Abstract
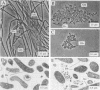
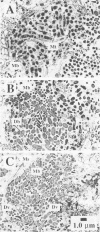
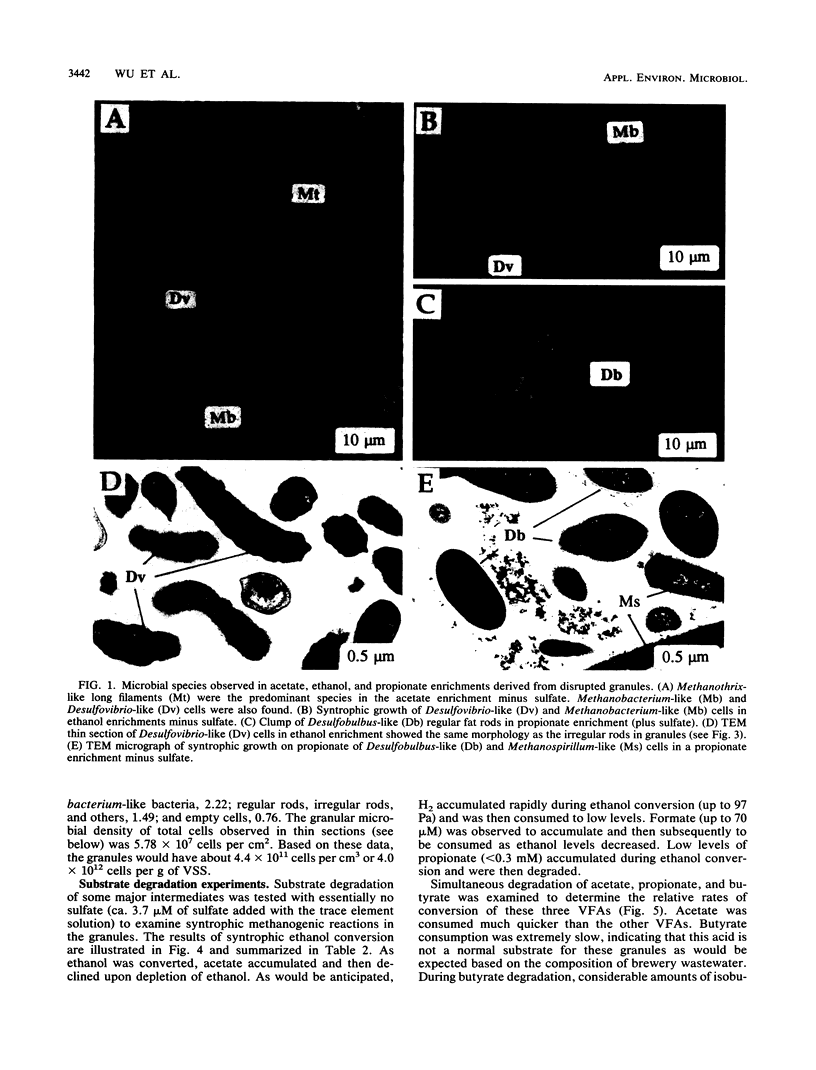
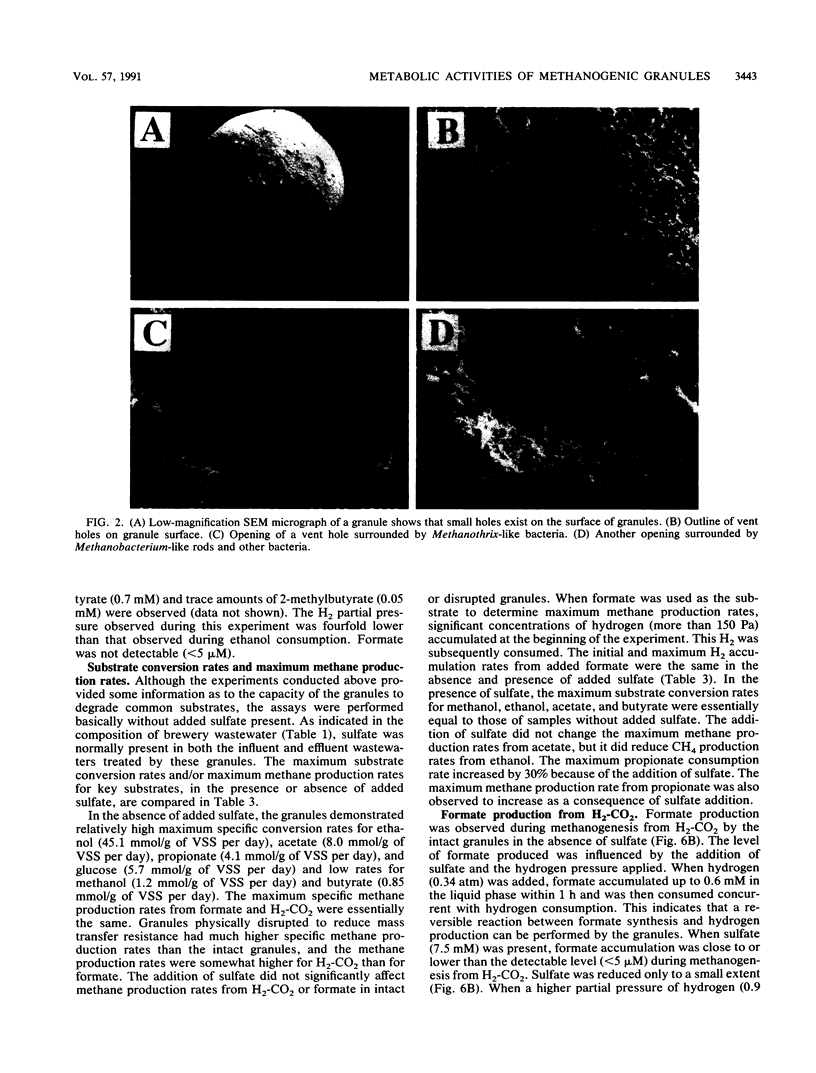
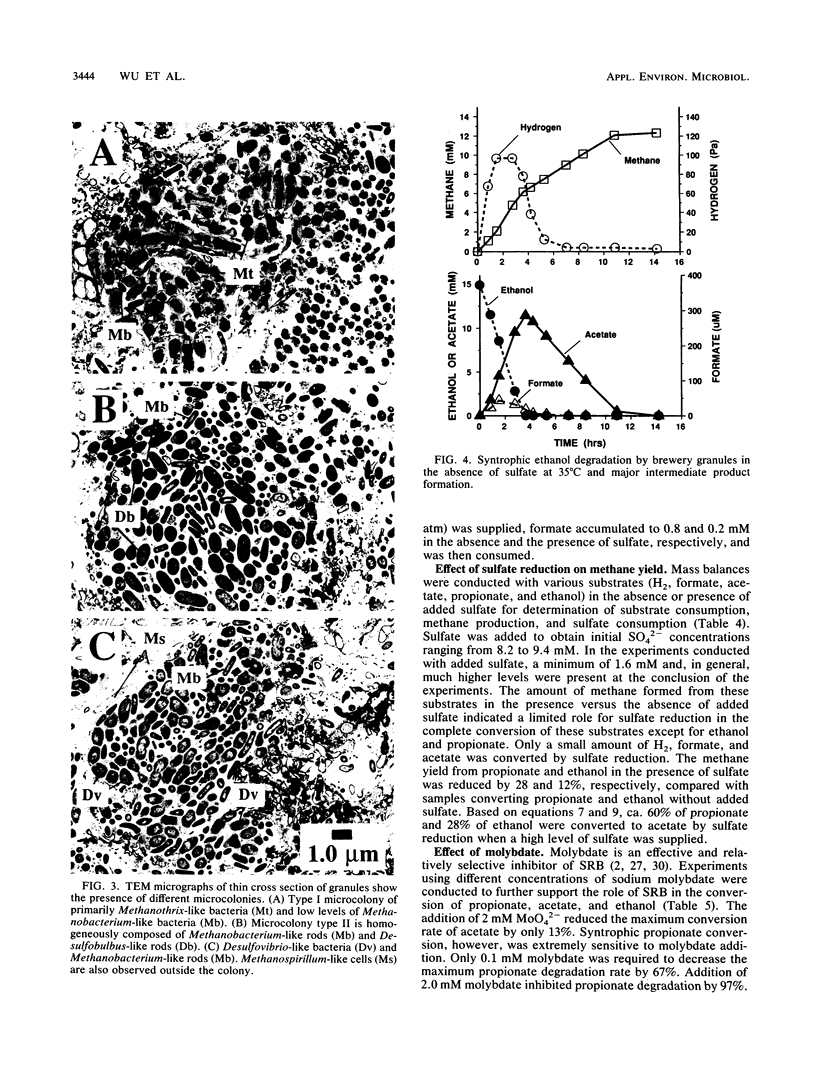
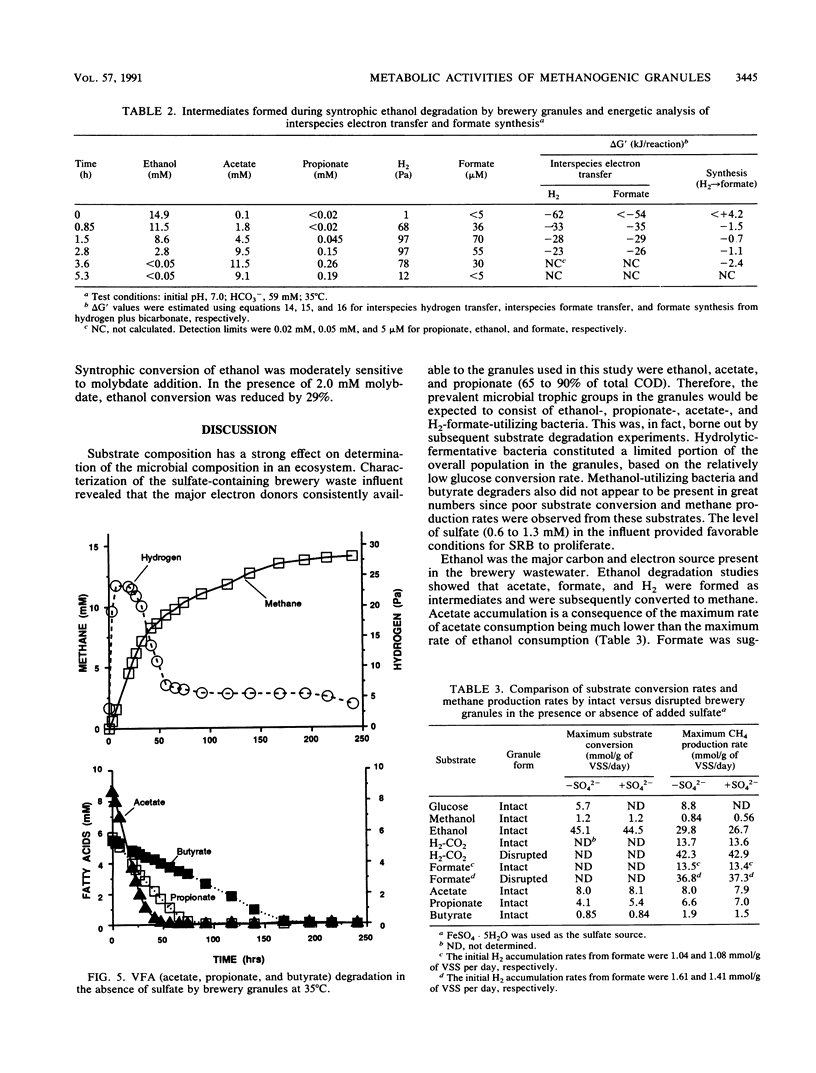
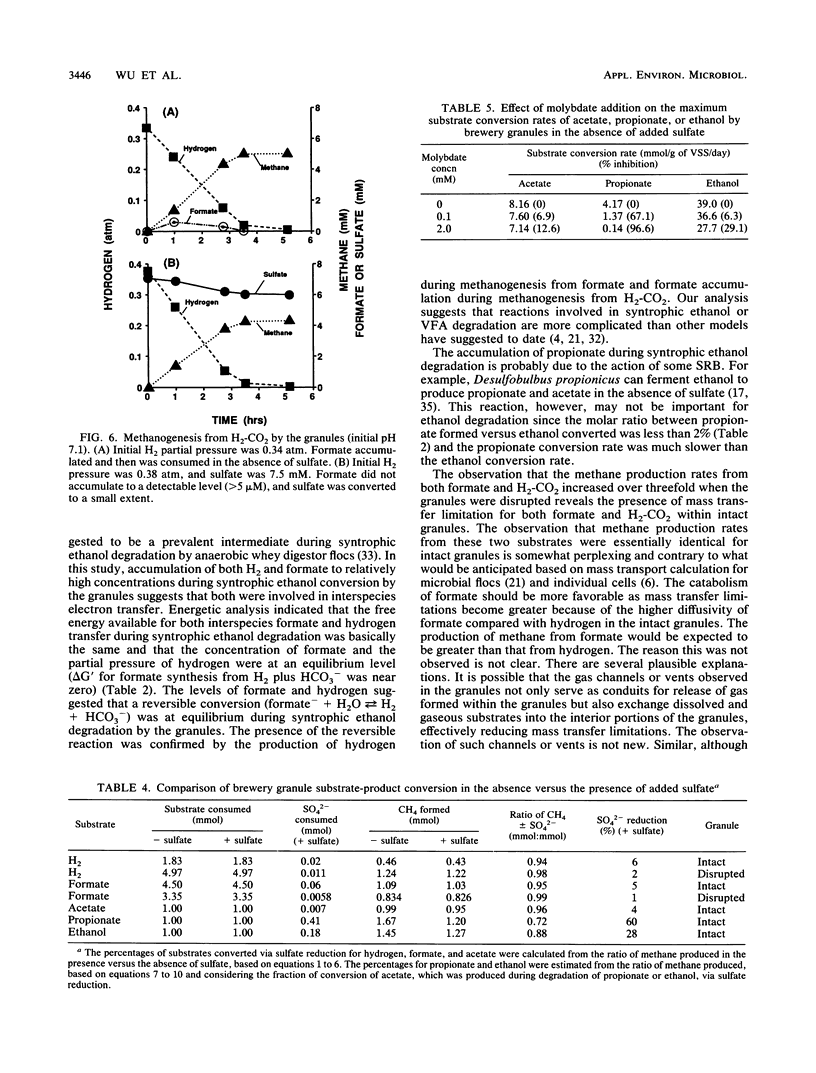
Granules from an upflow anaerobic sludge blanket system treating a brewery wastewater that contained mainly ethanol, propionate, and acetate as carbon sources and sulfate (0.6 to 1.0 mM) were characterized for their physical and chemical properties, metabolic performance on various substrates, and microbial composition. Transmission electron microscopic examination showed that at least three types of microcolonies existed inside the granules. One type consisted of Methanothrix-like rods with low levels of Methanobacterium-like rods; two other types appeared to be associations between syntrophic-like acetogens and Methanobacterium-like organisms. The granules were observed to be have numerous vents or channels on the surface that extended into the interior portions of the granules that may be involved in release of gas formed within the granules. The maximum substrate conversion rates (millimoles per gram of volatile suspended solids per day) at 35 degrees C in the absence of sulfate were 45.1, 8.04, 4.14, and 5.75 for ethanol, acetate, propionate, and glucose, respectively. The maximum methane production rates (millimoles per gram of volatile suspended solids per day) from H2-CO2 and formate were essentially equal for intact granules (13.7 and 13.5) and for physically disrupted granules (42 and 37). During syntrophic ethanol conversion, both hydrogen and formate were formed by the granules. The concentrations of these two intermediates were maintained at a thermodynamic equilibrium, indicating that both are intermediate metabolites in degradation. Formate accumulated and was then consumed during methanogenesis from H2-CO2. Higher concentrations of formate accumulated in the absence of sulfate than in the presence of sulfate. The addition of sulfate (8 to 9 mM) increased the maximum substrate degradation rates for propionate and ethanol by 27 and 12%, respectively. In the presence of this level of sulfate, sulfate-reducing bacteria did not play a significant role in the metabolism of H2, formate, and acetate, but ethanol and propionate were converted via sulfate reduction by approximately 28 and 60%, respectively. In the presence of 2.0 mM molybdate, syntrophic propionate and ethanol conversion by the granules was inhibited by 97 and 29%, respectively. The data show that in this granular microbial consortium, methanogens and sulfate-reducing bacteria did not compete for common substrates. Syntrophic propionate and ethanol conversion was likely performed primarily by sulfate-reducing bacteria, while H2, formate, and acetate were consumed primarily by methanogens.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banat I. M., Lindström E. B., Nedwell D. B., Balba M. T. Evidence for coexistence of two distinct functional groups of sulfate-reducing bacteria in salt marsh sediment. Appl Environ Microbiol. 1981 Dec;42(6):985–992. doi: 10.1128/aem.42.6.985-992.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Johnson R. L., Liu Y. Diffusion of the Interspecies Electron Carriers H(2) and Formate in Methanogenic Ecosystems and Its Implications in the Measurement of K(m) for H(2) or Formate Uptake. Appl Environ Microbiol. 1989 Jul;55(7):1735–1741. doi: 10.1128/aem.55.7.1735-1741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W., Zeikus J. G. Influence of corrinoid antagonists on methanogen metabolism. J Bacteriol. 1981 Apr;146(1):133–140. doi: 10.1128/jb.146.1.133-140.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P. Anaerobic Degradation of Lactate by Syntrophic Associations of Methanosarcina barkeri and Desulfovibrio Species and Effect of H(2) on Acetate Degradation. Appl Environ Microbiol. 1981 Feb;41(2):346–354. doi: 10.1128/aem.41.2.346-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps T. J., Conrad R., Zeikus J. G. Sulfate-Dependent Interspecies H(2) Transfer between Methanosarcina barkeri and Desulfovibrio vulgaris during Coculture Metabolism of Acetate or Methanol. Appl Environ Microbiol. 1985 Sep;50(3):589–594. doi: 10.1128/aem.50.3.589-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W., Akin D. E., Nordstedt R. A., Thomas M. V., Aldrich H. C. Light and electron microscopic examinations of methane-producing biofilms from anaerobic fixed-bed reactors. Appl Environ Microbiol. 1984 Jul;48(1):127–136. doi: 10.1128/aem.48.1.127-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele Jurgen H., Zeikus J. Gregory. Control of Interspecies Electron Flow during Anaerobic Digestion: Significance of Formate Transfer versus Hydrogen Transfer during Syntrophic Methanogenesis in Flocs. Appl Environ Microbiol. 1988 Jan;54(1):20–29. doi: 10.1128/aem.54.1.20-29.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore A. S., Fardeau M. L., Hatchikian C. E., Le Gall J., Belaich J. P. Energetics of Growth of a Defined Mixed Culture of Desulfovibrio vulgaris and Methanosarcina barkeri: Interspecies Hydrogen Transfer in Batch and Continuous Cultures. Appl Environ Microbiol. 1983 Nov;46(5):1152–1156. doi: 10.1128/aem.46.5.1152-1156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]