Abstract
Entorhinal cortex lesions induce significant reorganization of several homotypic and heterotypic inputs to the hippocampus. This investigation determined whether surviving heterotypic inputs after bilateral entorhinal lesions would support the acquisition of a learned alternation task. Rats with entorhinal lesions or sham operations were trained to acquire a spatial alternation task. Although the sham-operated rats acquired the task within about three weeks postsurgery, rats with bilateral entorhinal lesions failed to learn the task after 12 consecutive weeks of training despite heterotypic sprouting of the cholinergic septodentate pathway and the expansion of the commissural/associational fiber plexus within the dentate gyrus. Thus, heterotypic sprouting failed to ameliorate significantly the effects of bilateral entorhinal lesions. Rather, entorhinal lesions produce a persistent impairment of spatial memory, characterized by a mixture of random error production and perseverative responding.
INTRODUCTION
Understanding the mechanisms enabling recovery of function after lesions of the central nervous system remains a major goal within the neuroscientific community (Stein & Hoffman, 2003; Ward, 2005; Rijntjes et al, 2006). A frequently invoked mechanism accounting for recovery is axonal sprouting. Following the deafferentation of some common terminal field, remaining neighboring afferents may undergo terminal proliferation and establish synaptic contacts with the denervated zone, i.e., undergo axonal sprouting (see Deller & Frotscher, 1997, for review). For example, lesions of the entorhinal cortex (EC), which innervates approximately 86% of the outer two-thirds of the molecular layer of the dentate gyrus (Matthews, Cotman & Lynch, 1976), result in a dramatic reorganization of several of the remaining inputs to the dentate molecular layer from one to four weeks postlesion (see Ramirez, 2001, for review). This reorganization includes the heterotypic inputs that normally project to the molecular layer but originate from sources other than the EC, such as the cholinergic septodentate pathway and the commissural/associational fiber plexus.
Published empirical reports show that bilateral EC lesions result in significant retention impairments on spatial memory tasks, despite sprouting by several of the heterotypic inputs to the dentate gyrus (Loesche & Steward, 1977; Steward, Loesche, & Horton 1977; Ramirez & Stein, 1984; Ramirez, Labbe & Stein, 1988). This study investigated whether bilateral electrolytic lesions of the entorhinal area in rats would interfere with the acquisition of a spatial memory task during the period in which heterotypic sprouting occurs most vigorously.
MATERIALS AND METHODS
Subjects
The subjects were adult male Sprague-Dawley rats (300–400 g; Hilltop Breeding Laboratories, USA). Rats were housed individually on a diurnal (12h light/12h dark) light cycle and were maintained at approximately 80% of their weight at the beginning of the investigation, allowing weight gain of up to 5 g per week throughout testing. The rats had ad libitum access to water.
Apparatus
Spatial alternation testing was conducted with a gray Y-maze. A guillotine door separated each goal arm (13 cm high x 13 cm wide x 47 cm long) from the approach alley (13 cm high x 13 cm wide x 40 cm long). Upon entering the goal arm, rats were reinforced with 45 mg Noyes food pellets (P. J. Noyes, Lancaster, NH) that were dispensed semi-automatically.
Experimental Design and Behavioral Testing
Subjects were randomly assigned to one of two treatment conditions: (i) bilateral electrolytic EC lesion (BECX, n = 7); or (ii) sham operation (Sham, n = 9). Following random assignment, the animals were pre-trained and acclimated to the Y-maze using a random forced-choice (i.e., one goal arm blocked) schedule for seven days. To avoid the development of a position bias during pre-training, the rats were trained to traverse the maze in a pre-determined random order (Gellerman series; Gellerman, 1933) for a total of 10 traversals, five to the right or left, and were reinforced with two 45 mg Noyes pellets per trial.
Behavioral training/testing on a learned alternation Y-maze task began three days postsurgery to ensure that reinforced-alternation training/testing was initiated at the outset of hippocampal sprouting. The rats were tested in sessions of 11 trials per day for 10 alternations reinforced with two 45 mg Noyes pellets per correct trial. The rats were reinforced for the first trial of each session and they were reinforced on subsequent trials for correctly alternating their choice of goal arm. The experimenters were blind to the subjects’ study condition. This is similar to the approach described in Ramirez and Stein (1984), with the exception that here behavioral training/testing occurred postlesion and an intertrial interval (ITI) of 40 sec was used. All subjects were tested for 12 consecutive weeks (7 days a week for the first two weeks, then 5 days a week thereafter). We recorded (cf. Ramirez & Stein, 1984) both errors (repeating entry into a previously rewarded goal arm) as well as perseverative errors (repeating an error in consecutive trials beyond the first error), which are often observed after hippocampal injury (Isaacson, 1982). The criterion for acquisition was operationally defined as two or fewer errors for three consecutive days (cf. Ramirez & Stein, 1984).
Surgical Procedures
The subjects received surgery within 24 hours of completing behavioral pre-training. Surgical procedures, stereotaxic coordinates, and bilateral electrolytic lesion parameters have been described previously (Loesche & Steward, 1977; Ramirez & Stein, 1984). Briefly, subjects were anesthetized with an intraperitoneal injection of 0.1 ml of atropine sulfate (0.54 mg/ml) followed by sodium pentobarbital (Nembutal, 50 mg/kg). A 1 mA current was passed through an insulated stainless steel electrode angled at 10 deg away from midline for 45 sec at each of the following stereotaxic sites: 1.5 mm anterior to transverse sinus; 3, 4, and 5 mm lateral to sagittal suture; 2, 4, and 6 mm ventral to dura. Sham surgeries followed the same procedure, but electrodes were not inserted into the brain.
Histological Procedures
Within 24 hours of completing behavioral testing the subjects were euthanized with Nembutal (100 mg/ml) and perfused intracardially with 10% buffered formalin solution. The tissue was frozen-sectioned in 40 μm thick slices in the horizontal plane. Every 6th section was stained with cresyl violet acetate to assess the extent of the EC lesions. For qualitative inspection, every 7th section was stained with Naik acetylcholinesterase (AChE) histochemical technique to ascertain sprouting by the AChE-containing, cholinergic septodentate pathway in the outer molecular layer of the dentate gyrus and to examine the presence of the pale-staining zone in the inner molecular layer that corresponds to the fiber plexus of the commissural/associational input to the dentate gyrus (cf. West et al., 1982; Fass & Ramirez, 1984).
RESULTS
Histological Results
Histological assessment indicated that all cases in the BECX group sustained injury to the retrohippocampal area characterized by extensive EC injury bilaterally (see Figure 1C). In addition to the entorhinal injury evident in all our cases, four cases sustained from some to extensive injury to the para- and pre-subiculum and two cases sustained at least some injury to the para- and pre-subiculum, subiculum, and the perirhinal cortex. A qualitative visual inspection of tissue prepared with AChE histochemistry confirmed that all BECX animals exhibited intensified staining in the outer molecular layer of the dentate gyrus, indicative of septodentate sprouting, and a widening of the pale-staining zone of the inner molecular layer, which is associated with sprouting of the commissural/associational fiber plexus – findings consistent with a well-established body of literature on septodentate and commissural/associational sprouting in the dentate gyrus after entorhinal injury (Figure 1B; West, et al., 1982; reviewed in Deller & Frotscher, 1997, and in Ramirez, 2001).
Figure 1.

Examples of the minimum (black) and maximum (gray) extent of lesions in the retrohippocampal area are represented in the horizontal plane (C). In the right panel in (B), note the intensification of the AChE stain in the outer molecular layer in a rat with a bilateral EC lesion (white arrow), which indicates septodentate sprouting, compared to the AChE stain in a rat with a sham operation shown in the left panel (white arrow). In the right panel in (B), also note the widening of the pale-staining zone of the commissural/associational fiber plexus, which may be taken to indicate the expansion of the commissural/associational inputs to the inner molecular layer (black arrow; West et al., 1982), compared to the pale-staining zone in the left panel (black arrow). The open arrows indicate the upper margin of the dentate supragranular zone. (The photomicrographs in (B) are from the horizontal plane of the dentate gyrus indicated in the box shown in (A) at Bregma –3.1. Illustrations adapted from Paxinos and Watson (1986). White scale bar in right panel of (B) = 60 μm).
Behavioral Results
We calculated the daily errors committed by the rats as an average score for 12 one-week blocks. We conducted a two-way mixed ANOVA to compare sham-operated and lesioned groups across those 12 blocks. Lesioned rats made more errors ( M=5.391 ) than sham-operated rats ( M=1.823 ) ( F(1,14)= 320.495, MSE= 1.876, p<.001, η2= .958 ). There was a main effect for blocks, wherein errors decreased over time ( F(11,154)= 5.305, MSE= .547, p<.001, η2= .275 ). There was no statistical interaction. As seen in Figure 2A, a significant linear trend ( F(1,14)= 25.835, MSE= 1.663, p= .001, η2 = .536 ) shows that errors decreased over time, and a significant quadratic trend ( F(1,14)= 7.640, MSE= .329, p=.015, η2= .353 ) reflects errors decreasing for both groups after the first three blocks. We preceded all of these comparisons with appropriate multivariate F-tests. We adjusted alpha for post hoc comparisons of groups at each of the 12 blocks. All the mean differences between groups at each time block shown in Table 1 and Figure 2A are statistically significant.
Figure 2.
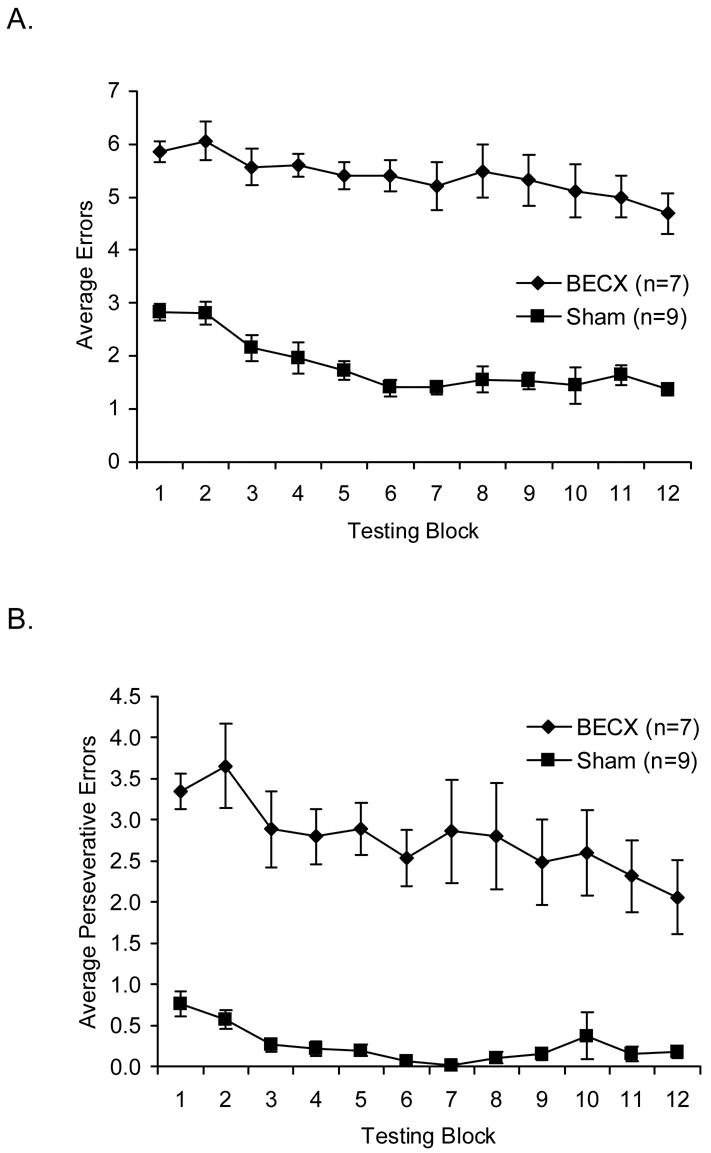
Rats that sustained bilateral entorhinal lesions exhibited profound impairments in spatial alternation performance. The BECX group committed significantly more errors (A) and perseverative errors (B) than the sham-operates. (Error bars = ±S.E.M.).
Table 1.
Mean Daily Errors of Groups by Time Block
| Groups
|
||
|---|---|---|
| Block | Lesion | Control |
| 1 | 5.857 | 2.825 |
| 2 | 6.061 | 2.810 |
| 3 | 5.571 | 2.156 |
| 4 | 5.600 | 2.000 |
| 5 | 5.400 | 1.733 |
| 6 | 5.400 | 1.400 |
| 7 | 5.200 | 1.400 |
| 8 | 5.486 | 1.556 |
| 9 | 5.314 | 1.533 |
| 10 | 5.114 | 1.444 |
| 11 | 5.000 | 1.644 |
| 12 | 4.686 | 1.378 |
All mean differences from Blocks 1 to 12 are statistically significant at p<.05.
As seen in Figure 2B, the trends for perseverative errors are essentially the same as for total errors. Correlations between total and perseverative errors across all rats at each time block ranged from .940 to .961.
DISCUSSION
Corroborating earlier findings (Loesche & Steward, 1977; Steward et al., 1977; Ramirez & Stein, 1984; Ramirez, Labbe, & Stein, 1988), bilateral retrohippocampal lesions centering on the entorhinal area resulted in significant impairments on a spatial alternation task. Our findings further demonstrate that acquisition of the learned alternation task is as sensitive as retention testing to bilateral entorhinal injury. Previous work in our laboratory (Ramirez & Stein, 1984; Ramirez, Labbe, & Stein, 1988;) indicated that rats with bilateral entorhinal injury tested on an alternation task with a 0 sec ITI may eventually recover from the behavioral deficits. However, others (Loesche & Steward, 1977; Steward et al., 1977) have shown that bilateral EC lesions produce persistent impairments in spatial retention tasks, which likely resulted from lengthier ITIs than those previously used in our laboratory. This study indicates that, with a 40 sec ITI, deficits in spatial memory (as reflected in errors and perseverative errors) may persist in an acquisition paradigm up to 12 weeks postlesion – suggesting that a 40 sec ITI exceeds the capacity of the areas surviving after an entorhinal injury to adequately compensate for the loss of the entorhinal area. Similarly, whereas perseverative responding in a retention task significantly diminishes within one and two weeks of beginning training with a 0 sec ITI (Ramirez et al., 1988), a 40 sec ITI with the acquisition paradigm used here resulted in perseverative behavior that lasted as long as 12 weeks postoperatively. In combination with the bilateral EC lesion, either the acquisition nature of the task or the 40 sec ITI might be responsible for the persistent perseverative behavior. Taken together, these observations indicate that as the length of the ITI increases, the demands being placed on the functional system contributing to spatial working memory concomitantly increases. With a compromised entorhinal component it seems mnemonic function required to perform tasks with delays exceeding some maximum performance limit is degraded. Based on our studies using this behavioral paradigm, this delay is likely bracketed somewhere between a 0 and a 40 sec ITI. Future work exploring the parameters of differential delays in both retention and acquisition settings after entorhinal injury would further elucidate the relation between ITI duration on this spatial alternation task and postlesion behavioral performance, and might in turn more precisely demonstrate features of memory.
That bilateral EC lesions produce persistent behavioral impairments despite axonal sprouting by several of the remaining surviving afferents (e.g., the septodentate pathway) suggests that heterotypic hippocampal sprouting is insufficient to promote recovery of mnemonic functions after extensive loss of the major cortical input to the hippocampus. Indeed, heterotypic sprouting under certain conditions may be detrimental to the recovery of function following cortical injury. We have previously reported, for example, that the reduction of AChE-containing septodentate sprouting was correlated with the enhanced recovery of performance on a differential low-rate response (DRL-20) task and open-field locomotor activity after bilateral EC lesions (Ramirez et al., 1998). In contrast, it is noteworthy that when unilateral entorhinal lesions are performed, rats recover from the spatial impairments in a time course paralleling homotypic sprouting by the contralateral homologue of the damaged entorhinal area (i.e., the crossed temporodentate pathway that emerges from the same cell type and layer of the temporodentate perforant path – CTD; Loesche & Steward, 1977; Reeves & Smith, 1987; also reviewed in Ramirez, 2001). Moreover, when CTD sprouting is accelerated, animals with unilateral lesions exhibit spared memory function when tested on a delayed alternation task (Ramirez et al., 1996).
Because the lesions in our rats extended into subiculum, pre- or parasubiculum, or perirhinal cortex, we cannot rule out the possibility that these structures also contributed to the mnemonic functions disrupted in this study. Evidence from numerous studies indicates that several components of the parahippocampal region are implicated in mnemonic function that is also disrupted by hippocampal injury (for reviews, see Witter & Wouterlood, 2002). Nonetheless, we consistently and commonly injured the entorhinal area in all cases in this experiment. Moreover, the one case that sustained the most limited damage to the EC did not differ substantially from the other rats sustaining more extensive retrohippocampal injury (data not shown).
For several years behavioral studies of entorhinal injury have yielded varied and often conflicting accounts of entorhinal contribution to mnemonic function. Mechanical or electrolytic EC lesions consistently impair performance on various memory-sensitive spatial tests – often, though not always – similar to hippocampal injury, including the induction of perseverative behavior (e.g., Ramirez et al., 1988; Ramirez et al., 1995; Ramos, 2002). Yet neurotoxic EC lesions, which spare axons-of-passage, yield outcomes varying by the task, the type of neurotoxin used, and/or the age of the rats (Marighetto, Yee & Rawlins, 1998; Pouzet et al., 1999; Schmadel, Schwabe, & Koch, 2004). This study and recent reports by Steffenach et al. (2005), Parron, Poucet, and Save (2006), and Sargolini et al. (2006) continue to implicate the entorhinal and retrohippocampal region in spatial memory function; indeed these recent reports strongly suggest that the entorhinal area in particular is a key element in the circuitry underlying spatial memory processing. Clearly, additional research is required to dissect the contribution that the entorhinal area makes to learning and memory, as well as to ascertain the functional significance of heterotypic sprouting.
Acknowledgments
Supported by grants from the National Institutes of Health (MH60608) and the Howard Hughes Medical Institute (52005120) to JJR. We would like to thank Joseph Taylor for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Deller T, Frotscher M. Lesion-induced plasticity of central neurons: Sprouting of single fibres in the rat hippocampus after unilateral entorhinal cortex lesion. Progress in Neurobiology. 1997;53:687–727. doi: 10.1016/s0301-0082(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Fass B, Ramirez JJ. Effects of ganglioside treatments on lesion-induced behavioral impairments and sprouting in the CNS. Journal of Neuroscience Research. 1984;12:445–458. doi: 10.1002/jnr.490120228. [DOI] [PubMed] [Google Scholar]
- Gellerman LW. Chance orders of alternative stimuli in visual discrimination experiments. Journal of Genetic Psychology. 1933;42:206–208. [Google Scholar]
- Isaacson RL. The limbic system. New York: Plenum Press; 1982. [Google Scholar]
- Loesche J, Steward O. Behavioral correlates of denervation and reinnervation of the hippocampal formation of the rat: recovery of alternation performance following unilateral entorhinal cortex lesions. Brain Research Bulletin. 1977;2:31–39. doi: 10.1016/0361-9230(77)90022-3. [DOI] [PubMed] [Google Scholar]
- Marighetto A, Yee BK, Rawlins JN. The effects of cytotoxic entorhinal lesions and electrolytic medial septal lesions on the acquisition and retention of a spatial working memory task. Experimental Brain Research. 1998;119:517–528. doi: 10.1007/s002210050368. [DOI] [PubMed] [Google Scholar]
- Matthews DA, Cotman C, Lynch G. An electron microscopic study of lesion-induced synaptogenesis in the dentate gyrus of the adult rat. II. Reappearance of morphologically normal synaptic contacts. Brain Research. 1976;115:23–41. doi: 10.1016/0006-8993(76)90820-9. [DOI] [PubMed] [Google Scholar]
- Parron C, Poucet B, Save E. Cooperation between the hippocampus and the entorhinal cortex in spatial memory: A disconnection study. Behavioural Brain Research. 2006;170:99–109. doi: 10.1016/j.bbr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Welzl H, Gubler MK, Broersen L, Veenman CL, Feldon J, Rawlins JN, Yee BK. The effects of NMDA-induced retrohippocampal lesions on performance of four spatial memory tasks known to be sensitive to hippocampal damage in the rat. European Journal of Neuroscience. 1999;11:123–140. doi: 10.1046/j.1460-9568.1999.00413.x. [DOI] [PubMed] [Google Scholar]
- Ramos JM. Training method dramatically affects the acquisition of a place response in rats with neurotoxic lesions of the hippocampus. Neurobiology of Learning and Memory. 2002;77:109–118. doi: 10.1006/nlme.2000.3997. [DOI] [PubMed] [Google Scholar]
- Ramirez JJ. The role of axonal sprouting in functional reorganization after CNS injury: lessons from the hippocampal formation. Restorative Neurology and Neuroscience. 2001;19:237–262. [PubMed] [Google Scholar]
- Ramirez JJ, Labbe R, Stein DG. Recovery from perseverative behavior after entorhinal cortex lesions in rats. Brain Research. 1988;459:153–156. doi: 10.1016/0006-8993(88)90296-x. [DOI] [PubMed] [Google Scholar]
- Ramirez JJ, MacDonald K, Mañibo J, Payne J, Tuite C. GM1-ganglioside suppresses septodentate sprouting and enhances recovery from entorhinal cortex lesions on DRL performance and locomotor behavior in rats. Restorative Neurology and Neuroscience. 1998;12:203–211. [PubMed] [Google Scholar]
- Ramirez JJ, Martin C, McQuilkin ML, MacDonald KA, Valbuena M, O'Connell JM. Bilateral entorhinal cortex lesions impair DRL performance in rats. Psychobiology. 1995;23:37–44. [Google Scholar]
- Ramirez JJ, McQuilkin M, Carrigan T, MacDonald K, Kelley MS. Progressive entorhinal cortex lesions accelerate hippocampal sprouting and spare spatial memory in rats. Proceedings of the National Academy of Sciences U S A. 1996;93:15512–15517. doi: 10.1073/pnas.93.26.15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JJ, Stein DG. Sparing and recovery of spatial alternation performance after entorhinal cortex lesions in rats. Behavioural Brain Research. 1984;13:53–61. doi: 10.1016/0166-4328(84)90029-9. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Smith DC. Reinnervation of the dentate gyrus and recovery of alternation behavior following entorhinal cortex lesions. Behavioral Neuroscience. 1987;101:179–186. doi: 10.1037//0735-7044.101.2.179. [DOI] [PubMed] [Google Scholar]
- Rijntjes M. Mechanisms of recovery in stroke patients with hemiparesis or aphasia: new insights, old questions and the meaning of therapies. Current Opinion in Neurology. 2006;19:76–83. doi: 10.1097/01.wco.0000203886.28068.38. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Schmadel S, Schwabe K, Koch M. Effects of neonatal excitotoxic lesions of the entorhinal cortex on cognitive functions in the adult rat. Neuroscience. 2004;128:365–374. doi: 10.1016/j.neuroscience.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Stein DG, Hoffman SW. Concepts of CNS plasticity in the context of brain damage and repair. Journal of Head Trauma Rehabilitation. 2003;18:317–341. doi: 10.1097/00001199-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Steward O, Loesche J, Horton WC. Behavioral correlates of denervation and reinnervation of the hippocampal formation of the rat: open field activity and cue utilization following bilateral entorhinal cortex lesions. Brain Research Bulletin. 1977;2:41–48. doi: 10.1016/0361-9230(77)90023-5. [DOI] [PubMed] [Google Scholar]
- Ward NS. Neural plasticity and recovery of function. Progress in Brain Research. 2005;150:527–535. doi: 10.1016/S0079-6123(05)50036-0. [DOI] [PubMed] [Google Scholar]
- West JR, Lind MD, Demuth RM, Parker ES, Alkana RL, Cassell M, Black AC. Lesion-induced sprouting in the rat dentate gyrus is inhibited by repeated ethanol administration. Science. 1982;218:808–810. doi: 10.1126/science.218.4574.808. [DOI] [PubMed] [Google Scholar]
- Witter M, Wouterlood F. The parahippocampal region: Organization and role in cognitive function. New York: Oxford University Press; 2002. [Google Scholar]


