Abstract
Social experiences, both positive and negative, may influence cardiovascular regulation. Prairie voles (Microtus ochrogaster) are socially monogamous rodents that form social bonds similar to those seen in primates, and this species may provide a useful model for investigating neural and social regulation of cardiac function. Cardiac regulation has not been studied previously in the prairie vole. Radiotelemetry transmitters were implanted into adult female prairie voles under anesthesia, and electrocardiographic parameters were recorded. Autonomic blockade was performed using atenolol (8 mg/kg ip) and atropine methyl nitrate (4 mg/kg ip). Several variables were evaluated, including heart rate (HR), HR variability and the amplitude of respiratory sinus arrhythmia. Sympathetic blockade significantly reduced HR. Parasympathetic blockade significantly increased HR, and reduced HR variability and the amplitude of respiratory sinus arrhythmia. Combined autonomic blockade significantly increased HR, and reduced HR variability and respiratory sinus arrhythmia amplitude. The data indicate that autonomic function in prairie voles shares similarities with primates, with a predominant vagal influence on cardiac regulation. The current results provide a foundation for studying neural and social regulation of cardiac function during different behavioral states in this socially monogamous rodent model.
Keywords: Arvicolinae, Atenolol, Atropine, Autonomic blockade, Cardiac, Heart rate variability, Respiratory sinus arrhythmia
1. Introduction
Psychological and physiological responses to several environmental and social stimuli have been linked directly to cardiovascular regulation [1–6]. Reactions to the social context may play a role in brain-heart interactions, and the mechanisms of these influences are best understood through knowledge of the underlying neurobiological processes. To this end, an integrative research program involving animal models is useful. The prairie vole (Microtus ochrogaster) is a rodent species that may provide a valuable model for studying the mechanisms of social behavior and the role of social experiences in mediating cardiovascular regulation. This species exhibits traits of social behavior that are similar to primates, including an active engagement in and reliance on the social environment, the formation of social bonds, display of biparental care, and living in extended families [7–9].
Relevant to our understanding of social behavior and its consequences on cardiovascular regulation is the study of autonomic and cardiac function. Previous research from Grippo and colleagues [6,10–12] indicates that exposure to mild, unpredictable environmental and social stressors in rats leads to behavioral changes, basal cardiac rate and rhythm disturbances, exaggerated cardiovascular reactivity to novel stressors, and a disruption of autonomic balance. Cardiovascular function is determined by several central and peripheral nervous system factors, including a balance of sympathetic and parasympathetic influences [13–15], and the functions of the autonomic nervous system may vary across species [16–19]. While social behavior has been well studied in the prairie vole [see for instance 7–9], neither cardiac nor autonomic function has been investigated previously in this rodent species.
The current study was designed to investigate basal autonomic and cardiac function in prairie voles. The social behaviors of prairie voles are atypical for small mammals and parallel the strong social bonds observed in humans. Therefore we predicted, consistent with theories of social behavior and vagal regulation of the heart [see 20], that the prairie vole would exhibit high basal cardiac vagal tone. The current results may provide a foundation for investigating neural and social regulation of the heart in socially monogamous rodent models.
2. Methods
2.1 Animals
Eight adult, reproductively naïve, female prairie voles (40–50 grams) were used for the experimental procedures. Animals were descendants of a wild stock originally caught near Champaign, IL. Animals were maintained on a 14/10 h light/dark cycle (lights on at 6:30 am), with a temperature of 25 ± 1° C and a relative humidity of 24 ± 1 g/m3. All animals were allowed food (Purina rabbit chow) and water ad libitum. Offspring were housed with breeding pairs in large polycarbonate cages (25x45x60 cm) with cotton nesting material until 21 days of age, at which time they were removed and housed in same-sex sibling pairs in smaller cages (12x18x28 cm).
Females were chosen for these initial experiments because they have been studied extensively for their social behavior in our laboratory [21–26], and they do not show a spontaneous puberty or estrous cycle [27,28]. In this species, the ovaries remain inactive until the female has physical contact with a male. Therefore removal of potential hormonal influences on cardiovascular regulation (e.g., via ovariectomy) is not required in reproductively naïve female prairie voles.
Experimental procedures were carried out when the animals were approximately 60–120 days of age. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
2.2 Telemetric Transmitter Implantation
Telemetric transmitters were implanted subcutaneously under aseptic conditions, during the light period, for long-term electrocardiogram (ECG) recordings. Prairie voles were anesthetized with ketamine (67 mg/kg sc; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg sc; NLS Animal Health, Owings Mills, MD) and placed under a warming lamp. Ophthalamic ointment was applied to the eyes to maintain moisture, and was reapplied as necessary. The procedures for implantation of the transmitter were similar to methods described previously for the mouse [29]. A midline incision was made on the back along the spine, and a wireless radiofrequency transmitter (DataSciences International, St. Paul, MN; model TA10ETA-F20; 2.1 cm length, 3.9 g weight, 1.9 cm3 volume) was inserted into a subcutaneous tissue pocket. The leads were directed caudally using a trochar with a sleeve. The negative lead was brought through the sleeve, and anchored to the muscle on the right side of the heart with a permanent suture. The positive lead was brought through the sleeve to rest caudal to the rib cage on the left side of the heart, and anchored to the muscle with a permanent suture. All skin incisions were sutured closed using sterile 5-0 nylon suture. Animals were administered subcutaneous fluids as necessary, and monitored carefully to avoid adverse effects. After immediate recovery from anesthesia, animals were housed individually for 5 days to permit adequate healing of sutures (animals were housed in custom-designed divided cages adjacent to a same-sex sibling during this time period, which permit the siblings to interact with one another, but prevent the uninstrumented animal from picking at the wounds of the instrumented animal). Animals then were returned to the standard home cages (with the sibling) and allowed to recover for an additional 2–5 days before the onset of experimentation.
2.3 Radiotelemetric Recordings
ECG signals were recorded continuously with a radiotelemetry receiver (DataSciences International, St. Paul, MN). The analog signal from the receiver was digitized with 12-bit precision at a sampling rate of 5 kHz. Activity level was monitored via the telemetry receiver at a sampling rate of 256 Hz. Parameters were recorded continuously in all animals during an undisturbed baseline period (3–5 days) and during and following all experimental procedures.
2.4 Selective Pharmacological Autonomic Blockade
The study of HR and heart rhythms was performed on a subset of animals (n = 6) under conditions of pharmacological autonomic blockade. Heart rate, HR variability, and amplitude of respiratory sinus arrhythmia were measured under the following conditions: (a) during β-adrenergic receptor blockade with atenolol (8 mg/kg ip; Sigma-Aldrich, St. Louis, MO), (b) during cholinergic receptor blockade with atropine methyl nitrate (i.e., atropine; 4 mg/kg ip; Sigma-Aldrich, St. Louis, MO), and (c) during dual autonomic blockade with a combination of atenolol and atropine (8 mg/kg ip and 4 mg/kg ip, respectively). These drug doses were chosen for their ability to effectively and completely block the respective autonomic inputs to the heart according to previously published results from voles [19], and were examined for effectiveness in prairie voles prior to experimentation.
All drug treatments were administered during the light period, between the hours of 8:00 am and 10:00 am, and all animals were exposed to all three drug treatments over a 6-day period, with 48 hours between each drug treatment. The order of drug administration was counterbalanced across animals such that half of the animals received atenolol on the first day of drug treatment and atropine on the second day of drug treatment, and half of the animals received the reverse administration. All animals received both atenolol and atropine on the third day of drug treatment. ECG and activity data were recorded continuously following the drug treatment (during the light period), and animals were quiet after the first few minutes following the injections.
2.5 Quantification of Radiotelemetric Recordings
Quantification of Telemetric Variables
Multiple segments of 30 seconds to 5 minutes of stable, continuous ECG data were used to evaluate HR, HR variability, and the amplitude of respiratory sinus arrhythmia during resting conditions and following selective autonomic blockade. Multiple segments of 30 seconds to 5 minutes of stable activity data were used to evaluate activity level. Data segments were matched across subjects and time points, and were used to calculate all ECG and activity variables (i.e., the parameters of HR, HR variability, respiratory sinus arrhythmia, and activity were all calculated from the same data segments, to ensure comparability of the parameters).
Baseline Parameters
Activity level was evaluated with software provided by the vendor (DataSciences International, St. Paul, MN), and included a gross estimation of locomotor activity based on the strength of the signal of the radiotelemetry transmitter from a centralized point in the receiver. Data are reported in counts/minute (cpm). HR was evaluated with software provided by the vendor (DataSciences International, St. Paul, MN), and was verified with custom-designed software to ensure that each R wave was detected and included in the HR output. Data are reported in beats/minute (bpm).
Calculations of HR and activity were conducted using 30-second continuous segments of data collected every 30 minutes for a total of 3 days (undisturbed). This resulted in a total of 96–144 30-second segments of accumulated HR and activity data from each animal. Sequential segments of data are presented over time. Baseline parameters are presented as: (a) 24-hour activity and HR (average of each 24-hour period), (b) day activity and HR (average of each 14-hour period between the hours of 6:30 am and 8:30 pm), and (c) night activity and HR (average of each 10-hour period, between the hours of 8:30 pm and 6:30 am).
Resting ECG Parameters
Because activity level was high in prairie voles and occurred in short bouts throughout the light and dark periods, resting ECG parameters (HR, HR variability, and respiratory sinus arrhythmia) were evaluated from ECG data sampled during a period of at least 1 hour of minimal activity, during the 3-day undisturbed baseline period (i.e., when activity counts were 2.0 cpm or lower), and included the average of 30-second intervals of continuous ECG data collected every 30 minutes. This resulted in 2–6 30-second segments of accumulated data from each animal, for each parameter. Sequential segments were averaged to provide one resting value for each HR, HR variability, and respiratory sinus arrhythmia from each subject.
Variations in heart period were analyzed using software provided by the vendor (DataSciences International, St. Paul, MN) and custom-designed software. HR variability was statistically analyzed by calculating the standard deviation of all R-R (normal-to-normal; N-N) intervals from each individual animal (SDNN index, [30]). Respiratory sinus arrhythmia was assessed from the ECG signal with custom-designed software, using a modification of the procedures described in Yongue et al. [31]. The methodology to extract the amplitude of respiratory sinus arrhythmia represents an index of the impact of myelinated vagal efferent pathways originating in the nucleus ambiguus. These pathways have a respiratory rhythm and are assumed to provide the primary vagal input to the heart’s pacemaker. The measure has been validated with pharmacological and physiological manipulations in other species [see 32]. The raw ECG signal was exported into a data file, where the data were examined to ensure that all R waves were detected. Preliminary spectral analyses were conducted to identify spectral peaks within the approximate frequency band in which breathing is observed in mammals of similar size (i.e., mouse and common vole). Spectral analyses confirmed that prairie voles express a spectral peak in the 1–4 Hz range, similar to that which is reported in both mice and common voles [19,29]. The R-R intervals were resampled at a rate of 20 Hz and, to comply with the assumption of stationarity, filtered with a 51-point cubic moving polynomial to remove low frequency (trend) components below 0.5 Hz. The residuals of this procedure were free of aperiodic and slow periodic processes in the data that may have violated the assumption of stationarity. A bandpass filter was applied to define respiratory sinus arrhythmia by extracting only the variance in the HR spectrum between the frequencies of 1 and 4 Hz.
Autonomic Blockade
The parameters of HR, HR variability (SDNN index), and. respiratory sinus arrhythmia amplitude were evaluated during the peak HR response beginning 30 minutes following each drug injection (atenolol, atropine, and both drugs), using stable, continuous ECG data. The data were manually examined to determine the peak HR response during a window of 1 hour that included a stable ECG recording that was not confounded by movement artifact (e.g., data were analyzed during a resting state). This resulted in approximately 3–10 minutes of stable ECG data for each parameter, from each animal. Sequential segments were averaged to provide one value for each HR, HR variability (SDNN index) and respiratory sinus arrhythmia (for each drug treatment) from each subject, and these values were compared to the respective resting values.
2.6 Data Analysis
The data are presented as means ± standard error of the mean (SEM). Two animals were excluded from the autonomic blockade experiments due to technical difficulties with the radiotelemetry transmitters. For all analyses, care was taken not to include any periods of ECG involving animal movement artifact (with the exception of 24-hour, day, and night HR values, for which movement has not been excluded). All data were analyzed with single factor repeated measures analyses of variance (ANOVA) and a priori Student’s t-tests, with a Bonferroni correction for all multiple comparisons. A probability value of P < 0.05 was considered to be statistically significant.
3. Results
3.1 Baseline Telemetric Variables and Body Weight
Baseline body weight was 47 ± 4 g. Baseline HR and activity were assessed over 3 days during the undisturbed baseline period. Figure 1 illustrates one 24-hour period of mean HR (Panel A) and mean spontaneous activity (Panel B). Table 1 presents the mean 24-hour, day, and night HR and activity level for all animals over the 3-day period. Neither HR nor activity level varied significantly from day to night (P > 0.05 for both analyses). These data suggest that the prairie voles described here do not show a noticeable circadian rhythm of either spontaneous activity or HR, but rather show an ultradian rhythm with a period of approximately 2–4 hours (refer to Figure 1).
Figure 1.
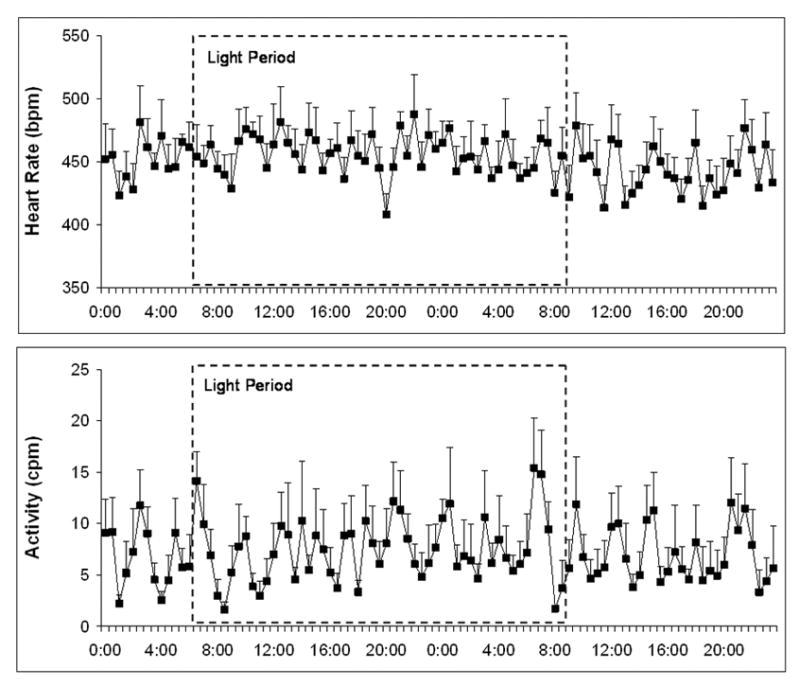
Mean (± SEM) heart rate (Panel A) and spontaneous activity (Panel B) in prairie voles during one undisturbed 24-hour period. The light period is demarcated with dotted lines.
Table 1.
Baseline heart rate and activity in prairie voles.
| 24-Hour | Day | Night | |
|---|---|---|---|
| Heart Rate (bpm) | 448 ± 10 | 449 ± 11 | 455 ± 11 |
| Activity Level (cpm) | 7 ± 2 | 7 ± 2 | 7 ± 2 |
Note: Data are shown as means ± SEM. Day parameters are represented during the light period (between the hours of 6:30 am and 8:30 pm); Night parameters are represented during the dark period (between the hours of 8:30 pm and 6:30 am).
3.2 Resting ECG Parameters
Prairie voles were active in short bouts throughout the light and dark periods (refer to Figure 1), and as expected HR was positively correlated with activity level (Pearson’s r = 0.40; data not shown); therefore resting ECG parameters were assessed during periods of minimal activity (i.e., periods when activity counts were 2.0 cpm or lower). Resting HR was 389 ± 10 bpm. Resting SDNN index was 14 ± 2 ms. Resting respiratory sinus arrhythmia amplitude was 3.44 ± 0.36 ln(ms2).
3.3 Selective Pharmacological Autonomic Blockade
Figure 2 displays the resting HR, SDNN index, and respiratory sinus arrhythmia amplitude, and the responses of these parameters during the peak HR response following selective and dual autonomic blockade, in a subset of animals (n = 6). Autonomic blockade with atenolol, atropine, and a combination of both drugs produced significant changes in HR. The ANOVA yielded a significant main effect of drug treatment on HR [F(3,20) = 19.85, P < 0.05]. Compared to resting HR, sympathetic blockade with atenolol led to a slight, yet significant decrease in HR [t(5) = 3.63; P < 0.05] versus the resting value. Parasympathetic blockade with atropine and combined autonomic blockade with both drugs both increased HR [atropine: t(5) = 5.98, P < 0.05; both drugs: t(5) = 4.59, P < 0.05]. The HR response following atropine was slightly greater than, but not significantly different from, the HR response to both drugs (P > 0.05).
Figure 2.
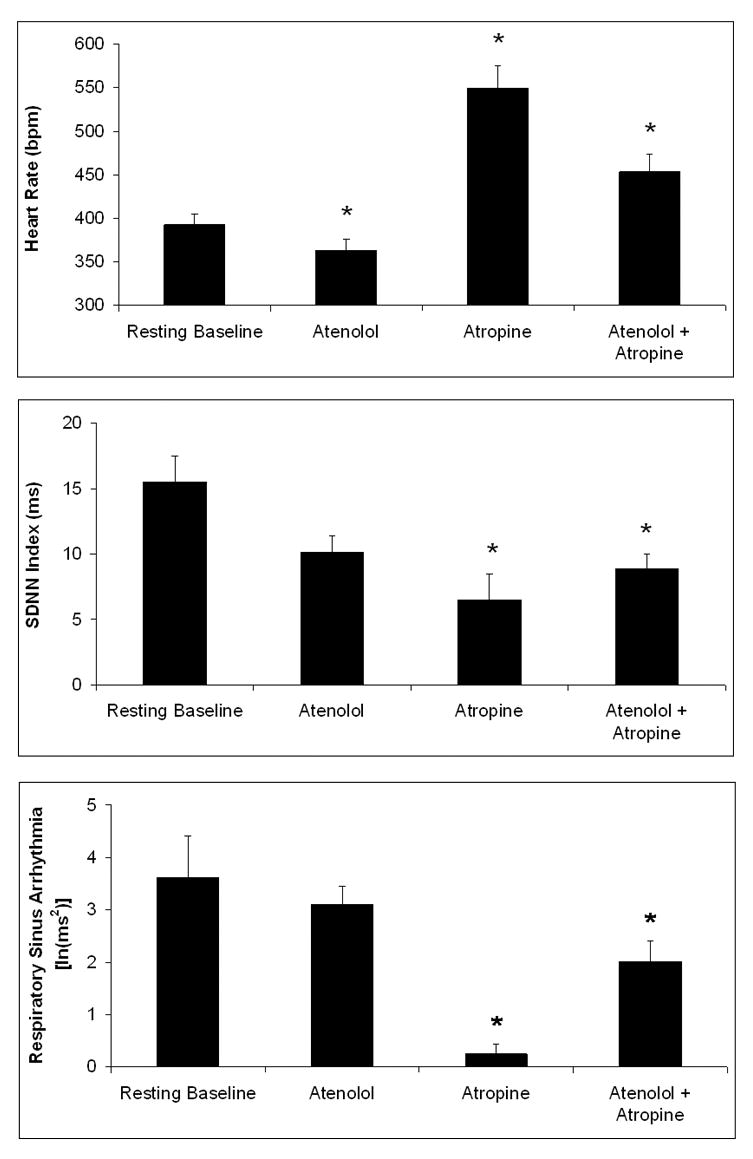
Mean (± SEM) heart rate (Panel A), SDNN index (Panel B), and respiratory sinus arrhythmia amplitude (Panel C) during a resting baseline period and following β-adrenergic receptor blockade with atenolol (8 mg/kg ip), cholinergic receptor blockade with atropine (4 mg/kg ip), and combined autonomic blockade with both drugs. Note the scale differences among the three panels. *P < 0.05 vs. respective resting baseline value.
Autonomic blockade significantly altered HR variability. The ANOVA yielded a significant main effect of drug treatment on SDNN index [F(3,20) = 5.43; P < 0.05]. Compared to the resting SDNN index, sympathetic blockade with atenolol did not significantly alter the SDNN index (P > 0.05). Parasympathetic blockade with atropine and combined autonomic blockade with both drugs both led to a significant decrease in SDNN index [atropine: t(5) = 4.07, P < 0.05; both drugs: t(5) = 3.81, P < 0.05] compared to the resting value. The SDNN index response to atropine was slightly greater than, but not significantly different from, the response to both drugs (P > 0.05).
Autonomic blockade significantly altered respiratory sinus arrhythmia amplitude. The ANOVA yielded a significant main effect of drug treatment on amplitude of respiratory sinus arrhythmia [F(3,20) = 19.40; P < 0.05]. Compared to resting respiratory sinus arrhythmia amplitude, sympathetic blockade with atenolol did not produce a significant change in respiratory sinus arrhythmia amplitude (P > 0.05). Parasympathetic blockade with atropine led to a significant decrease in respiratory sinus arrhythmia amplitude [t(5) = 9.18; P < 0.05] versus the resting value. Combined autonomic blockade with both drugs led to a slight, yet significant, decrease in respiratory sinus arrhythmia amplitude [t(5) = 3.79; P < 0.05] compared to the resting value. The response to atropine alone was greater than the response to both drugs [t(5) = 5.17; P < 0.05], suggesting a potential pharmacologic interaction between atenolol and atropine.
Figure 3 illustrates the effect of autonomic blockade on HR and the spectral density distribution. In response to atropine administration, HR increased by approximately 200 bpm and the amplitude of respiratory sinus arrhythmia was greatly attenuated. In contrast, in response to atenolol administration, HR slowed by approximately 50–75 bpm, and the amplitude of respiratory sinus arrhythmia remained high. Inspection of the spectral plots provides additional information regarding the effects of autonomic blockade. Under all conditions, there is a peak within the frequency band of spontaneous breathing in voles (1–4 Hz). However, the spectral density associated with the atropine peak is approximately 1% of the size of the peak under either baseline or atenolol (refer to Panels G-I in Figure 3).
Figure 3.
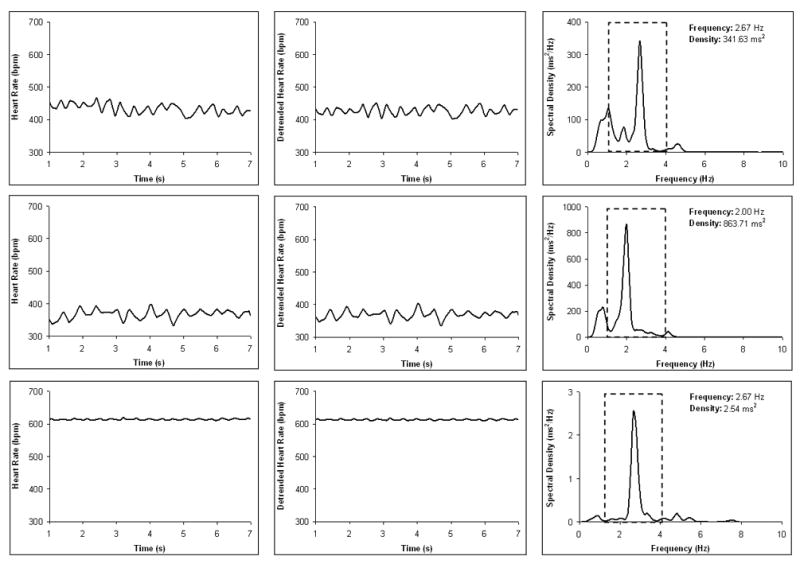
An example from one prairie vole showing time sampled heart rate (Panels A–C), detrended (i.e., cubic moving polynomial) heart rate pattern illustrating respiratory sinus arrhythmia (Panels D–F), and spectral density distribution of the detrended time sampled beat-to-beat heart period data (Panels G–I) during a resting baseline period (Panels A, D, and G) and following atenolol (Panels B, E, and H) and atropine administration (Panels C, F, and I). In Panels G–I, the band of spontaneous breathing in the prairie vole (1–4 Hz) is demarcated with dotted lines, and the peak response within this range is noted. Note the scale differences in Panels G–I.
4. Discussion
This is the first study to describe cardiac function in socially monogamous prairie voles. The present results suggest that the prairie vole may exhibit unique neural and autonomic regulation of the heart. Compared to other small rodents such as rats and mice, prairie voles have a low resting HR, high resting HR variability, and high amplitude of respiratory sinus arrhythmia. Additionally, this species demonstrates a predominant vagal tone to the heart, shown via the high amplitude of respiratory sinus arrhythmia at rest and large changes in HR and HR variability in response to cholinergic receptor blockade with atropine. Prairie voles do not appear to show a noticeable circadian rhythm of HR or activity, as these parameters did not differ from day to night. Short bouts of activity are exhibited throughout both the light and dark periods, mirrored by similar changes in HR during these periods. To our knowledge, this study provides the first description of basal, undisturbed locomotor activity in prairie voles monitored via radiotelemetry. The data described here are consistent with previous reports of prairie voles and other Microtus species, suggesting that these animals show an ultradian rhythm of locomotor activity and feeding (period length of 2–3 hours) [33–37]. However, common voles (Microtus arvalis) show circadian modulation of locomotor activity when allowed access to running wheels [34,37].
Resting HR and autonomic balance differ across species; basal HR in rodents, with few exceptions, is negatively correlated with body mass based on allometric scaling principles (HR is proportional to body mass to the negative quarter power) [see 38]. For illustrative purposes, Table 2 compares the body weight and resting HR data from prairie voles in the current study with parameters from rats, mice, and common voles (non-social voles). Interestingly, while prairie voles are similar in size to mice and common voles, resting HR in prairie voles is similar to rats (which are approximately nine times greater in body weight than prairie voles).
Table 2.
Comparison of body weight and basal heart rate among four rodent species.
| n | Body Weight (g) | Heart Rate (bpm) | Reference | |
|---|---|---|---|---|
| Prairie Voles | 8 | 47 ± 4 | 392 ± 17 | Current |
| Common Voles | 15 | 46 ± 1 | 432 ± 5 | [57] |
| Mice | 12 | 46 ± 1 | 646 ± 14 | [57] |
| Rats | 10 | 408 ± 3 | 364 ± 8 | [10] |
Note: Data are shown as means ± SEM. The data presented in this table were chosen from studies with comparable methodology to the current study. Data from common voles, mice, and rats are compiled from the control values reported in the respective references.
The specific functions of the autonomic nervous system may vary across species, with possible species-typical sympathovagal balance [15–17,39–42]. The prairie voles studied here exhibit a resting autonomic balance characterized by high parasympathetic tone to the heart. β-Adrenergic receptor blockade with atenolol led to a slight (yet significant) decrease in HR, but did not alter SDNN index or respiratory sinus arrhythmia. As expected, cholinergic receptor blockade with atropine led to a significant increase in HR, and a significant reduction in SDNN index and respiratory sinus arrhythmia amplitude, versus the respective resting parameters. This suggests that a prevalent vagal influence mediates HR, HR variability, and respiratory sinus arrhythmia in prairie voles. A predominant vagal influence on resting cardiac function has been reported in dogs, humans, and common voles [16–19,39]. In contrast, resting HR and HR variability may be modulated largely by sympathetic tone in rats [see for instance control groups in references 10,11] and mice [19].
Consistent with the responses to pharmacological autonomic blockade is the observation that the amplitude of respiratory sinus arrhythmia is very high in prairie voles. Respiratory sinus arrhythmia is a sensitive and specific measure of inputs to the heart via the myelinated vagus; therefore, this parameter might provide a sensitive measure of parasympathetic nervous system changes during specific behavioral states [see for instance 43]. The data described here imply a strong “vagal brake” on HR in prairie voles. Vagal influence, in turn, may be associated with or promote high levels of social behavior [43,44], in contrast to the defensive fight-flight behaviors mediated by the sympathetic nervous system. The prairie voles displayed respiratory sinus arrhythmia cycles in which the heart period rapidly shifted approximately 25–50 ms during a resting state; oscillations of this magnitude are observed in healthy humans at rest and in dogs [20,44–46]. Furthermore, calculations of effect size based on Cohen’s d [47] indicate that the effect of atropine treatment was largest for amplitude of respiratory sinus arrhythmia (d = 4.49), compared with the measures of SDNN index (d = 0.76) and HR (d = 0.33). In these analyses, Cohen’s d represents the number of standard deviations that the mean of each drug treatment shifts from resting baseline. These effect sizes in response to atropine administration are similar to those observed in humans [48]. These findings confirm, in the prairie vole, that respiratory sinus arrhythmia is mediated by the vagus nerve [15,43,49,50]. Future studies should evaluate the convergence of respiratory sinus arrhythmia with respiration in prairie voles, as has been described in other mammals, including humans [31,51].
However, the influence of combined autonomic blockade on respiratory sinus arrhythmia in prairie voles is puzzling. There may be complex interactions between atenolol and atropine at the specific doses employed here, leading to an amplitude of respiratory sinus arrhythmia that differs from that following atropine alone. It is not clear whether this effect is due to the combined influence of the drugs at the level of the receptor, a specific pharmacokinetic interaction, or changes in the baroreceptor reflex. Previous reports of pharmacological autonomic blockers on respiratory sinus arrhythmia in humans suggest a facilitatory influence of β-adrenergic blockade on cardiac vagal nerve activity, perhaps due to alterations in baroreceptor reflex function [41,52,53]. Further research should investigate these drug interactions in prairie voles. Alternatively, other methods of vagal blockade such as cooling or surgery could be administered prior to or following β-adrenergic blockade in prairie voles.
The cardiac characteristics of the prairie voles described here provide a foundation for investigating specific research questions related to the role of the social environment in mediating cardiovascular regulation. Prairie voles exhibit social interactions that share features with those of humans, including living in family groups and the capacity to form pair bonds [7–9]. Thus, studies involving both behavioral and cardiovascular regulation may be useful in this rodent species, with the potential for translation to human conditions. The precise mechanisms that underlie the observed autonomic balance specific to prairie voles and other socially monogamous mammals remain to be investigated. Future research will benefit from focusing on specific central mechanisms such as neurotransmitter and neuropeptide function, and peripheral mechanisms such as endocrine and autonomic function, underlying the association of social behavior and cardiac regulation. It is possible that a high level of parasympathetic activity contributes to the prolonged social interactions that characterize the behavior of socially monogamous mammals. For instance, Porges [20] has proposed that neural mechanisms regulating the myelinated vagus play a role in regulating the “social engagement system,” and that the physiological state of the body mediates emotional experiences and social behavior (i.e., polyvagal theory). Also, active engagement in the social environment may exert a positive influence on cardiac regulation via afferent feedback, perhaps exhibiting cardioprotective properties. Indeed, the lack of positive social interactions in humans can lead to several behavioral and physiological alterations, including affective disorders, neuroendocrine dysfunction, and cardiovascular pathophysiology [for instance 1,2,54,55,56], among others. Further studies may indicate that prairie voles can provide a useful model for investigating experimental questions relating to the interactions of social behavior and autonomic and cardiac function.
Acknowledgments
This research was supported by National Institute of Mental Health MH 73233 (AJG), MH 67446 (SWP) and MH 72935 (CSC), and National Institute of Child Health and Human Development HD 48390 (CSC). The authors would like to thank Dr. John Denver, Dr. Hossein Nazarloo, and Mr. Eric Schmidt for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams KB, Sanders S, Auth EA. Loneliness and depression in independent living retirement communities: risk and resilience factors. Aging Ment Health. 2004;8:475–85. doi: 10.1080/13607860410001725054. [DOI] [PubMed] [Google Scholar]
- 2.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 3.Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bartolomucci A, Palanza P, Costoli T, Savani E, Laviola G, Parmigiani S, Sgoifo A. Chronic psychosocial stress persistently alters autonomic function and physical activity in mice. Physiol Behav. 2003;80:57–67. doi: 10.1016/s0031-9384(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 5.Viewig WVR, Hubbard JR. Mental stress and the cardiovascular system. In: Hubbard JR, Workman EA, editors. Handbook of stress and medicine: an organ system approach. New York: CRC Press; 1998. pp. 17–43. [Google Scholar]
- 6.Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, Johnson AK. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286:H619–H626. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 8.Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 9.Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Horm Brain Behav. 2002;1:299–337. [Google Scholar]
- 10.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 11.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 12.Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–1170. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 14.Rowell LB. Human cardiovascular control. New York: Oxford University Press; 1993. [Google Scholar]
- 15.Japundzic N, Grichois ML, Zitoun P, Laude D, Elghozi JL. Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J Auton Nerv Syst. 1990;30:91–100. doi: 10.1016/0165-1838(90)90132-3. [DOI] [PubMed] [Google Scholar]
- 16.Evans JM, Randall DC, Funk JN, Knapp CF. Influence of cardiac innervation on intrinsic heart rate in dogs. Am J Physiol Heart Circ Physiol. 1990;258:H1132–H1137. doi: 10.1152/ajpheart.1990.258.4.H1132. [DOI] [PubMed] [Google Scholar]
- 17.Randall DC, Brown DR, Raisch RM, Yingling JD, Randall WC. SA nodal parasympathectomy delineates autonomic control of heart rate power spectrum. Am J Physiol Heart Circ Physiol. 1991;260:H985–H988. doi: 10.1152/ajpheart.1991.260.3.H985. [DOI] [PubMed] [Google Scholar]
- 18.Higgins CB, Vatner SF, Braunwald E. Parasympathetic control of the heart. Pharmacol Rev. 1973;25:119–155. [PubMed] [Google Scholar]
- 19.Ishii K, Kuwahara M, Tsubone H, Sugano S. Autonomic nervous function in mice and voles (Microtus arvalis): investigation by power spectral analysis of heart rate variability. Lab Animals. 1996;30:359–364. doi: 10.1258/002367796780739880. [DOI] [PubMed] [Google Scholar]
- 20.Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 21.DeVries AC, DeVries MB, Taymans SE, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeVries AC, Johnson CL, Carter CS. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster) Can J Zool. 1997;75:295–301. [Google Scholar]
- 23.Cushing BS, Carter CS. Prior exposure to oxytocin mimics the effects of social contact and facilitates sexual behaviour In females. J Neuroendocrinol. 1999;11:765–769. doi: 10.1046/j.1365-2826.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- 24.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 25.Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- 26.Grippo AJ, Kramer KM, Carter CS, Porges SW. Social isolation in prairie voles is associated with anhedonia and stress-related increases in oxytocin and vasopressin. Soc Neurosci Abstr. 2005 http://sfn.ScholarOne.com/itin2005/main.html.
- 27.Carter CS, Getz LL. Monogamy and the prairie vole. Scientific Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- 28.Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- 29.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279:H733–H740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- 30.Task Force of the European Society of Cardiology; North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 31.Yongue BG, McCabe PM, Porges SW, Rivera M, Kelley SL, Ackles PK. The effects of pharmacological manipulations that influence vagal control of the heart on heart period, heart-period variability and respiration in rats. Psychophysiology. 1982;19:426–432. doi: 10.1111/j.1469-8986.1982.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 32.Porges SW, McCabe PM, Yongue BG. Respiratory-heart rate interactions: psychophysiological implications for pathophysiology and behavior. In: Cacioppo J, Petty R, editors. Perspectives in cardiovascular psychophysiology. New York: Guilford Publications, Inc.; 1982. pp. 223–264. [Google Scholar]
- 33.Gerkema MP, van der Leest F. Ongoing ultradian activity rhythms in the common vole, Microtus arvalis, during deprivation of food, water and rest. J Comp Physiol. 1991;168:591–597. doi: 10.1007/BF00215081. [DOI] [PubMed] [Google Scholar]
- 34.Gerkema MP, Groos GA, Daan S. Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. J Biol Rhythms. 1990;5:81–95. doi: 10.1177/074873049000500201. [DOI] [PubMed] [Google Scholar]
- 35.Gerkema MP, Daan S, Wilbrink M, Hop MW, van der Leest F. Phase control of ultradian feeding rhythms in the common vole (Microtus arvalis): the roles of light and the circadian system. J Biol Rhythms. 1993;8:151–171. doi: 10.1177/074873049300800205. [DOI] [PubMed] [Google Scholar]
- 36.Voltura MB, Wunder BA. Effects of ambient temperature, diet quality, and food restriction on body composition dynamics of the prairie vole, Microtus ochrogaster. Physiol Zool. 1998;71:321–328. doi: 10.1086/515929. [DOI] [PubMed] [Google Scholar]
- 37.van der Veen DR, Le Minh N, Gos P, Arneric M, Gerkema MP, Schibler U. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc Natl Acad Sci. 2006;103:3393–3398. doi: 10.1073/pnas.0507825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noujaim SF, Lucca E, Muñoz V, Persaud D, Berenfeld O, Meijler FL, Jalife J. From mouse to whale: a universal scaling relation for the PR interval of the electrocardiogram of mammals. Circulation. 2004;110:2802–2808. doi: 10.1161/01.CIR.0000146785.15995.67. [DOI] [PubMed] [Google Scholar]
- 39.Matsunaga T, Harada T, Mitsui T, Inokuma M, Hashimoto M, Miyauchi M, Murano H, Shibutani Y. Spectral analysis of circadian rhythms in heart rate variability of dogs. Am J Vet Res. 2001;62:37–42. doi: 10.2460/ajvr.2001.62.37. [DOI] [PubMed] [Google Scholar]
- 40.Kuwahara M, Yayou K, Ishii K, Hashimoto S, Tsubone H, Sugano S. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J Electrocardiol. 1994;27:333–337. doi: 10.1016/s0022-0736(05)80272-9. [DOI] [PubMed] [Google Scholar]
- 41.Kollai M, Jokkel G, Bonyhay I, Tomcsanyi J, Naszlady A. Relation between tonic sympathetic and vagal control of human sinus node function. J Auton Nerv Syst. 1994;46:273–280. doi: 10.1016/0165-1838(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 42.Larson SK, Porges SW. The ontogeny of heart period patterning in the rat. Dev Psychobiol. 1982;15:519–528. doi: 10.1002/dev.420150604. [DOI] [PubMed] [Google Scholar]
- 43.Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal "brake" predicts child behavior problems: a psychobiological model of social behavior. Dev Psychobiol. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 44.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 45.Bryne EA, Fleg JL, Vaitkevicius PV, Wright J, Porges SW. Role of aerobic capacity and body mass index in the age-associated decline in heart rate variability. J Appl Physiol. 1996;81:743–750. doi: 10.1152/jappl.1996.81.2.743. [DOI] [PubMed] [Google Scholar]
- 46.Scher AM, Young AC. Reflex control of heart rate in the unanesthetized dog. Am J Physiol. 1970;218:780–789. doi: 10.1152/ajplegacy.1970.218.3.780. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 48.Porges SW. The polyvagal theory: a phylogenetic perspective. Biol Psychol. 2006 in press. [Google Scholar]
- 49.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 50.Weise F, Heydenreich F, Runge U. Contributions of sympathetic and vagal mechanism to the genesis of heart rate fluctuations during orthostatic load: a spectral analysis. J Auton Nerv Syst. 1987;21:127–134. doi: 10.1016/0165-1838(87)90015-4. [DOI] [PubMed] [Google Scholar]
- 51.Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol. 2006 doi: 10.1016/j.biopsycho.2005.09.00. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coker R, Koziell A, Oliver C, Smith SE. Does the sympathetic nervous system influence sinus arrhythmia in man? Evidence from combined autonomic blockade. J Physiol. 1984;356:459–464. doi: 10.1113/jphysiol.1984.sp015476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deering AH, Harron DG, Riddell JG, Shanks RG. Effect of acute administration of propranolol and atenolol on baroreflex function in normal man. Eur J Clin Pharmacol. 1988;35:607–612. doi: 10.1007/BF00637596. [DOI] [PubMed] [Google Scholar]
- 54.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 55.Ploog DW. The place of the Triune Brain in psychiatry. Physiol Behav. 2003;79:487–493. doi: 10.1016/s0031-9384(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 56.MacMahon KMA, Lip GYH. Psychological factors in heart failure: a review of the literature. Arch Int Med. 2002;162:509–516. doi: 10.1001/archinte.162.5.509. [DOI] [PubMed] [Google Scholar]
- 57.Ishii K, Kuwahara M, Tsubone H, Sugano S. The telemetric monitoring of heart rate, locomotor activity, and body temperature in mice and voles (Microtus arvalis) during ambient temperature changes. Lab Animals. 1996;30:7–12. doi: 10.1258/002367796780744992. [DOI] [PubMed] [Google Scholar]


