Adjusted means (least-squares means) were calculated by linear models. We used conditional logistic regression models to estimate odds ratios (ORs) of prostate cancer. Serum selenium was modeled as quartiles based on the distribution among the controls. We used the continuous variable to estimate for linear trend. All P values are two-sided. The analyses were conditioned on the matching factors (age, time since initial screening, and year of blood draw) and adjusted for study center. We evaluated confounding due to potential risk factors for prostate cancer, including average numbers of prostate cancer screening, family history of prostate cancer, educational attainment, physical activity, BMI, aspirin and ibuprofen use, diabetes, alcohol, smoking, energy, fat, tomatoes, fruit and vegetable intake, dairy products, red meat, heterocyclic amines from meat, vitamin E, β -carotene, lycopene, and calcium. None of the factors changed the β coefficient of the risk estimates of selenium by > 10%, and, therefore, none of these factors were included in the analyses. To explore potential effect modification by smoking, reported intake of antioxidants, and multivitamin use, we performed stratified analyses and evaluated the statistical significance of multiplicative interactions by comparison of the − 2 log likelihood statistics of the main effect model for selenium with that of the joint effects model, including cross-product terms. All analyses were carried out with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
The average age of controls was 65 y and did not vary significantly by quartile of serum selenium. Reported intake of β -carotene, lycopene, and vitamin E tended to be higher in men with high selenium concentrations than in men with low selenium concentrations, whereas BMI, energy, and red meat and alcohol intake was lower in men with high serum selenium concentrations (Table 1). Other baseline study characteristics were not significantly different across quartiles of serum selenium. Compliance with the PLCO screening protocol also did not vary significantly by selenium concentrations and was very high because the average number of screens per year was close to 1—the goal for the screening intervention. The average serum selenium concentrations in the study population (controls) was 141.3 ng/mL, with mean serum selenium concentrations significantly higher in areas with high soil selenium content (146.8 ng/mL) than in those with intermediate and low soil selenium content areas (136.8 ng/mL; P for mean difference < 0.0001; Table 2). Median serum selenium concentrations of the 4th quartile (170.4 ng/mL) was 50% higher than the 1st quartile (113.7 ng/mL; Table 3).
TABLE 1.
Distribution of study characteristics in the control subjects according to quartile (Q) of serum selenium1
| Serum selenium
|
|||||
|---|---|---|---|---|---|
| Q1 (50.5 to < 126.8) (n = 219) | Q2 (≥ 126.8 to < 141.9) (n = 220) | Q3 (≥ 141.9 to < 158.0) (n = 220) | Q4 (≥ 158.0 to 253.0) (n = 220) | P for trend | |
| Median (ng/mL) | 113.7 | 135.3 | 149.4 | 170.4 | |
| Age (y) | 64.8 ± 1.42 | 64.7 ± 1.4 | 64.7 ± 1.4 | 64.7 ± 1.4 | 0.34 |
| Prostate cancer screens (no./y)3 | 0.95 ± 0.1 | 0.97 ± 0.1 | 0.96 ± 0.1 | 0.95 ± 0.1 | 0.79 |
| Family history of prostate cancer (%) | 6.2 | 3.6 | 5.0 | 7.6 | 0.45 |
| Some college-level education (%) | 75.7 | 78.1 | 70.2 | 77.3 | 0.82 |
| BMI (kg/m2) | 27.8 ± 3.9 | 27.4 ± 3.9 | 27.2 ± 3.9 | 27.2 ± 3.9 | 0.07 |
| Vigorous physical activity ≥ 1 h/wk (%) | 67.5 | 70.5 | 68.6 | 70.9 | 0.56 |
| Aspirin use ≥ 1 times/wk (%) | 47.3 | 44.7 | 47.0 | 50.4 | 0.45 |
| Ibuprofen use ≥ 1 times/wk (%) | 16.0 | 10.8 | 12.4 | 15.9 | 0.91 |
| Smoking status (%) | |||||
| Never | 34.1 | 24.2 | 33.2 | 32.8 | 0.73 |
| Current | 8.7 | 11.2 | 7.9 | 7.7 | 0.46 |
| Former | 47.5 | 53.2 | 49.6 | 54.0 | 0.30 |
| Pipe and cigar (never cigarettes) | 9.7 | 11.4 | 9.3 | 5.6 | 0.11 |
| Alcohol use ≥ 15 g/d (%) | 33.5 | 33.6 | 30.9 | 27.9 | 0.17 |
| Energy (kcal/d) | 2380 ± 930 | 2409 ± 928 | 2385 ± 928 | 2228 ± 916 | 0.09 |
| Total fat (g/d) | 79.6 ± 17.2 | 78.4 ± 17.1 | 80.9 ± 16.9 | 78.1 ± 17.3 | 0.70 |
| Fruit (servings/d) | 3.4 ± 2.2 | 3.5 ± 2.2 | 3.5 ± 2.2 | 3.7 ± 2.2 | 0.08 |
| Vegetables (servings/d) | 5.4 ± 2.0 | 5.5 ± 2.0 | 5.3 ± 2.0 | 5.7 ± 2.0 | 0.46 |
| Red meat intake (g/d) | 103.2 ± 58.1 | 97.7 ± 58.0 | 98.1 ± 57.3 | 91.7 ± 58.5 | 0.06 |
| Dietary lycopene (μg/d) | 11 166 ± 6137 | 11 594 ± 6127 | 11 424 ± 6048 | 11 631 ± 6181 | 0.51 |
| Dietary β -carotene (μg/d) | 5041 ± 2576 | 4980 ± 2572 | 4942 ± 2539 | 5351 ± 2594 | 0.27 |
| Total vitamin E intake (IU/d) | 128 ± 242 | 141 ± 242 | 153 ± 239 | 198 ± 244 | 0.004 |
| Calcium (mg/d) | 1171 ± 452 | 1176 ± 452 | 1188 ± 446 | 1180 ± 456 | 0.79 |
All values other than age were standardized for age and study center. Total fat, fruit, vegetables, red meat, dietary lycopene, dietary β-carotene, total vitamin E, and calcium intakes were also standardized for energy intake.
x¯ ± SE (all such values).
Average number of prostate cancer screening examinations (prostate-specific antigen, digital rectal examination, or both) from enrollment until the year of diagnosis (cases) or until the study year in which a control was selected. Maximal period is limited to period of active screening (years 0–5).
TABLE 2.
Mean (± SD) serum selenium concentrations by study center in control subjects
| Selenium (ng/mL)1 | |
|---|---|
| Overall | 141.3 ± 26.0 |
| Soil selenium content2 | |
| Intermediate or low | 136.8 ± 27.2 |
| Birmingham, AL | 128.8 ± 28.3 |
| Washington, DC | 133.8 ± 26.9 |
| Pittsburgh, PA | 132.2 ± 27.0 |
| Detroit, MI | 139.9 ± 27.4 |
| Salt Lake City, UT | 139.7 ± 27.3 |
| High | 146.8 ± 27.23 |
| Marshfield, WI | 146.4 ± 27.0 |
| Minneapolis, MN | 150.0 ± 27.0 |
| Denver, CO | 140.8 ± 27.0 |
Conditioned on matching factors of age, time since initial screening, and year of blood draw.
Defined on the basis of the National Geochemical Survey (46).
Significantly different from low or intermediate soil selenium content, P < 0.0001.
TABLE 3.
Odds ratios (ORs) of prostate cancer according to quartiles (Q) of serum selenium, overall and by tumor characteristics1
| Serum selenium
|
|||||
|---|---|---|---|---|---|
| Q12 (50.5 to < 126.8 ng/mL) | Q2 (≥ 126.8 to < 141.9 ng/mL) | Q3 (≥ 141.9 to < 158 ng/mL) | Q4 (≥ 158 to 253 ng/mL) | P for trend3 | |
| Median (ng/mL) | 113.7 | 135.3 | 149.4 | 170.4 | |
| Overall | |||||
| Controls (n) | 219 | 220 | 220 | 220 | |
| Cases (n) | 195 | 189 | 198 | 142 | |
| OR | 1.00 | 0.95 | 1.13 | 0.84 | 0.70 |
| 95% CI | — | 0.72, 1.27 | 0.85, 1.51 | 0.62, 1.14 | |
| Nonadvanced4 | |||||
| Cases (n) | 118 | 118 | 111 | 88 | |
| OR | 1.00 | 0.98 | 1.03 | 0.85 | 0.61 |
| 95% CI | — | 0.70, 1.37 | 0.73, 1.46 | 0.60, 1.22 | |
| Advanced5 | |||||
| Cases (n) | 72 | 71 | 84 | 51 | |
| OR | 1.00 | 0.97 | 1.31 | 0.84 | 0.73 |
| 95% CI | — | 0.65, 1.46 | 0.88, 1.95 | 0.54, 1.30 | |
| Stage III or IV | |||||
| Cases (n) | 26 | 19 | 36 | 14 | |
| OR | 1.00 | 0.71 | 1.53 | 0.62 | 0.57 |
| 95% CI | — | 0.37, 1.37 | 0.85, 2.73 | 0.30, 1.29 | |
ORs were obtained from multivariate-adjusted conditional logistic regression analysis including age, time since initial screening, year of blood draw, and study center.
Reference category.
Obtained by using serum selenium as a continuous variable.
Defined as stage I or II and Gleason score < 7 (n = 435).
Defined as stage III or IV or Gleason score ≥ 7 (n = 278).
Serum selenium was not associated significantly with prostate cancer incidence overall: men in the highest quartile had a non-significant 16% reduction in prostate cancer risk compared with men in the lowest quartile of serum selenium, and there was no suggestion of a linear trend (P for trend = 0.70; Table 3). Similarly, no significant association with serum selenium was observed for advanced prostate cancer (stage III and IV OR in a comparison of the highest with the lowest quartile: 0.62; P for trend = 0.57). When stratified by study areas with high and intermediate or low soil selenium content, serum selenium was not significantly associated with prostate cancer in either group (study regions with high soil selenium content: OR for the highest compared with the lowest quartile of selenium: 0.68; 95% CI: 0.42, 1.09; P for trend = 0.42; study regions with intermediate or low soil selenium content: OR for the highest compared with the lowest quartile of selenium: 0.96; 95% CI: 0.63, 1.47; P for trend = 0.82).
The association between serum selenium and incident prostate cancer did not differ significantly by total reported intakes of vitamin C, β-carotene, or lycopene (Table 4); however, an inverse association between serum selenium and prostate cancer (OR for the highest compared with the lowest quartile: 0.58; 95% CI: 0.37, 0.91; P for trend = 0.05) was observed in men who reported a high intake of total vitamin E (equal to or more than the median, which was 28.0 IU/d—a dose similar to the one used in the Alpha-tocopherol, Beta-carotene Trial), showing a significant interaction between vitamin E and selenium (P for interaction = 0.01). High serum selenium was also nonsignificantly associated with a lower risk of prostate cancer in men taking multivitamins (OR for the highest compared with the lowest quartile of selenium: 0.61; 95% CI: 0.36, 1.04; P for trend = 0.06), but not in nonusers (P for interaction = 0.05). Because most vitamin E supplementation was in the form of multivitamins, we could not separate the effects of these 2 vitamin sources.
TABLE 4.
Odds ratios (ORs) of prostate cancer according to quartiles (Q) of serum selenium stratified by low and high intake of antioxidative vitamin and carotenoids and by multivitamin use1
| Serum selenium
|
||||||
|---|---|---|---|---|---|---|
| Q1 (50.5 to < 126.8 ng/mL) | Q2 (≥ 126.8 to < 141.9 ng/mL) | Q3 (≥ 141.9 to < 158.0 ng/mL) | Q4 (≥ 158.0 to 253.0 ng/mL) | P for trend2 | P for interaction | |
| Vitamin C3 | 0.79 | |||||
| Low | ||||||
| Cases/controls (n) | 97/120 | 101/110 | 91/107 | 68/94 | ||
| OR4 | 1.00 | 1.02 | 1.18 | 0.96 | 0.705 | |
| 95% CI | — | 0.68, 1.55 | 0.77, 1.82 | 0.61, 1.51 | ||
| High | ||||||
| Cases/controls (n) | 89/94 | 85/105 | 101/111 | 68/121 | ||
| OR4 | 1.00 | 0.87 | 1.10 | 0.71 | 0.705 | |
| 95% CI | — | 0.56, 1.36 | 0.71, 1.71 | 0.45, 1.13 | ||
| Vitamin E3 | 0.01 | |||||
| Low | ||||||
| Cases/controls (n) | 86/125 | 96/115 | 91/102 | 64/89 | ||
| OR4 | 1.00 | 1.26 | 1.44 | 1.34 | 0.14 | |
| 95% CI | — | 0.83, 1.94 | 0.93, 2.25 | 0.84, 2.14 | ||
| High | ||||||
| Cases/controls (n) | 100/89 | 90/100 | 101/116 | 72/126 | ||
| OR4 | 1.00 | 0.80 | 0.84 | 0.58 | 0.05 | |
| 95% CI | — | 0.51, 1.24 | 0.55, 1.30 | 0.37, 0.91 | ||
| β-Carotene3 | 0.86 | |||||
| Low | ||||||
| Cases/controls (n) | 93/111 | 87/106 | 94/114 | 62/100 | ||
| OR4 | 1.00 | 0.99 | 1.21 | 0.89 | 0.705 | |
| 95% CI | — | 0.64, 1.53 | 0.79, 1.87 | 0.56, 1.41 | ||
| High | ||||||
| Cases/controls (n) | 93/103 | 99/109 | 98/104 | 74/115 | ||
| OR4 | 1.00 | 1.00 | 1.04 | 0.85 | 0.705 | |
| 95% CI | — | 0.66, 1.52 | 0.68, 1.58 | 0.54, 1.32 | ||
| Lycopene3 | 0.72 | |||||
| Low | ||||||
| Cases/controls (n) | 95/110 | 100/95 | 86/110 | 73/117 | ||
| OR4 | 1.00 | 1.30 | 1.12 | 0.95 | 0.705 | |
| 95% CI | — | 0.85, 2.00 | 0.72, 1.73 | 0.61, 1.49 | ||
| High | ||||||
| Cases/controls (n) | 91/104 | 86/120 | 106/108 | 63/98 | ||
| OR4 | 1.00 | 0.81 | 1.26 | 0.84 | 0.705 | |
| 95% CI | — | 0.53, 1.24 | 0.80, 1.89 | 0.53, 1.34 | ||
| Multivitamin use | 0.05 | |||||
| No | ||||||
| Cases/controls (n) | 109/143 | 110/134 | 101/121 | 78/120 | ||
| OR4 | 1.00 | 1.12 | 1.29 | 1.13 | 0.16 | |
| 95% CI | — | 0.76, 1.65 | 0.87, 1.93 | 0.74, 1.71 | ||
| Yes | ||||||
| Cases/controls (n) | 77/71 | 76/81 | 91/97 | 58/95 | ||
| OR4 | 1.00 | 0.89 | 1.00 | 0.61 | 0.06 | |
| 95% CI | — | 0.54, 1.49 | 0.61, 1.63 | 0.36, 1.04 | ||
Twenty-four cases and 17 controls without data on dietary and supplemental intake were excluded from all analyses in this table. Quartile 1 was the reference category.
Conducted by using serum selenium as a continuous variable.
Low and high are defined as below or above the median, respectively. The median for vitamin C was 241.7 mg/d, that for vitamin E was 28.0 IU/d, that for β-carotene was 4817.8 μg/d, and that for lycopene was 9693.6 μg/d. Vitamin C, vitamin E, and β-carotene are based on dietary and supplemental intakes, and lycopene is based on dietary intake from a 137-item food-frequency questionnaire that included 14 additional questions on intakes of vitamin and mineral supplements (43).
Obtained from a multivariate conditional logistic regression analysis adjusted for age, time since initial screening, year of blood draw, and study center.
For stratified analyses with no significant interaction, the P for trend for the combined analysis per Table 3 (P for trend = 0.70) was provided.
An analysis stratified by smoking status is shown in Table 5. We observed an inverse association between selenium and prostate cancer in smokers (OR for the highest compared with the lowest quartile of selenium: 0.65; 95% CI: 0.44, 0.97; P for trend = 0.09), and selenium-related risks tended to increase nonsignificantly in men who never smoked (P for interaction = 0.007).
TABLE 5.
Odds ratios (ORs) of prostate cancer according to quartile (Q) of serum selenium stratified by smoking status1
| Quartile of Serum Selenium
|
||||||
|---|---|---|---|---|---|---|
| Smoking status | Q12 (50.5 to < 126.8 ng/mL) | Q2 (≥ 126.8 to < 141.9 ng/mL) | Q3 (≥141.9 to < 158.0 ng/mL) | Q4 (≥158.0 to 253.0 ng/mL) | P for trend3 | P for interaction |
| None | 0.007 | |||||
| Cases/controls (n) | 64/76 | 70/53 | 80/72 | 55/72 | ||
| OR4 | 1.00 | 1.65 | 1.50 | 1.32 | 0.15 | |
| 95% CI | — | 0.95, 2.86 | 0.86, 2.59 | 0.72, 2.40 | ||
| Smokers (current and former) | ||||||
| Cases/controls (n) | 119/123 | 103/142 | 100/127 | 76/135 | ||
| OR4 | 1.00 | 0.70 | 0.91 | 0.65 | 0.09 | |
| 95% CI | — | 0.48, 1.03 | 0.65, 1.35 | 0.44, 0.97 | ||
Men smoking pipe or cigar were excluded (57 cases and 79 controls).
Reference category.
Obtained by using serum selenium as a continuous variable.
Obtained from a multivariate conditional logistic regression analysis adjusted for age, time since initial screening, year of blood draw, and study center.
DISCUSSION
In this nested case-control study, which included 724 incidence prostate cancer cases and 879 controls, we observed no overall association between prediagnostic selenium concentrations and prostate cancer. However, greater serum selenium concentrations were associated with lower risks of this disease in men who reported a high vitamin E intake, in multivitamin users, and in smokers.
The strongest support for a chemopreventive effect of selenium in human prostate carcinogenesis comes from the Nutritional Prevention of Cancer Trial, a randomized study to evaluate selenium supplementation (200 μg/d) and skin cancer prevention, which found, as secondary endpoints, reduced risks of total cancer mortality (50%) and prostate cancer incidence (52% reduced risk; average intervention period 6.4 y, with 64 prostate cancer cases) (1, 3). In a second trial (SU.VI.MAX), no overall association was found with selenium supplementation; however, among men with a normal baseline PSA (< 3 ng/mL), the risk of prostate cancer was 48% lower in the group of selenium-treated men than in the group of placebo-treated men (47). The result of this study cannot be attributed directly to selenium (dose: 100 μg/d), because 5 other antioxidative vitamins and minerals were given simultaneously as a multivitamin supplement.
Several (12, 48–52), but not all (53, 54), case-control studies nested in prospective cohorts also showed inverse associations between serum selenium and prostate cancer risk, with several reporting stronger associations for advanced prostate cancer (most studies defined advanced cancer as stage III and IV disease)(48–51, 53). Of 3 retrospectively designed population-based case-control studies (55–57), only one (56) found a non-significant inverse association between serum selenium and prostate cancer.
Because the enzyme activity of some selenoenzymes, such as the glutathione peroxidases, tend to plateau at high serum selenium concentrations (58, 59), selenium supplementation may be most effective in populations with low selenium exposure. The Nutritional Prevention of Cancer Trial, which was conducted specifically in areas with low selenium intake, supported this hypothesis showing the strongest inverse associations with prostate cancer in men with low baseline serum selenium concentrations (1st tertile: < 106 ng/mL, and 2nd tertile: 106–121 ng/mL) and no association in men with high baseline concentrations (3rd tertile: > 121 ng/mL) (4). However, the inverse selenium-prostate cancer associations observed in epidemiologic studies do not appear to be limited to settings with low mean serum selenium concentrations (Figure 1 and Figure 2), and, from our study, the strongest inverse associations were noted in areas with high soil selenium content. Furthermore, it is unknown how these circulating concentrations translate to the prostate, which also expresses selenoenzymes not found in the circulating system, eg, selenoprotein 15 (60–62). In addition, selenium may also prevent prostate cancer directly through active selenium metabolites, in particular methylated forms; however, such effects, as shown in experimental studies, are achieved only at supranutritive doses (16, 20, 21).
FIGURE 1.
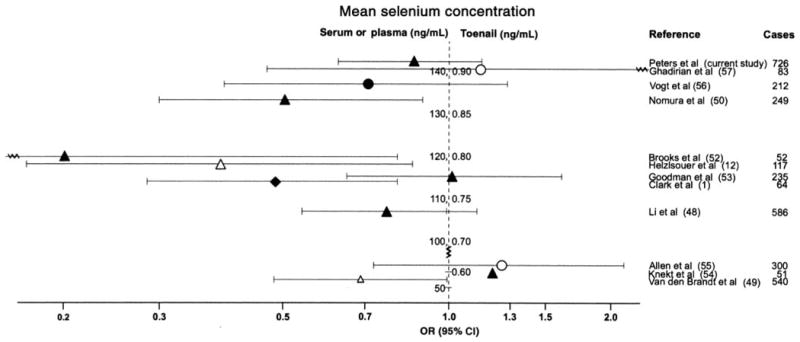
Clinical trial and observational studies on selenium and the risk of prostate cancer listed by mean or median selenium concentrations. ORs and 95% CIs were obtained in a comparison of the highest with the lowest quantile of selenium. ◆, randomized clinical trials with selenium concentrations obtained from serum or plasma; ▲, nested case-control study (CCS) and case cohort study with selenium concentrations obtained from serum or plasma; ●, population-based CCS with selenium concentrations obtained from serum or plasma; △, nested CCS and case cohort study with selenium obtained from toenails; ○, population-based CCS with selenium obtained from toenails. Observational studies were excluded if they used a questionnaire-based assessment of selenium intake, included patients with benign prostatic hyperplasia as controls, or included < 15 prostate cancer cases.
FIGURE 2.
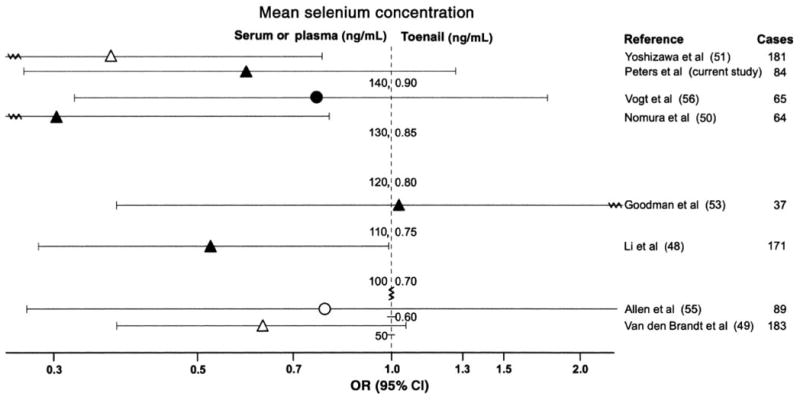
Observational studies on selenium and risk of advanced prostate cancer (1) listed by mean or median selenium concentrations. ORs and 95% CIs were obtained in a comparison of the highest with the lowest quantile of seleniuim. ▲, nested case-control study (CCS) and case cohort study with selenium concentrations obtained from serum or plasma; ●, population-based CCS with selenium concentrations obtained from serum or plasma; △, nested CCS and case cohort study with selenium concentrations obtained from toenails; ○, population-based CCS with selenium concentrations obtained from toenails. Observational studies were excluded if they used a questionnaire-based assessment of selenium intake, included patients with benign prostatic hyperplasia as controls, or included < 15 prostate cancer cases. Advanced cancer was defined as a tumor that extends through the prostatic capsule or as a metastatic disease (stage C or D, stage III or IV, or regional or distant stage); the exception was Vogt et al (55), who defined advanced cancer as regional or distant stage, Gleason Score ≥ 7, or both.
Our results suggest a synergistic relation between selenium and vitamin E, showing little evidence that one antioxidative nutrient can replace the other in prostate cancer prevention. Our finding is consistent with 2 observational studies (12, 13) and a nutritional intervention trial conducted in Linxian China (5), although not all observational studies (49, 51, 55, 56) found such interactions. However, our study lacks specificity on this point because most vitamin E supplementation was in the form of multivitamins, making it difficult to separate the effects of vitamin E from those of other multivitamin constituents. Because the Selenium and Vitamin E Cancer Prevention Trial, one of the largest ongoing intervention trials, makes a multivitamin without vitamin E and selenium, available to trial participants who prefer to continue using multivitamin while participating in the trial, this trial will be able to further explore interaction between selenium, vitamin E, and multivitamins. The trial is expected to be completed in 2013 (7).
We also observed a strong inverse association between serum selenium and prostate cancer risk in smokers. Although smoking itself was not associated with prostate cancer risk in the present study (data not shown) and several other studies (63), it is noteworthy that another antioxidant, vitamin E, is associated with reduced risk of this disease primarily in smokers, as seen in this cohort (64) and most other studies (65–70). Additional exploration of a 3-way interaction between vitamin E, smoking, and selenium was beyond the scope of the present study, because the numbers of cases and controls in these subgroups were small. Effect modification of the selenium-prostate cancer association by smoking was found in 3 observational studies (49, 50, 56), but not in another (51) and not in an investigation of prostate cancer risk in smokers and asbestos workers (53). Smoking results in increased exposure to radical oxidative species (35–38), and selenium inhibits the damaging effect of oxidative species on DNA and other biomolecules. The protective role of selenium in smokers could also be enhanced by the presence of oxidative-response elements in the promoter regions of genes encoding for selenoenzymes, such as GPX1 (71), and their increased transcription related to exposure to oxidative stressors (72–74).
Given an increasing nationwide distribution of foods, we were somewhat surprised to observe statistically significant differences in serum selenium concentrations by regional soil selenium content. However, this difference was not accounted for by regional differences in dietary pattern [eg, differences in the level of consumption of foods high in selenium, such as grains, eggs, meat, and fish (75)], because adjustment for these and other foods did not significantly change the results (data not shown).
A limitation of our study was the relatively short follow-up, to a maximum of 8 y. To avoid potential effects of disease on selenium concentrations, we only included cases diagnosed ≥ 1 y after blood draw; excluding cases diagnosed within the first 2 y showed similar results (data not shown). We only measured selenium at a single point in time, and multiple measurements ideally over the entire period of cancer development would have reduced the possibility of attenuated risk estimates due to random error. Stratified analysis by antioxidative nutrients was based on questionnaire data, which may introduce measurement error. Correlations of serum selenium with BMI and intakes of alcohol, red meat, vitamin E, β-carotene, lycopene, and energy suggest that combined lifestyle factors may contribute to prostate cancer prevention and that observational studies such as ours only incompletely control for unmeasured confounding. Clinical trials with selenium as an intervention could address this.
The present study was large (Figures 1 and 2), and the men studied had a broad range of serum selenium concentrations [almost as wide as the intervention effect in the Nutritional Prevention of Cancer Trial, in which mean serum selenium concentrations rose from 114 ng/mL at baseline to 190 ng/mL at the end of the intervention (1)]. By restricting our analysis to men randomly assigned to the screening arm of the trial, disease detection bias was limited. Compliance with the PLCO protocol for prostate cancer screening was very high and similar across quartiles of selenium. Our large sample size ensured sufficient power to observe ORs of ≤ 0.68 in comparisons of the 4th with the 1st quartile, similar to the summary OR of a recent meta-analysis (OR: 0.72) (76) and within the range of expected associations (Figure 1).
In conclusion, overall we observed no inverse association between prediagnostic serum selenium concentrations and the risk of prostate cancer in this large cohort, which was followed up by standardized screening procedures. However, higher serum selenium may be associated with lower prostate cancer risk in men who report a high intake of vitamin E, in multivitamin users, and in smokers.
Footnotes
From the Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (UP); the Department of Epidemiology, University of Washington, Seattle, WA (UP); the Johns Hopkins University School of Medicine, Baltimore, MD (CBF); the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Rockville, MD (NC, AS, and RBH); the Marshfield Clinic Research Foundation, Marshfield, WI (DR); the Washington University, St Louis, MO (GLA); the University of Colorado Health Sciences Center, Denver, CO (EDC); the Institute of Analytical Chemistry, The Danish University of Pharmaceutical Sciences, Copenhagen, Denmark (SS); and the Core Genotype Facility, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Gaithersburg, MD (SJC).
Supported by the US Department of Health and Human Services, National Cancer Institute/National Institutes of Health grant (PLCO Cancer Screening Trial) and Intramural Research Program.
Reprints not available. Address correspondence to U Peters, Cancer Prevention Program, Fred Hutchinson Cancer Research Center Research, PO Box 19024, 1100 Fairview Avenue North M4-B402, Seattle, WA 98109-1024. E-mail: upeters@fhcrc.org.
UP designed the study, supervised all aspects of the serum selenium analysis, conducted the statistical analysis of the data, and wrote the manuscript. CBF, AS, and SJC provided input in study design, data interpretation, and manuscript preparation. NC provided guidance in the statistical analysis, study design, data interpretation, and manuscript preparation. DR, GLA, and EDC were involved in the acquisition of data, interpretation of the data, and manuscript preparation. SS conducted the serum selenium analysis and provided input in data interpretation and manuscript preparation. RBH was instrumental in the study conception, data acquisition, study design, data interpretation, manuscript preparation, and overall supervision. None of the authors has a conflict of interest with the funding organization of this study.
References
- 1.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 2.Combs GF., Jr Status of selenium in prostate cancer prevention. Br J Cancer. 2004;91:195–9. doi: 10.1038/sj.bjc.6601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–9. [PubMed] [Google Scholar]
- 4.Clark LC, Dalkin B, Krongrad A, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–4. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 5.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 6.Horvath PM, Ip C. Synergistic effect of vitamin E and selenium in the chemoprevention of mammary carcinogenesis in rats. Cancer Res. 1983;43:5335–41. [PubMed] [Google Scholar]
- 7.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 8.Combs GF, Scott ML. Nutritional interrelationships of vitamin E and selenium. BioScience. 1977;27:467–73. [Google Scholar]
- 9.Richards LR, Benghuzzi H, Tucci M, Hughes J. The synergistic effect of conventional and sustained delivery of antioxidants on LNCaP prostate cancer cell line. Biomed Sci Instrum. 2003;39:402–7. [PubMed] [Google Scholar]
- 10.Venkateswaran V, Fleshner NE, Klotz LH. Synergistic effect of vitamin E and selenium in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2004;7:54–6. doi: 10.1038/sj.pcan.4500707. [DOI] [PubMed] [Google Scholar]
- 11.Zu K, Ip C. Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003;63:6988–95. [PubMed] [Google Scholar]
- 12.Helzlsouer KJ, Huang HY, Alberg AJ, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–23. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 13.Hartman TJ, Albanes D, Pietinen P, et al. The association between baseline vitamin E, selenium, and prostate cancer in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 1998;7:335–40. [PubMed] [Google Scholar]
- 14.Dong Y, Lee SO, Zhang H, Marshall J, Gao AC, Ip C. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 15.Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 16.Jiang C, Wang Z, Ganther H, Lu J. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61:3062–70. [PubMed] [Google Scholar]
- 17.Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–82. [PubMed] [Google Scholar]
- 18.Venkateswaran V, Klotz LH, Fleshner NE. Selenium modulation of cell proliferation and cell cycle biomarkers in human prostate carcinoma cell lines. Cancer Res. 2002;62:2540–5. [PubMed] [Google Scholar]
- 19.Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001;61:7071–8. [PubMed] [Google Scholar]
- 20.Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res. 2003;63:52–9. [PubMed] [Google Scholar]
- 21.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–6. [PubMed] [Google Scholar]
- 22.Zhao H, Whitfield ML, Xu T, Botstein D, Brooks JD. Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells. Mol Biol Cell. 2004;15:506–19. doi: 10.1091/mbc.E03-07-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo YR, Kelley MR, Smith ML. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc Natl Acad Sci USA. 2002;99:14548–53. doi: 10.1073/pnas.212319799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leinfelder W, Zehelein E, Mandrand-Berthelot MA, Bock A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988;331:723–5. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- 25.Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–43. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 26.Gladyshev VN, Hatfield DL. Selenocysteine-containing proteins in mammals. J Biomed Sci. 1999;6:151–60. doi: 10.1007/BF02255899. [DOI] [PubMed] [Google Scholar]
- 27.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–35. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvo A, Xiao N, Kang J, et al. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325–35. [PubMed] [Google Scholar]
- 29.Lincoln DT, li Emadi EM, Tonissen KF, Clarke FM. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anti-cancer Res. 2003;23:2425–33. [PubMed] [Google Scholar]
- 30.Moschos MP. Selenoprotein P. Cell Mol Life Sci. 2000;57:1836–45. doi: 10.1007/PL00000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zini A, Schlegel PN. Expression of glutathione peroxidases in the adult male rat reproductive tract. Fertil Steril. 1997;68:689–95. doi: 10.1016/s0015-0282(97)00283-5. [DOI] [PubMed] [Google Scholar]
- 32.Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst. 1997;89:40–8. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- 33.Iynem AH, Alademir AZ, Obek C, Kural AR, Konukoglu D, Akcay T. The effect of prostate cancer and antiandrogenic therapy on lipid peroxidation and antioxidant systems. Int Urol Nephrol. 2004;36:57–62. doi: 10.1023/b:urol.0000032676.31470.b2. [DOI] [PubMed] [Google Scholar]
- 34.Tam NN, Gao Y, Leung YK, Ho SM. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and anti-oxidant defense machinery during prostatic involution and regrowth. Am J Pathol. 2003;163:2513–22. doi: 10.1016/S0002-9440(10)63606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J. 1991;277:133–8. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–26. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pryor WA, Hales BJ, Premovic PI, Church DF. The radicals in cigarette tar: their nature and suggested physiological implications. Science. 1983;220:425–7. doi: 10.1126/science.6301009. [DOI] [PubMed] [Google Scholar]
- 38.Pryor WA, Stone K, Zang LY, Bermudez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998;11:441–8. doi: 10.1021/tx970159y. [DOI] [PubMed] [Google Scholar]
- 39.Hayes RB, Sigurdson A, Moore L, et al. Methods for etiologic and early marker investigations in the PLCO trial. Mutat Res. 2005;592:147–54. doi: 10.1016/j.mrfmmm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Fleming ID, Cooper JS, Henson ED, et al., editors. AJCC cancer staging manual. Philadelphia, PA: Lippincott-Raven; 1997. Prostate; pp. 219–24. [Google Scholar]
- 41.Rothman KJ, Greeenland S. Modern epidemiology. Philadelphia, PA: Lippincott Raven; 1998. [Google Scholar]
- 42.Stürup S, Hayes RB, Peters U. Development and application of a simple routine method for the determination of selenium in serum by octopole reaction system ICPMS. Anal Bioanal Chem. 2005;381:686–94. doi: 10.1007/s00216-004-2946-x. [DOI] [PubMed] [Google Scholar]
- 43.Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. [(accessed 27 October 2006)]; Internet: http://www3.cancer.gov/prevention/plco/DQX.pdf.
- 44.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152:279–86. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 45.Tippett KS, Cypel YS. Continuing Survey of Food Intakes by Individuals 1994–96. Washington, DC: US Department of Agriculture, Agricultural Research Service; 1997. Design and operation: the Continuing Survey of Food Intakes by Individuals and the Diet and Health Knowledge Survey, 1994–96. Nationwide Food Surveys Report no. 96-1. [Google Scholar]
- 46.Grossman JN, Grosz AE, Schweitzer PN, Schruben PN The National Geochemical Survey Team. The National Geochemical Survey—database and documentation. [(accessed 27 October 2006)];US Geological Survey Open-File Report 2004-1001. Internet: http://tin.er.usgs.gov/geochem/doc/averages/se/usa.html.
- 47.Meyer F, Galan P, Douville P, et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU. VI. MAX trial Int J Cancer. 2005;116:182–6. doi: 10.1002/ijc.21058. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Stampfer MJ, Giovannucci EL, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004;96:696–703. doi: 10.1093/jnci/djh125. [DOI] [PubMed] [Google Scholar]
- 49.Van den Brandt PA, Zeegers MP, Bode P, Goldbohm RA. Toenail selenium levels and the subsequent risk of prostate cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2003;12:866–71. [PubMed] [Google Scholar]
- 50.Nomura AM, Lee J, Stemmermann GN, Combs GF., Jr Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:883–7. [PubMed] [Google Scholar]
- 51.Yoshizawa K, Willett WC, Morris SJ, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90:1219–24. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 52.Brooks JD, Metter EJ, Chan DW, et al. Plasma selenium level before diagnosis and the risk of prostate cancer development. J Urol. 2001;166:2034–8. [PubMed] [Google Scholar]
- 53.Goodman GE, Schaffer S, Bankson DD, Hughes MP, Omenn GS. Predictors of serum selenium in cigarette smokers and the lack of association with lung and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1069–76. [PubMed] [Google Scholar]
- 54.Knekt P, Aromaa A, Maatela J, et al. Serum selenium and subsequent risk of cancer among Finnish men and women. J Natl Cancer Inst. 1990;82:864–8. doi: 10.1093/jnci/82.10.864. [DOI] [PubMed] [Google Scholar]
- 55.Allen NE, Morris JS, Ngwenyama RA, Key TJ. A case–control study of selenium in nails and prostate cancer risk in British men. Br J Cancer. 2004;90:1392–6. doi: 10.1038/sj.bjc.6601701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogt TM, Ziegler RG, Graubard BI, et al. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int J Cancer. 2003;103:664–70. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- 57.Ghadirian P, Maisonneuve P, Perret C, et al. A case-control study of toenail selenium and cancer of the breast, colon, and prostate. Cancer Detect Prev. 2000;24:305–13. [PubMed] [Google Scholar]
- 58.Hill KE, Xia Y, Akesson B, Boeglin ME, Burk RF. Selenoprotein P concentration in plasma is an index of selenium status in selenium-deficient and selenium-supplemented Chinese subjects. J Nutr. 1996;126:138–45. doi: 10.1093/jn/126.1.138. [DOI] [PubMed] [Google Scholar]
- 59.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol. 1995;9:65–73. doi: 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- 60.Gladyshev VN, Jeang KT, Wootton JC, Hatfield DL. A new human selenium-containing protein. Purification, characterization, and cDNA sequence. J Biol Chem. 1998;273:8910–5. doi: 10.1074/jbc.273.15.8910. [DOI] [PubMed] [Google Scholar]
- 61.Kumaraswamy E, Malykh A, Korotkov KV, et al. Structure-expression relationships of the 15-kDa selenoprotein gene. Possible role of the protein in cancer etiology. J Biol Chem. 2000;275:35540–7. doi: 10.1074/jbc.M004014200. [DOI] [PubMed] [Google Scholar]
- 62.Pang ST, Dillner K, Wu X, Pousette A, Norstedt G, Flores-Morales A. Gene expression profiling of androgen deficiency predicts a pathway of prostate apoptosis that involves genes related to oxidative stress. Endocrinology. 2002;143:4897–906. doi: 10.1210/en.2002-220327. [DOI] [PubMed] [Google Scholar]
- 63.Hickey K, Do KA, Green A. Smoking and prostate cancer. Epidemiol Rev. 2001;23:115–25. doi: 10.1093/oxfordjournals.epirev.a000776. [DOI] [PubMed] [Google Scholar]
- 64.Kirsh VA, Hayes RB, Mayne ST, et al. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J Natl Cancer Inst. 2006;98:245–54. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 65.Chan JM, Stampfer MJ, Ma J, Rimm EB, Willett WC, Giovannucci EL. Supplemental vitamin E intake and prostate cancer risk in a large cohort of men in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8:893–9. [PubMed] [Google Scholar]
- 66.Eichholzer M, Stahelin HB, Ludin E, Bernasconi F. Smoking, plasma vitamins C, E, retinol, and carotene, and fatal prostate cancer: seventeen-year follow-up of the prospective basel study. Prostate. 1999;38:189–98. doi: 10.1002/(sici)1097-0045(19990215)38:3<189::aid-pros3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 67.Gann PH, Ma J, Giovannucci E, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–30. [PubMed] [Google Scholar]
- 68.Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from beta-carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev. 2003;12:518–26. [PubMed] [Google Scholar]
- 69.Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with alpha-tocopherol and beta- carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–6. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 70.Weinstein SJ, Wright ME, Pietinen P, et al. Serum alpha-tocopherol and gamma-tocopherol in relation to prostate cancer risk in a prospective study. J Natl Cancer Inst. 2005;97:396–9. doi: 10.1093/jnci/dji045. [DOI] [PubMed] [Google Scholar]
- 71.Cowan DB, Weisel RD, Williams WG, Mickle DA. Identification of oxygen responsive elements in the 5'-flanking region of the human glutathione peroxidase gene. J Biol Chem. 1993;268:26904–10. [PubMed] [Google Scholar]
- 72.de Haan JB, Bladier C, Griffiths P, et al. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998;273:22528–36. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- 73.Fuchs O. Effects of intracellular chelatable iron and oxidative stress on transcription of classical cellular glutathione peroxidase gene in murine erythroleukemia cells. Neoplasma. 1997;44:184–91. [PubMed] [Google Scholar]
- 74.Jornot L, Junod AF. Hyperoxia, unlike phorbol ester, induces glutathi-one peroxidase through a protein kinase C-independent mechanism. Biochem J. 1997;326:117–23. doi: 10.1042/bj3260117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dumont E, Vanhaecke F, Cornelis R. Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem. 2006;385:1304–23. doi: 10.1007/s00216-006-0529-8. [DOI] [PubMed] [Google Scholar]
- 76.Etminan M, Fitzgerald J, Gleave M, Chambers K. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control. 2005;16:1125–31. doi: 10.1007/s10552-005-0334-2. [DOI] [PubMed] [Google Scholar]


