Summary
More than half of the nascent B cells in humans initially express autoreactive antibodies. However, most of these autoantibodies are removed from the repertoire at two checkpoints before maturation into naïve B cells. A third checkpoint excludes remaining autoantibodies from the antigen-experienced IgM+ memory B cell pool. Nevertheless, low-affinity self-reactive antibodies are frequently found in the serum of normal humans. To determine the source of these antibodies we cloned and expressed antibodies from circulating human IgG+ memory B cells. Surprisingly, we found that self-reactive antibodies including anti-nuclear antibodies were frequently expressed by IgG+ memory B cells in healthy donors. Most of these antibodies were created de-novo by somatic hypermutation during the transition between mature naïve and IgG+ memory B cells.
Keywords: tolerance, self-reactivity, repertoire, memory, B cell
Introduction
Random immunoglobulin (Ig) gene recombination frequently produces self-reactive antibodies: as many as ~75% of newly generated B cells in the bone marrow show reactivity to HEp-2 cells in clinical anti-nuclear antibody (ANA) ELISA or immunoflourescence (Wardemann et al., 2003). A significant fraction of these autoantibodies are polyreactive and many recognize nuclear self-antigens. However, polyreactive antibodies and true ANAs are tightly regulated by central and peripheral self-tolerance mechanisms. Thus, few self-reactive naïve B cells persist and these express polyreactive antibodies or HEp-2 reactive antibodies with only low levels of reactivity for cytoplasmic antigens but not nuclear antigens (Wardemann et al., 2003).
Mature naïve B cells responding to antigen differentiate into antibody secreting plasma cells and memory B cells (McHeyzer-Williams and McHeyzer-Williams, 2005; Meffre et al., 2000; Radbruch et al., 2006; Rajewsky, 1996; Shapiro-Shelef and Calame, 2005). In humans, two types of memory B cells have been described: IgM+ memory B cells and class-switched memory B cells (Agematsu et al., 1997; Klein et al., 1998; Tangye et al., 1998). Little is known about the origin of IgM+ memory B cells but by analogy to the mouse it has been proposed that these cells are products of T cell independent immune responses (Weller et al., 2004; Weller et al., 2001). We have shown that the transition from naïve B cells into circulating IgM+ memory B cells is accompanied by efficient counter-selection against self-reactive naïve B cells before the onset of somatic hypermutation and that the few self-reactive IgM+ memory B cells present in the circulation of healthy humans gain self-reactivity as a result of somatic hypermutation (Tsuiji et al., 2006).
In contrast, development of most class-switched memory B cells depends on the germinal center reaction and T cell help. Ig class-switching is accompanied by extensive Ig gene somatic hypermutation that could change antibody affinity or create self-reactive antibodies (Ray et al., 1996; Shlomchik et al., 1990; van Es et al., 1991).
To examine the establishment of self-tolerance in class-switched IgG+ memory B cells we cloned, expressed, and measured the reactivity of 141 antibodies from human IgG+ memory B cells isolated from peripheral blood of three healthy donors. Surprisingly, we found that self-reactive antibodies including true ANAs and polyreactive antibodies were significantly enriched in circulating IgG+ memory B cells relative to naïve B cells and IgM+ memory B cells.
Results
Features of IgG+ memory B cell antibodies
To characterize the antibody gene repertoire of IgG+ memory B cells we isolated single IgG+CD27+CD19+CD10- peripheral blood cells from three healthy donors and cloned their IgH and IgL chains (Figures 1 and S1 and Tables S1–S3) (Agematsu et al., 1997; Klein et al., 1998; Plebani et al., 1989; Tangye et al., 1998). B cells from all three donors showed an Ig subclass distribution reflecting that of human serum IgG antibodies with IgG1>IgG2>IgG3>IgG4 (Figure 1A; on average 63.4% IgG1, 25.5% IgG2, 8.9% IgG3 and 2.2% IgG4) and were somatically mutated with an average 18.0 ± 8.1, 9.7 ± 5.4, and 7.7 ± 4.0 mutations for IgH, Igκ and Igλ V genes respectively (Figures 1B and 1C). There were no significant differences in IgV gene mutation frequencies among IgG isotypes (data not shown) and as expected replacement mutations were more frequent than silent mutations in heavy and light chain V gene complementary determining regions (CDRs) compared to framework regions (FWRs), suggesting antigen mediated selection (Figure 1C). Overall, IgH and Igκ/λ light chain gene usage was similar between IgG memory and IgM mature naïve B cell antibodies, but antibodies with positively charged IgH CDR3 regions, a feature associated with self-reactivity, were enriched in IgG+ memory B cells (Figure 1D; P=0.014) (Jang et al., 1998; Radic et al., 1993)
Figure 1. IgH and IgL chain gene features from IgG memory B cell antibodies.
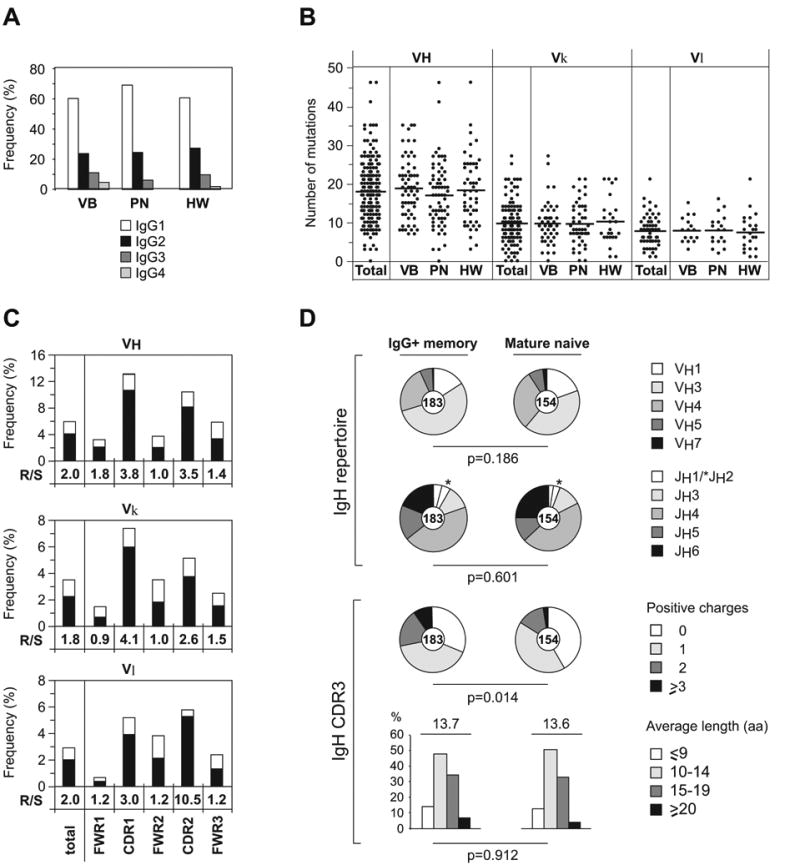
(A) IgG subclass distribution in single IgG+CD27+ memory B cells from three healthy donors.
(B) The number of mutations in VH, Vκ, and Vλ genes in antibodies from IgG+ memory B cells.
(C) The frequency of mutations in VH, Vκ, and Vλ genes in antibodies from IgG+ memory B cells calculated from the number of replacement (R; black bar) and silent (S; white bar) nucleotide exchanges per base pair in FWRs and CDRs. The R/S ratio for each region is indicated.
(D) IgH V and J gene repertoire and IgH CDR3 positive charges and length in amino acids (aa) of antibodies from IgG+ memory B cells compared to mature naïve B cells. Pie charts depict VH and JH family usage and the proportion of IgH CDR3s with 0, 1, 2, or ≥3 positive charges. Bar graphs show frequencies of IgH CDR3s with 9 aa (white bars), 10–14 aa (light gray bars), 15–19 aa (dark gray bars), and ≥20 aa (black bars). The absolute number of sequences analyzed is indicated in the center of each pie chart. Values for mature naïve B cells in this and other figures were published previously and are shown here for comparison (Tsuiji et al., 2006; Wardemann et al., 2003).
IgG+ memory B cells frequently express self-reactive antibodies
Human epithelial cell line, HEp-2, ELISA and indirect immunoflourescence assays (IFAs) are used as diagnostic tests to detect self-reactive antibodies in the serum of patients with autoimmune diseases (Egner, 2000). We used both assays to determine the frequency of self-reactive IgG memory B cell antibodies and to define their subcellular staining patterns (Figure 2 and Tables S1–S3). Nearly half of the 141 IgG memory B cell antibodies from three healthy donors showed low levels of HEp-2-reactivity (Figure 2A; 46.8%). Although there was individual variation (Figure 2A; 62.8% for VB, 46.3% for PN and 31.8% for HW) the frequency of HEp-2 self-reactive antibodies was higher in IgG+ memory B cells in all donors compared to antibodies from mature naïve B cells and IgM+ memory B cells (Figure 2B) (Tsuiji et al., 2006; Wardemann et al., 2003). Some mature naïve B cells do express HEp-2 reactive antibodies but these are rarely true ANAs and usually recognize cytoplasmic antigens (Wardemann et al., 2003). In contrast, a significant fraction of the self-reactive antibodies produced by IgG+ memory B cells from all three donors showed true ANA staining patterns by IFA including nuclear and nucleolar patterns typically associated with autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, or scleroderma (Figure 2). It is important to note that all three donors studied showed normal serum ANA IgG titres compared to patients with SLE (Figure 2C). We conclude that in healthy humans low-level self-reactive antibodies including ANAs are more abundant in the IgG+ memory B cells than in mature naïve B cells and IgM+ memory B cells.
Figure 2. Self-reactive antibodies are enriched in the IgG+ memory B cell pool.
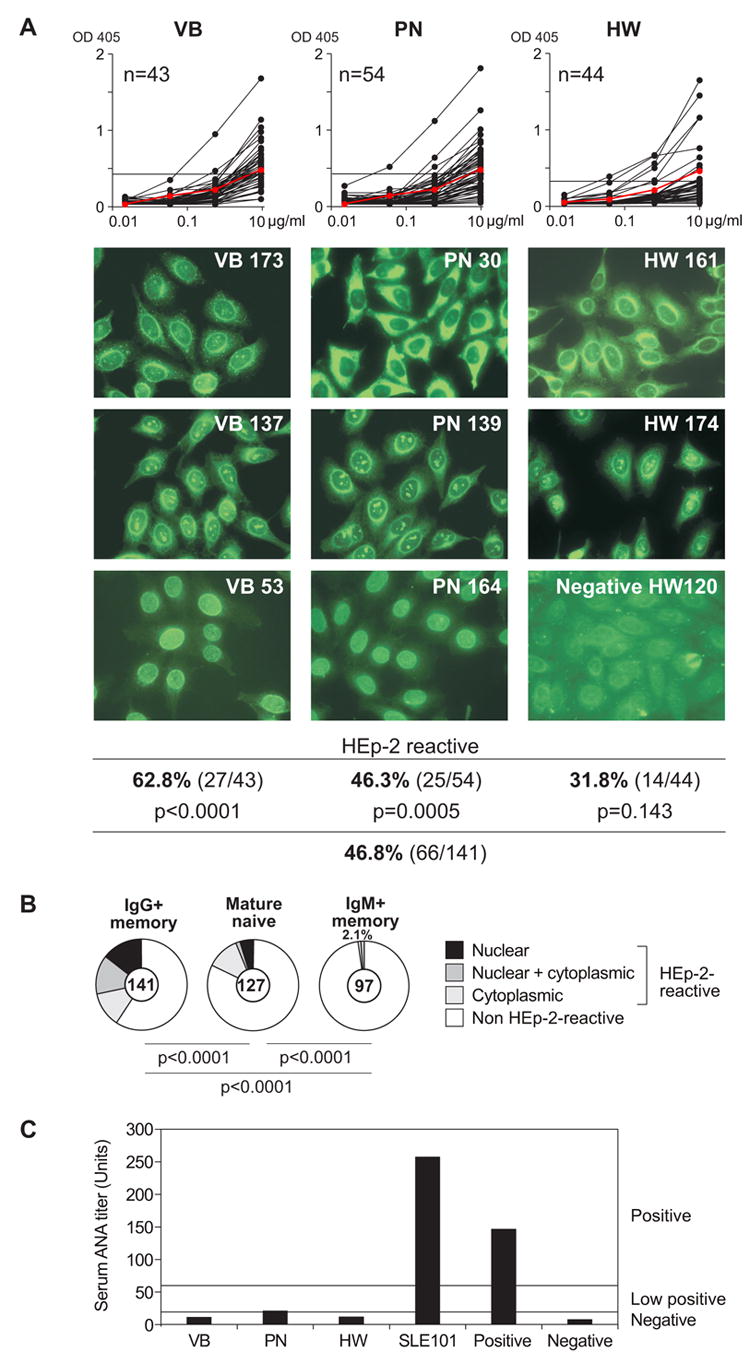
(A) IgG+ memory B cell antibodies from healthy donors were tested for self-reactivity by HEp-2 cell lysate ELISA and IFA. Horizontal line shows cut-off OD405 for positive reactivity determined by comparison to low positive control serum (red line). Typical HEp-2 cell IFA staining patterns of antibodies cloned from IgG+ memory B cells are shown.
(B) Pie charts summarizing the frequency of HEp-2 self-reactive IgG+ memory B cell antibodies with nuclear (black), nuclear plus cytoplasmic (dark gray), and cytoplasmic (light gray) IFA staining patterns, and the frequency of non-reactive antibodies (white) in comparison to mature naïve and IgM+ memory B cell clones (Tsuiji et al., 2006; Wardemann et al., 2003). The number of tested antibodies is indicated in each pie chart center. P-values are in comparison to mature naïve B cells and IgM+ memory B cells (Tsuiji et al., 2006; Wardemann et al., 2003).
(C) Serum IgG ANA levels of three healthy donors and one patient with Systemic lupus erythematosus (SLE101; Yurasov et al., 2005) were determined by HEp-2-ANA-ELISA. The manufacturer’s instructions were followed to calculate relative units based on internal positive and negative control sera. Horizontal lines show positive, low positive or negative cut-off titres as indicated
Polyreactive antibodies expressed by IgG+ memory B cells
To screen for polyreactive antibodies we tested the 141 IgG memory B cell antibodies for reactivity against a panel of defined antigens including double-stranded and single-stranded DNA (dsDNA/ssDNA), insulin and lipopoysaccharide (LPS; Figure 3). We found that on average 22.7% of the antibodies were polyreactive (Figure 3; 23.3% for VB, 22.2% for PN and 22.7% for HW) and these antibodies recognized several strains of bacteria and bacterial antigens (Figure S2 and Tables S1–S3). In addition, a substantial fraction of IgG memory B cell antibodies reacted with several strains of bacteria but not with self-antigens (20.9% for VB, 20.4% for PN and 15.9% for HW; Figure S2 and Tables S1–S3) and 1/141 antibodies showed high levels of specific reactivity with Staphylococcus aureus but not with any of the other antigens tested (HW 224; Figure S2 and Table S3). We conclude that the frequency of polyreactive antibodies is significantly higher in the IgG+ memory B cell compartment than in mature naïve B cells (22.7% vs. 6.2% in mature naïve; P<0.0001, Figure 3; Wardemann et al., 2003) or IgM+ memory B cells (22.7% vs. 1.0% in IgM+ memory; P<0.0001; Figure 3; Tsuiji et al., 2006) with no significant differences between isotype subclasses (Tables S1–S3 and data not shown).
Figure 3. Polyreactive antibodies contribute to the IgG+ memory B cell compartment.
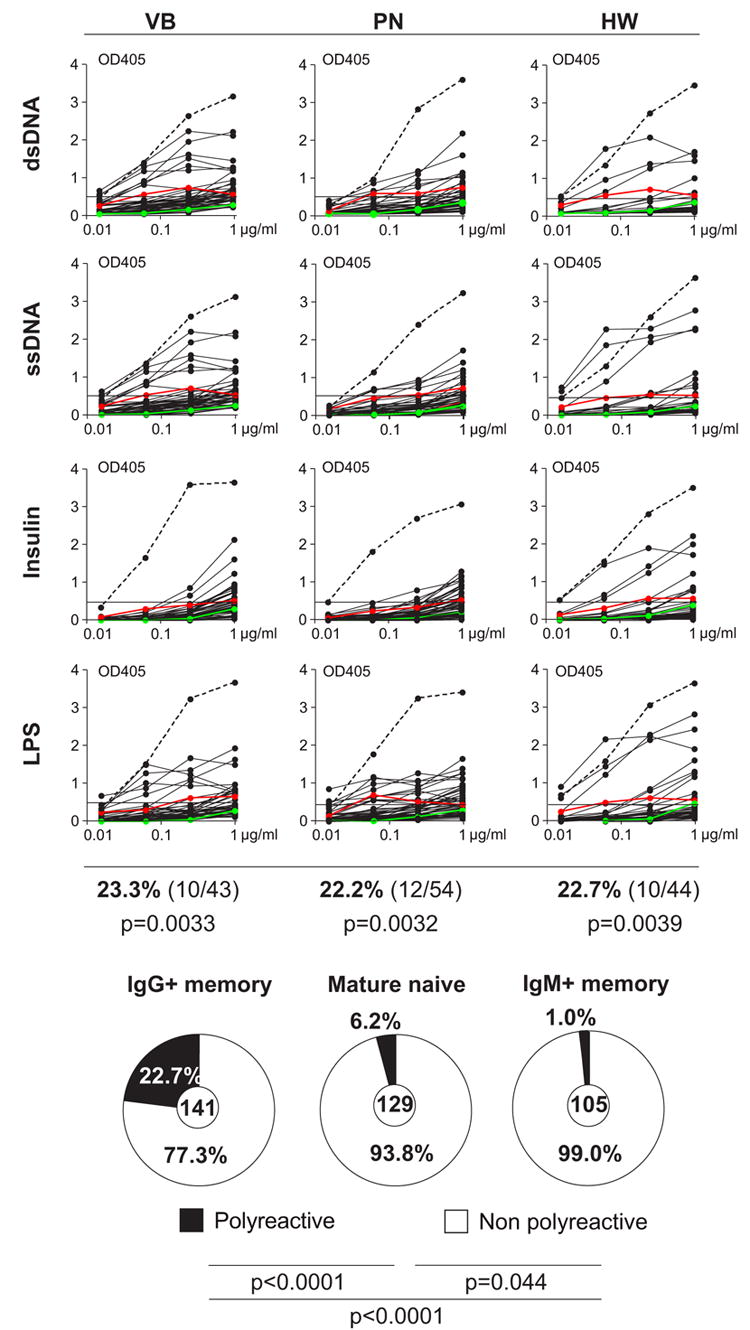
IgG+ memory B cell antibodies from healthy donors were tested for polyreactivity by ELISA with ds/ssDNA, LPS, and insulin. Dotted lines represent the high positive control antibody ED38 (Meffre et al., 2004). Horizontal lines show cut-off OD405 for positive reactivity as determined by comparison to the negative control antibody mGO53 (green line) and low positive control eiJB40 (red line; Wardemann et al., 2003). Pie charts show the frequency of polyreactive clones from IgG+ memory B cells from all three donors compared to mature naïve B cell and IgM+ memory B cell antibodies (Tsuiji et al., 2006; Wardemann et al., 2003). The number of tested antibodies is indicated in the center. P-values are in comparison to mature naïve B cells and IgM+ memory B cells (Tsuiji et al., 2006; Wardemann et al., 2003).
Somatic hypermutation creates polyreactivity and self-reactivity
The increase in self-reactivity during the transition between mature naïve and IgG+ memory B cells might be due to a selective advantage for pre-existing self-reactive cells, or selection for cells with self-reactive antibodies produced by somatic hypermutation. To determine the origin of the self-reactive antibodies we reverted the somatic mutations of 36 randomly chosen self-/polyreactive and non-reactive IgG memory B cell antibodies to their unmutated germline forms by PCR (Table S4; Herve et al., 2005; Tsuiji et al., 2006) and tested the recombinant antibodies for polyreactivity with ds/ssDNA, insulin and LPS (Figure 4 and Table S4 and data not shown). Twelve out of these 36 antibodies were initially polyreactive (Figure 4A, upper left panel). Of these, 3 (25%) still exhibited polyreactivity in the corresponding germline form, while the other 9 (75%) were completely negative (Figure 4A, upper right panel). Of the remaining twenty-four antibodies that were not polyreactive in their mutated form (Figure 4A, lower left panel), the vast majority (91.6%; 22/24) were also not polyreactive in the absence of mutations (Figure 4A, lower right panel). We found only two antibodies out of the initial 36 that showed polyreactivity in the germline but not in the mutated form (Figure 4A, lower right panel). Similar results were obtained when HEp-2 cell reactivity was analyzed by IFA and ELISA (Figures 4B and S3 and Table S4 and data not shown). We conclude that most self-reactive and polyreactive IgG antibodies originate from precursors that acquired reactivity by somatic hypermutation.
Figure 4. Somatic hypermutation contributes to self-reactivity in IgG memory B cell antibodies.
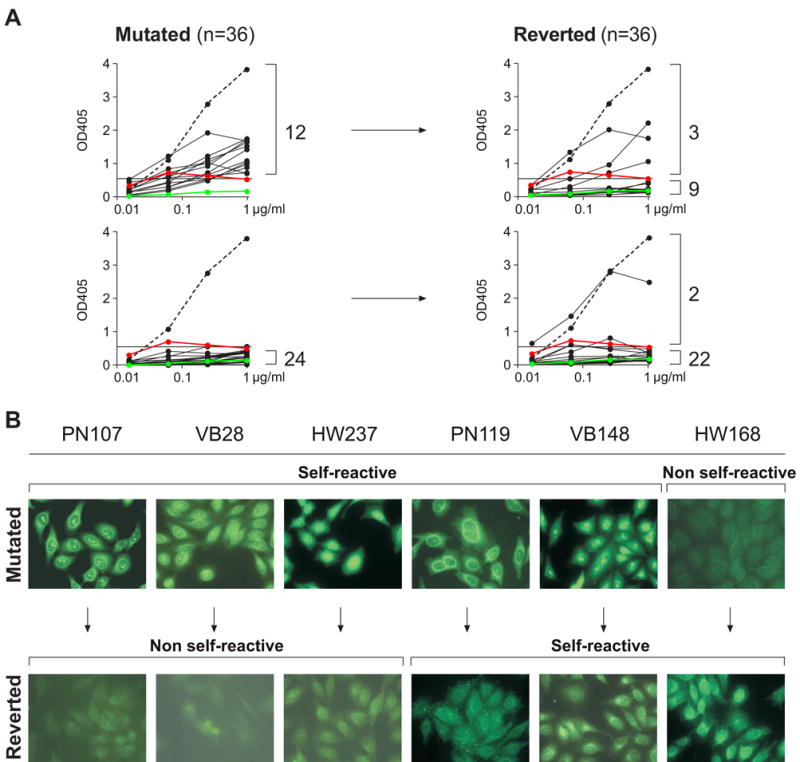
(A) IgH and IgL chains from IgG+ memory B cell antibodies were reverted into their germline counterparts by PCR. Recombinant polyreactive (upper left) and non-polyreactive (lower left) IgG+ memory B cell antibodies and their germline counterparts (right) were tested for polyreactivity by ELISA with ds/ssDNA, insulin and LPS. Representative graphs with dsDNA as antigen are shown. Dotted lines represent the high positive control antibody ED38 (Meffre et al., 2004). Horizontal lines show cut-off OD405 for positive reactivity as determined by comparison to the previously published control antibodies mGO53 (negative control: green line; Wardemann et al., 2003) and eiJB40 (low positive control: red line; Wardemann et al., 2003). (B) Typical HEp-2 cell IFA staining patterns of mutated IgG+ memory B cell antibodies (top) and their germline counterparts (bottom).
Serum IgM vs. IgG
Most polyreactivity in human serum has been attributed to IgM and not IgG (Coutinho et al., 1995; Guilbert et al., 1982; Seigneurin et al., 1988). However, secreted IgM is a pentamer, which has greater avidity than monomeric antibodies such as IgG. To determine the role of avidity in polyreactivity we reduced and purified monomeric human IgM from serum of pooled donors and from two of our individual donors (Figures 5 and S4). When tested at equal molar ratios in polyreactivity ELISAs with dsDNA, insulin and LPS monomeric IgMs were less reactive than purified serum IgG antibodies (Figure 5 and data not shown). In contrast, the pentameric IgM antibodies were more reactive than corresponding IgGs (Figure 5 and data not shown). Thus, the increased avidity of multimeric IgM is essential for their higher polyreactivity. We conclude that in humans, monomeric IgMs such as those found in the B cell antigen receptor expressed on naïve and IgM+ memory B cells are less polyreactive than the corresponding IgGs found on IgG+ memory B cells.
Figure 5. Monomeric IgM from human serum is less self-reactive than serum IgG.
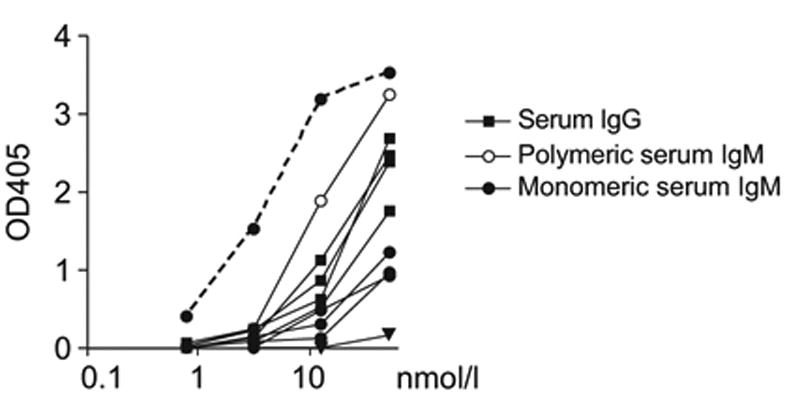
Polymeric IgM from pooled human plasma of healthy donors (open circles) and from serum of two healthy donors (PN, HW) was reduced under mild conditions to its monomeric IgM subunits (filled circles). Serum IgG antibodies from pooled human plasma of healthy donors and from three healthy donors (PN, HW, VB) were purified with Protein-G beads (squares). Equal molar quantities of the antibodies were tested by ELISA for reactivity against dsDNA. Control antibodies were the highly polyreactive ED38 (dotted line; Meffre et al., 2004) and negative mGO53 (triangles; Wardemann et al., 2003).
Discussion
Isolated VH genes cloned from unseparated peripheral human B cells show autoreactivity (Lecerf et al., 1998) when expressed in bacteria. However, the reactivity of the intact antibodies from which the VH genes were cloned could not be determined because they were expressed in absence of light chains which play a very important role in determining autoantibody reactivity (Wardemann et al., 2004). Furthermore, the representation of autoreactive B cells could not be assesed by such approaches since the amount of IgG mRNA produced varies with the stage of B cell differentiation and cloning from pools of cells would lead to over-representation by cells producing higher levels of IgG mRNA.
To examine the development of B cell tolerance in humans we cloned antibodies from developing, naïve and memory B cells and tested them for reactivity against a panel of defined antigens and HEp-2 cells (Tsuiji et al., 2006; Wardemann et al., 2003). We found that cells expressing self-reactive antibodies are efficiently excluded from the naive and circulating IgM+ memory B cell pool (Tsuiji et al., 2006; Wardemann et al., 2003). Surprisingly, development of IgG memory involved selective enrichment of B cells that produce antibodies that react with self-antigens. Many of these anti-self antibodies were authentic ANAs as determined by IFA on HEp-2 cells.
As many as one out of three healthy humans shows ANA reactivity at a screening dilution of 1/40 in clinical ELISA tests using HEp-2 cells (Egner, 2000). However, the cellular origin of these ANAs has never been determined. Although memory B cells do not produce high levels of secreted antibodies, they develop into plasma cells that do so (Bernasconi et al., 2002; Radbruch et al., 2006). Our experiments suggest that the ultimate source of the high levels of serum anti-self antibodies found in clinical ELISA assays is the large number of memory B cells that produce such antibodies.
What is the origin of the ANA expressing IgG+ memory B cells in normal humans? Only a few IgG memory B cell antibodies were polyreactive in their germline form, reflecting the paucity of such cells in the naïve B cell compartment (Wardemann et al., 2003). In addition, there were no significant differences in Ig gene usage between mature naïve B cells and IgG+ memory B cells including VH4-34, which is thought to have intrinsic autoreactivity (Cappione et al., 2005). Thus, there appears to be no initial selection for or against low levels of autoreactivity in entering the germinal center (GC). Instead, most of the selection occurs after somatic hypermutation either in the GC or after B cells emerge from the GC and enter the long lived IgG memory compartment. Irrespective of the compartment in which the cells are selected or the mechanism, it is likely that self-reactivity in the IgG+ memory B cell compartment represents a by-product of affinity maturation for binding to unknown foreign antigens (Casson and Manser, 1995; Diamond and Scharff, 1984; Liu et al., 1989; Pulendran et al., 1995, Han et al., 1995; Shokat and Goodnow, 1995; Wellmann et al., 2005). Consistent with this idea polyreactive IgGs are highly mutated with replacement/substitution ratios indicating antigen-mediated selection (Berek et al., 1991; Weiss and Rajewsky, 1990). In addition, there are well known examples of highly specific human antibodies that are also polyreactive e.g. broadly neutralizing anti-HIV antibodies (Haynes et al., 2005; Muster et al., 1993; Zwick et al., 2001).
In mice, both low and high affinity B cells are initially recruited to GCs but competition ensures that only high-affinity clones become memory cells (Paus et al., 2006; Shih et al., 2002). Our experiments suggest that there is also no initial selection against polyreactivity in seeding GCs. Thus, patients with SLE and rheumatoid arthritis that show abnormally high numbers of mature naïve B cells producing self-reactive and polyreactive antibodies may also have larger numbers of such cells in GCs, but whether this will impact on the nature of the memory repertoire in these patients remains to be determined (Samuels et al., 2005; Yurasov et al., 2006; Yurasov et al., 2005).
Finding large numbers of polyreactive B cells in the IgG memory compartment was surprising because most polyreactivity in sera has been attributed to IgM (Coutinho et al., 1995; Guilbert et al., 1982; Seigneurin et al., 1988). However, any direct comparison of monomeric IgG and pentameric IgM would naturally favor IgM because of the greater avidity of the latter. Comparison of monomeric serum IgM to IgG shows that it is the IgG fraction that contains higher levels of polyreactivity.
In mice, natural IgM antibodies with low levels of self-reactivity and polyreactivity are produced by B1 and marginal zone B cells and they are protective early in immune responses to a variety of pathogens (Baumgarth et al., 2000; Haas et al., 2005; Martin et al., 2001). The human equivalents of the mouse B1 cells have not been defined and human marginal zone B cells differ from mouse marginal zone B cells in that they show signs of antigen-mediated selection, display a memory B cell phenotype, and circulate in the bloodstream (Weller et al., 2004; Weller et al., 2001). Furthermore, the relative roles of IgM and IgG antibodies in early protection against infection have not been determined in mouse or human. However, a role for IgG in early protection in humans is suggested by increased susceptibility to bacterial infection in patients unable to produce IgG as a result of B cell intrinsic hyper-IgM syndrome, its reversal by passive transfer of pooled IgG, and by our finding that IgGs are frequently reactive against a number of different species of bacteria or bacterial antigens (Alachkar et al., 2006; Quartier et al., 2004). In addition to their role in immune protection, natural IgM antibodies have also been implicated in prevention of autoimmunity by binding and clearing of apoptotic cells (Kim et al., 2002). A similar role for IgG in preventing autoimmunity is suggested by the finding that patients with hyper-IgM type 2 syndrome are also highly susceptible to autoimmunity (Quartier et al., 2004). Despite the lower overall avidity of IgGs, these molecules may be important in early protection against infection and in prevention of autoimmunity because they can engage both the complement system and Fc receptors (Carroll, 2004; Ravetch and Clynes, 1998). This feature enables a series of important protective effector functions not available to IgMs, which do not bind to conventional Fc receptors.
In summary, our data demonstrate that a large number of circulating IgG+ memory B cells normally produce low affinity non-pathogenic autoantibodies. How many of these cells eventually develop into plasma cells and contribute to steady state antibody titers remains to be determined. Under normal conditions, autoreactive IgG+ memory B cells may be anergic. Alternatively, an additional and yet to be defined checkpoint may control the differentiation of self-reactive IgG+ memory B cells into antibody secreting plasma cells (Han et al., 1995; Shokat et al.,1995; Pulendran et al., 1995; Klinman, 1996). Nevertheless, abnormalities in checkpoint regulation or activation of peripheral self-reactive IgG+ memory B cells may contribute to the development of autoimmunity in susceptible individuals (Bernasconi et al., 2002; Kaneko et al., 2006; Mackay et al., 2006; Radbruch et al., 2006).
Experimental Procedures
Single B Cell Sorting
All samples were obtained after signed informed consent in accordance with IRB-reviewed protocols at the Rockefeller University. Control data from mature naïve B cells were previously published and are shown for comparison. Single CD19+CD10-CD27+IgG+ B cells were isolated as described (Wardemann et al., 2003) after staining with anti-CD19-APC, anti-CD10-PE, anti-CD27-FITC, anti-IgG-Biotin (BD Biosciences Pharmigen) and Streptavidin-PECy7 (Caltag Laboratories).
PCR Amplification and Expression Vector Cloning
Single cell cDNA synthesis was performed as described using Superscript III at 50 °C (Wardemann et al., 2003). Igγ and Igk/λ chain genes were amplified in two rounds of nested PCR (manuscript in preparation and Tsuiji et al., 2006; Wardemann et al., 2003; Yurasov et al., 2006; Yurasov et al., 2005). All PCR products were sequenced and analyzed for Ig gene usage and CDR3 analysis, number of V gene mutations (Ig-Blast; Tables S1–3) and for IgG isotype subclass (http://imgt.cines.fr). Second PCR products for Igλ genes contained restriction sites allowing direct cloning into expression vectors. For Igγ and Igk genes restriction sites for expression vector cloning were introduced after sequencing using gene specific primers and 1st PCR products as template. All IgH and IgL chain genes were sequenced after cloning to confirm identity with the original PCR products.
Antibody Production, ELISA and Indirect Immunofluorescence Assay
Antibodies were expressed and tested for polyreactivity with ds/ssDNA, insulin and LPS and for self-reactivity with HEp-2 cells by ELISA and IFA as described (Wardemann et al., 2003). ELISA against bacteria was described previously (Tsuiji et al., 2006). Threshold values for reactivity are indicated in the graphs and were set in all assays using our previously published control antibodies mGO53 (negative), eiJB40 (low positive), and ED38 (high positive) for polyreactivity ELISAs and additional positive and negative control sera for HEp-2 reactivity (Meffre et al., 2004; Wardemann et al., 2003). Data shown are representative for at least 2 independent experiments.
Reversion of Hypermutated Sequences to Germline
Antibodies for reversion experiments were chosen randomly and are listed in Table S4. Mutated IgH and IgL chain genes were reverted to their germline sequence by two separate PCR reactions for the V gene and the (D)J gene followed by a third overlap PCR to fuse the two PCR products as described (Herve et al., 2005; Tsuiji et al., 2006). All reverted IgH and IgL chain PCR products were sequenced before and after cloning into the corresponding expression vectors to confirm lack of mutations. Recombinant mutated and un-mutated antibodies were expressed and tested for polyreactivity and self-reactivity in comparison as described above.
Statistics
P-values for Ig gene repertoire analyses, analysis of positive charges in IgH CDR3, and antibody reactivity were calculated by 2 x 2 or 2 x 5 Fisher Exact test or Chi Square Test. P-values for IgH CDR3 length were calculated by Student’s t-test.
Serum IgM and IgG Purification and Reduction of IgM
IgM was purified from serum of three healthy donors as described (Cambier and Butler, 1974). In brief, 20 ml of clarified whole serum were dialyzed against 0.01M potassium phosphate buffer, pH 5.4, for 24h at 4°C. Precipitates were dissolved in 4ml 0.01M acetate buffer, pH 5.4, containing 0.15M NaCl. 0.1M ZnSO4 was added drop-wise to a final concentration of 10mM. The preparations were stirred at 25°C for 2h prior to centrifugation at 3000rpm for 15 min. Supernatants were subjected to a Superdex™ 200 column (GE Healthcare) equilibrated with 0.1M Tris-HCl buffer, pH8.6, containing 0.1M NaCl and eluted fractions were collected. IgG was purified from human serum of the same three donors with ProteinG beads (GE Healthcare) following the manufacturer’s instructions.
To obtain IgM subunits IgM was reduced in 0.5M Tris buffer, pH 8.6, containing 10mM cysteine for 2 – 4h at room temperature. The optimal incubation conditions were determined empirically for each IgM preparation. The reaction was stopped with 10% molar excess of iodoacteamide and applied to a Superdex™200 column (GE Healthcare) equilibrated with sodium borate buffer, pH 8.6. Size and purity of the preparations was monitored by non-reducing SDS-PAGE (see Figure S5). Monomeric IgM concentration was determined by serial dilution on SDS-PAGE with total human IgG (Sigma) standards. Equal molar amounts of IgMs (180kD) and IgG (156kD) were used in indirect ELISAs with ds/ssDNA, insulin and LPS as antigens as described above (Wardemann et al., 2003). Reactive antibodies were detected with anti-human IgM/IgG-HRP antibody (Jackson ImmunoResearch) and confirmed in independent experiments with either anti-human kappa-HRP or anti-human lambda-HRP (Biosource).
Supplementary Material
Acknowledgments
We thank all members of the Wardemann and Nussenzweig laboratory and Anna Gazumyan, Robert Hurwitz and Ralf Winter for technical advice on IgM purification and reduction and Eva Besmer for help with the manuscript. This work was supported by grants from the National Institutes of Health (MCN), the Dana Foundation (HW) and the Naito Foundation (MT). MCN is a Howard Hughes Medical Investigator. SY is supported by career development grants from the NIH and the New York Chapter of the Arthritis Foundation.
Abbreviations
- ANA
anti-nuclear antibody
- CDR
complementary determining regions
- FWR
framework region
- IFA
indirect immunoflourescence assay
- SLE
systemic lupus erythematosus
- GC
germinal center
Footnotes
Competing interests statement
The authors declare that they have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agematsu K, Nagumo H, Yang FC, Nakazawa T, Fukushima K, Ito S, Sugita K, Mori T, Kobata T, Morimoto C, Komiyama A. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997;27:2073–2079. doi: 10.1002/eji.1830270835. [DOI] [PubMed] [Google Scholar]
- Alachkar H, Taubenheim N, Haeney MR, Durandy A, Arkwright PD. Memory switched B cell percentage and not serum immunoglobulin concentration is associated with clinical complications in children and adults with specific antibody deficiency and common variable immunodeficiency. Clin Immunol. 2006;120:310–318. doi: 10.1016/j.clim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. The Journal of Experimental Medicine. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- Cambier JC, Butler JE. A rapid method for the purification of immunoglobulin M (IgM) from the sera of certain mammalian species. Prep Biochem. 1974;4:31–46. doi: 10.1080/00327487408068184. [DOI] [PubMed] [Google Scholar]
- Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Casson LP, Manser T. Random mutagenesis of two complementarity determining region amino acids yields an unexpectedly high frequency of antibodies with increased affinity for both cognate antigen and autoantigen. The Journal of Experimental Medicine. 1995;182:743–750. doi: 10.1084/jem.182.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Diamond B, Scharff MD. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci USA. 1984;81:5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol. 2000;53:424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779–2787. [PubMed] [Google Scholar]
- Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. The Journal of Experimental Medicine. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Herve M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT, Chiorazzi N, Meffre E. Unmutated and mutated chronic lymphocytic leukemia derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115:1636–1643. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, Sanford D, Chung HY, Baek SY, Stollar BD. The structural basis for DNA binding by an anti-DNA autoantibody. Molecular immunology. 1998;35:1207–1217. doi: 10.1016/s0161-5890(98)00095-9. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. The Journal of Experimental Medicine. 2002;196:655–665. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. The Journal of Experimental Medicine. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman NR. The "clonal selection hypothesis" and current concepts of B cell tolerance. Immunity. 1196;5:189–195. doi: 10.1016/s1074-7613(00)80314-3. [DOI] [PubMed] [Google Scholar]
- Lecerf JM, Chen Y, Richalet-Secordel P, Wang X, Stollar BD. Autoreactivity of human VH domains from cDNA libraries: analysis with a bacterial expression system. J Immunol. 1998;161:1274–1283. [PubMed] [Google Scholar]
- Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the Fc{gamma}IIB receptor on memory B cells in SLE. The Journal of Experimental Medicine. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- Meffre E, Casellas R, Nussenzweig MC. Antibody regulation of B cell development. Nature Immunol. 2000;1:379–385. doi: 10.1038/80816. [DOI] [PubMed] [Google Scholar]
- Meffre E, Schaefer A, Wardemann H, Wilson P, Davis E, Nussenzweig MC. Surrogate Light Chain Expressing Human Peripheral B Cells Produce Self-reactive Antibodies. The Journal of Experimental Medicine. 2004;199:145–150. doi: 10.1084/jem.20031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. The Journal of Experimental Medicine. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani A, Ugazio AG, Avanzini MA, Massimi P, Zonta L, Monafo V, Burgio GR. Serum IgG subclass concentrations in healthy subjects at different age: age normal percentile charts. Eur J Pediatr. 1989;149:164–167. doi: 10.1007/BF01958271. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Quartier P, Bustamante J, Sanal O, Plebani A, Debre M, Deville A, Litzman J, Levy J, Fermand JP, Lane P, et al. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to Activation-Induced Cytidine Deaminase deficiency. Clin Immunol. 2004;110:22–29. doi: 10.1016/j.clim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150:4966–4977. [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci U S A. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. The Journal of Experimental Medicine. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin JM, Guilbert B, Bourgeat MJ, Avrameas S. Polyspecific natural antibodies and autoantibodies secreted by human lymphocytes immortalized with Epstein-Barr virus. Blood. 1988;71:581–585. [PubMed] [Google Scholar]
- Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. The Journal of Experimental Medicine. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. The Journal of Experimental Medicine. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. The Journal of Experimental Medicine. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Gmelig Meyling FH, van de Akker WR, Aanstoot H, Derksen RH, Logtenberg T. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. The Journal of Experimental Medicine. 1991;173:461–470. doi: 10.1084/jem.173.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Hammersen J, Nussenzweig MC. Human autoantibody silencing by immunoglobulin light chains. The Journal of Experimental Medicine. 2004;200:191–199. doi: 10.1084/jem.20040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Weiss U, Rajewsky K. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. The Journal of Experimental Medicine. 1990;172:1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a pre-diversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, Hermine O, Fischer A, Reynaud CA, Weill JC. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurasov S, Tiller T, Tsuiji M, Velinzon K, Pascual V, Wardemann H, Nussenzweig MC. Persistent expression of autoantibodies in SLE patients in remission. The Journal of Experimental Medicine. 2006;203:2255–2261. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. The Journal of Experimental Medicine. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


