Abstract
Electrical synapses are abundant before and during developmental windows of intense chemical synapse formation, and might therefore contribute to the establishment of neuronal networks. Transient electrical coupling develops and is then eliminated between regenerating Helisoma motoneurons 110 and 19 during a period of 48-72 hours in vivo and in vitro following nerve injury. An inverse relationship exists between electrical coupling and chemical synaptic transmission at these synapses, such that the decline in electrical coupling is coincident with the emergence of cholinergic synaptic transmission. In this study, we have generated two- and three-cell neuronal networks to test whether predicted synaptogenic capabilities were affected by previous synaptic interactions. Electrophysiological analyses demonstrated that synapses formed in three-cell neuronal networks were not those predicted based on synaptogenic outcomes in two-cell networks. Thus, new electrical and chemical synapse formation within a neuronal network is dependent on existing connectivity of that network. In addition, new contacts formed with established networks have little impact on these existing connections. These results suggest that network-dependent mechanisms, particularly those mediated by gap junctional coupling, regulate synapse formation within simple neural networks.
INTRODUCTION
Electrical coupling is widespread in invertebrate nervous systems as well as in the developing mammalian brain. Neurons functionally coupled by electrical synapses possess coordinated patterns of spontaneous activity and changes in intracellular calcium concentration (Yuste et al., 1992). Some of these patterned activities are due to the presence of gap junctions, i.e., membrane channels that provide a conduit for the passage of electrical current (charged ions) and small cytoplasmic messengers, such as IP3 and cAMP, between contacting cells (Kandler and Katz, 1998). In many mammalian brain regions, electrical synapses are abundant before periods of chemical synapse formation and then decline in number as the extent of chemical neurotransmission increases (Bennett and Zukin, 2004). The appearance of this gap junctional communication, and the resulting synchronization of neuronal activities, is thought to be important for the development of neuronal assemblies (Kandler and Katz, 1995) and maturation of neuronal networks (Allen and Warner, 1991; Walton and Navarrete, 1991; Roerig and Feller, 2000; Peinado 2001; Personius et al., 2001; Montoro and Yuste, 2004). For example, dye coupling between magnocellular neurons of the rat hypothalamus increases in vivo during postnatal weeks one and two and then decreases during a period of intense chemical synapse formation at postnatal weeks three and four (Arumugam et al., 2005). Chronic block of NMDA receptors significantly reduces uncoupling among these hypothalamic neurons, demonstrating that chemical synaptic signaling can regulate the developmental uncoupling of gap junctions. Similarly, in spinal motoneurons, NMDA signaling mediates gap junction uncoupling (Mentis et al., 2002). A rapid developmental transition from gap junction-coupled to NMDA receptor-mediated synaptic transmission is also thought to function in cortical network formation (Dupont et al., 2006).
A sequential progression from electrical coupling to chemical synaptic transmission exists at regenerating synapses in the snail, Helisoma, both in vivo and in vitro. At a period of transition from electrical to chemical communication, the two types of synaptic transmission appear to be inversely related; i.e., gap junctional coupling is sustained when cholinergic neurotransmission is blocked with tubocurare and chemical synaptic transmission is increased when electrical coupling is reduced (Szabo et al., 2004). While many examples of the temporal progression from electrical to chemical neurotransmission have been reported (Kandler and Katz, 1998; Szabo et al., 2004; Arumagam et al, 2005; Dupont et al., 2006) and underlying mechanisms have been proposed (Arumugam et al., 2005; Neunuebel and Zoran, 2005), much is still unknown about the influence of these interactions on the formation of neuronal networks. To directly address this question, we have used the defined synapses formed between identified Helisoma neurons in vitro to determine if the process of transient electrical coupling affects subsequent synapse formation in three-cell neuronal networks.
Transient electrical synaptic connections develop between Helisoma motoneurons 110 and 19, forming and then disappearing over a 48-72 hour period (Szabo et al., 2004; Neunuebel and Zoran, 2005). The decline in electrical coupling is coincident with the emergence of unidirectional cholinergic synaptic transmission between presynaptic neuron 110 and postsynaptic neuron 19. In the current study, we have generated two- and three-cell neuronal networks to determine 1) whether new synaptic contacts impact extant network connectivity, and 2) whether the formation of new connections is affected by a neuron’s prior synaptic connectivity. Our results demonstrate that electrical and chemical synapses form in three-cell networks in a manner not predicted from two-cell networks, suggesting that gap junctional coupling and uncoupling plays an important role in regulating neuronal network formation in a cell-specific manner.
MATERIALS AND METHODS
Experiments were conducted on laboratory stocks of albino (red) pond snails, Helisoma trivolvis, which were maintained in 20-gallon aquaria at 26°C. Aquaria were kept on a controlled photoperiod of 12-hour light/12-hour dark. Animals were fed lettuce and trout chow daily.
Cell culture preparations
The removal of buccal neurons from the ganglia and their preparation for use in cell culture has been described (Haydon, 1988; Haydon and Zoran, 1991). Cells were initially transferred into non-adhesive, 35mm culture dishes (No. 1008 Falcon) containing 2ml of conditioned medium (CM). CM was bulk-cultured in silicone-treated (Sigmacote) glass petri dishes, before being transferred to culture dishes that had been made non-adhesive by pretreatment with a 0.5% solution of bovine serum albumin (BSA). Neurons were maintained in these non-adhesive culture conditions as single, spherical cells for 3d before being transferred into fresh BSA- or poly-L-lysine (PLL)-coated dishes (35mm, No. 3001 Falcon), also containing 2ml of CM. PLL-coated dishes caused neurons to adhere to the dish surface, which permitted neurite extension and specific identification of each cell at a later time-point based on its location in the dish. Neurons were plated either in isolation, or in contact with an appropriate partner(s) depending on the study. Neurons were maintained in these culture conditions for 1-5 days while extending processes and forming intercellular contacts. In some studies, neurons were cultured as pairs of neuronal somata, where neurite extension was suppressed (Haydon, 1988).
General electrophysiology
Electrophysiological properties of neurons were examined using intracellular recording techniques. All recordings were made from neurons plated into culture. Briefly, glass microelectrodes (borosilicate; FHC) were filled with 1.5M KCl and possessed tip resistances ranging from 10-20MΩ. Current-clamp recordings of neuronal membrane potentials were amplified using a bridge-balanced electrometer (Getting Instruments Inc.) and records were viewed on a storage oscilloscope (Tektronix). Neuronal membrane potential was maintained with base current injection (BCI) at approximately -70mV.
Electrical coupling was measured by injecting constant amplitude, hyperpolarizing current pulses (3s in duration) into one neuron and simultaneously recording membrane voltage changes in both the presynaptic (injected) neuron and the postsynaptic, electrically coupled, partner(s). Coupling coefficients were determined as the ratio of postsynaptic to presynaptic voltage changes (Bennett, 1977). Data analyses for coupling ratios and input resistance measurements were taken at the point of maximal membrane hyperpolarization. Analysis of unidirectional coupling data determined that electrical connections formed between all neuronal pairs were non-rectifying. Thus, all analyses presented were averages of bi-directional data and a single mean coupling coefficient was calculated for each pair. In some cases, day 5 data were normalized to levels present in the same networks on day 1.
An analysis of chemical connections from neuron 110 onto neuron 19 was conducted by injection of suprathreshold depolarizing current into presynaptic neurons. Four to five trains of 10 action potentials (APs, 1 per second) were evoked presynaptically in neuron 110, while the corresponding postsynaptic potentials (PSPs) in neuron 19 were recorded simultaneously. Criteria were developed to distinguish between electrical and chemical contributions (see Szabo et al., 2004). An analysis of 110-19 preparations possessing exclusively chemical connectivity indicated that the average latency from AP peak to PSP peak was 95msec. Stimulation of neuron 19, in contrast to presynaptic 110, never evoked chemical PSPs in co-cultured neurons. For three-cell experiments, simultaneous recordings were performed from all three cells and connectivity of the central cell with each of the other two was assessed. Data analyses for coupling ratios and input resistance measurements were taken at the peak membrane potential changes associated with hyperpolarizing current injections. Electrophysiological recordings were digitized by a MacLab A/D data acquisition system linked to a Macintosh computer using Chart software. Records were archived onto a magneto-optical disk for later analysis and printing.
Data analysis
Comparisons of electrical coupling were analyzed using either student’s t-tests (Microsoft Excel) or ANOVA, Fisher′s Least Significant Difference (LSD) (Statistica). Data are presented as mean plus or minus standard error of the mean (sem) unless otherwise indicated.
RESULTS
Buccal neurons 19 and 110 were cultured in conditions permitting neurite outgrowth, which assured contact among neurons of two- and three-cell networks. We first determined whether the sequence of synaptogenic events previously observed in these neurons lacking neurites (Szabo et al., 2004) also occurred in neurite-bearing networks. Cells were plated in each possible two-cell configuration, as 19-19, 19-110 and 110-110 partners, and were then incubated for 1 or 5 days of contact. Neurite extension in these cultures was elaborate and although all somata were initially plated in contact, in some cases neuritic fascicles formed between somata separated during process outgrowth (Fig. 1A and B).
Figure 1.
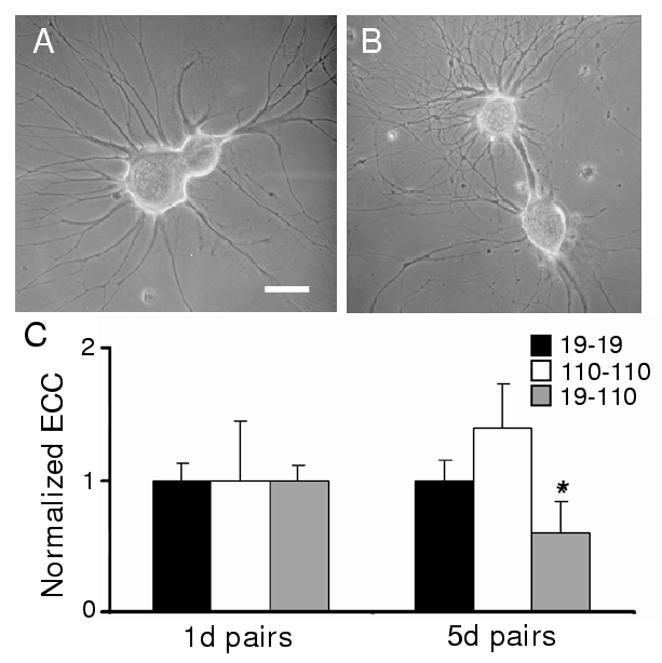
Formation of electrical synaptic connections between neurons paired in outgrowth-permissive conditions. (A) Neurons were plated in contacting pairs for 5d on PLL-coated dishes, which promoted adherence to the dish surface and outgrowth of processes. After 5d of outgrowth, neurons 19 (larger soma) and 110 exhibited extensive neuritic processes. (B) Although neurons were plated with contacting somata, in some pairs somata moved apart as a large fascicle of processes developed between the two cell bodies. (C) Electrical coupling coefficients (ECCs) were determined as the ratio of postsynaptic to presynaptic voltage changes. ECC values (normalized mean ± sem) for 19-19, 110-110, and 19-110 pairs, with 5d ECC values normalized to 1d values. (19-19: 1d ECC = 0.32 ± 0.06, n = 29 vs. 5d ECC = 0.32 ± 0.07, n = 28; NSD (no significant difference). 110-110: 1d ECC = 0.11 ± 0.05, n = 6 vs. 5d ECC = 0.15 ± 0.05, n = 6; NSD. 19-110: 1d ECC = 0.33 ± 0.06, n = 32 vs. 5d ECC = 0.20 ± 0.05, n = 19; *, p<0.05, student’s t-test).
Electrical and chemical neurotransmission in two-cell neuronal networks
Electrical connections formed in a manner similar to that seen previously in vivo (Szabo et al., 2004). After 24h of cell-cell contact, 19-19 and 19-110 pairs possessed coupling coefficients (ECCs) of 0.32 ± 0.06 (n = 29) and 0.33 ± 0.06 (n = 32), respectively. Two-cell 110-110 networks had an ECC of 0.11 ± 0.05 (n = 6). Following 5 days of contact, 19-19 and 110-110 pairs had strong electrical connections (Fig. 1C) while 19-110 pairs exhibited a reduction in coupling over this same period. Thus, electrical connections were maintained or strengthened at homotypic contacts, while heterotypic connections exhibited significant uncoupling. In addition, by day 5 of contact, an increasing percentage of 110-19 pairs possessed chemical connectivity and PSPs of greater amplitude (Fig. 2A). Cholinergic synaptic transmission between presynaptic 110 and postsynaptic 19 was present in 50% of cell pairs at day 2 (n=8) and 86% of pairs at day 4 (n=7). Chemical neurotransmission was not detected in 19-19 (d2, n=17; d5, n=28) or 110-110 pairs (d3, n=6; d7, n=6) where electrical coupling remains strong for at least 7 days in vitro. Therefore, synaptogenesis at neurite-bearing contacts was as previously described in soma-soma cultures.
Figure 2.
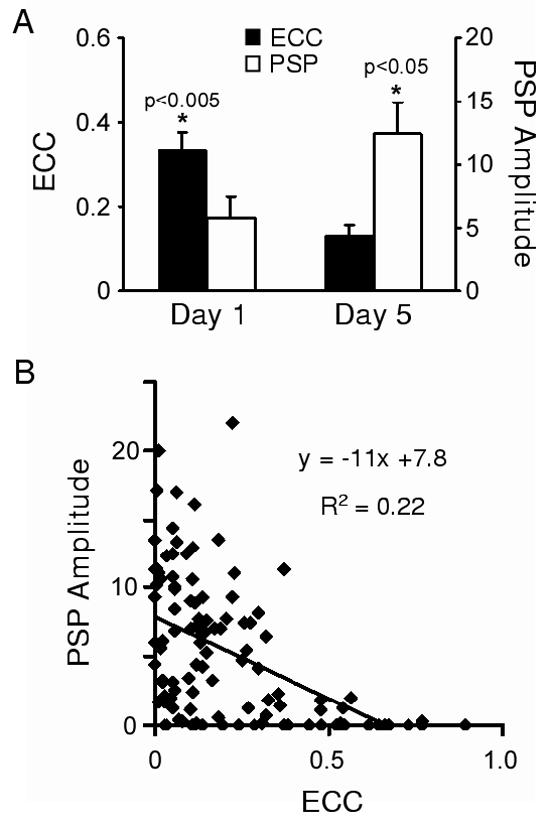
Temporal expression of chemical and electrical synaptic connections. (A) Histogram illustrating electrical coupling coefficients (ECC) and PSP amplitude measured at 95 msec after the peak of the presynaptic action potential on day 1 (n=32) and day 5 (n=19) in culture. Both the reduction in ECC values and increase in PSP amplitudes were significant (ECC: p<0.005; PSP: p<0.05; one-tailed student’s t-tests). (B) Scatterplot of 19-110 pairs from (A) and a linear fit of the relationship between ECC and PSP amplitude (n=51).
When strong electrical connections form between neurons, chemical synaptic transmission can become masked by junctional current flow (Liu and Nicholls, 1989; Bem et al., 2002). We therefore quantified chemical connectivity by measuring the amplitude of the postsynaptic potential at 95 ms after the peak of the presynaptic spike (Szabo et al., 2004). Although this approach underestimated chemical PSP amplitudes, it was necessary to faithfully distinguish chemical from electrical contributions at mixed synapses. As described above, electrical coupling at 19-110 pairs decreased over 5 days in culture, while PSP amplitudes increased significantly in strength (Fig. 2A). A negative correlation between these two modes of synaptic communication was observed for these 110-19 synapses (Fig. 2B).
Formation of three-cell networks
Having described the temporal sequence of synapse formation in two-cell configurations, we tested whether the generation of a three-cell network altered predicted synaptogenic outcomes. First, a pair of neurons was plated and allowed to extend processes. On day 4, a third neuron was plated contacting one of the original cells, and this three-cell network was then cultured for an additional day (Fig. 3A-C). The final network configuration used for electrophysiological analysis consisted of a central 5d-old neuron possessing a 1d connection on one side, and a 5d connection on the other (Fig. 3D). In this manner, eight three-cell networks were formed. Within these networks, four synaptic contacts were heterotypic 19-110, four were homotypic 110-110, and four were homotypic 19-19 (Fig. 4A and D; Table 1). Because of the temporal plating sequence employed, half of these contacts were established over 5 days and half over only 1 day.
Figure 3.
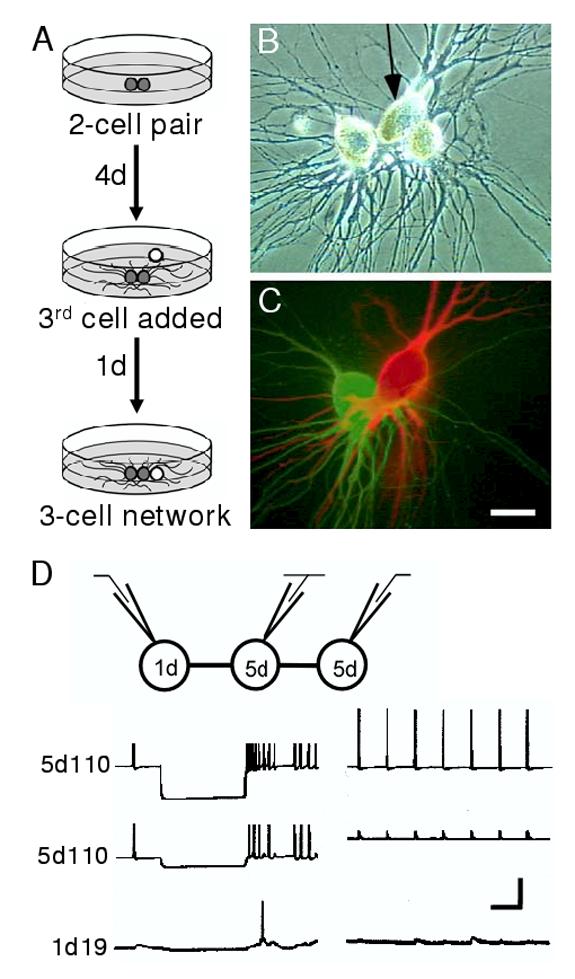
Experimental paradigm for investigating three-cell networks in culture. (A) Cells were plated into adhesive cell culture dishes in pairs (grey spheres). Following 4 days of contact and neurite outgrowth a third, younger cell (white sphere) was plated into contact with one cell of the pair for an additional day. (B) Phase-contrast micrograph showing neuronal somata plated in a row, with one central cell (arrow) paired to an older cell on one side, and a younger cell on the other. (C) Fluorescent dye-fill of the three-cell network in A. The central neuron 19 has been injected with Texas Red and the left neuron 110 with Lucifer yellow. Scale bar equals 30 μm. (D) Schematic of the recording configuration (top) illustrates that current was injected in one neuron of a network while simultaneous recordings from the other two cells were used to assess network connectivity. Hyperpolarizing (lower left traces) and depolarizing (lower right traces) current injections into one neuron (top traces) permitted assessment of connectivity with its 1 and 5d neighbors. In this case, hyperpolarizing current injected into a central 5d 110 elicited a hyperpolarization in a 5d 110 neighbor, but only weak electrical coupling was detected in the 1d 19 partner. Depolarizing current injection demonstrated weak chemical connectivity between the 5d 110 and the 1d 19. Note that the events recorded in the 5d 110 (middle trace, right panel) represent electrically-mediated synaptic potentials. Chemically-mediated synapses are actually inhibitory, but appear as depolarizing potentials due to the use of KCl electrodes that alter the chemical driving forces underlying these acetylcholine-gated channels (see Szabo et al., 2004). Vertical bar equals 40 mV (left traces), 20 mV (right traces). Horizontal bar equals 1 sec.
Figure 4.
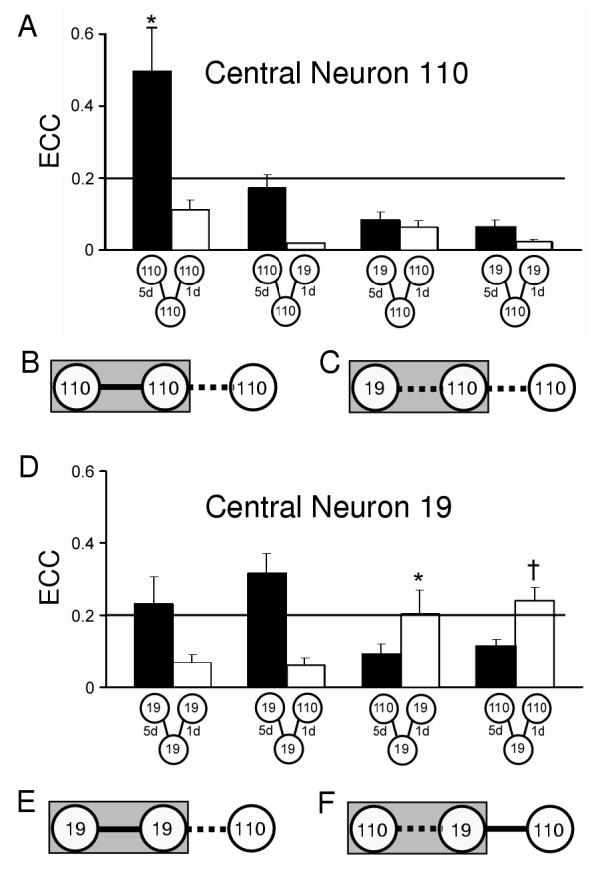
Electrical coupling between neurons in three-cell networks. (A) Histogram of ECC values for 5d (solid bars) and 1d connections (open bars) for three-cell networks with 110 as the central neuron (from left to right, 110-110-110, n=6; 110-110-19, n=9; 19-110-110, n=7; 19-110-110, n=17). (B and E) When electrical coupling was strong (solid line) at homotypic 5d contacts, newly formed 1d connections with a neuron 110 were weak (dashed line). Grey rectangle indicates 5d neuronal contacts. (C) When neuron 110 was the central cell of a heterotypic pair, it exhibited weak coupling at both its 5d and 1d contacts. (D) Histogram of ECC values for 5d and 1d connections with 19 as the central neuron (19-19-19, n=5; 19-19-110, n=8; 110-19-5d19, n=8; 110-19-110, n=14). An analysis across all 5d homotypic contacts yielded no significant differences, except at 110-110 connections (ANOVA, *, p<0.05). An analysis across all 5d heterotypic groups yielded no significant differences, (ANOVA, p=0.48). An analysis across all 1d contact groups indicated a significant difference (ANOVA, p<0.0001) at 5d19-1d19 (post hoc LSD,*, p<0.05) and 5d19-1d110 (post hoc LSD, †, p<0.05). (F) Heterotypic 5d contacts, where neuron 19 was the central cell, exhibited weak coupling and formed strong electrical connections at new 110 contacts. Overall, the presence of strong electrical coupling (e.g., B and E) appears to limit formation of new electrical synapses, while the presence of weak coupling (e.g., C and F) affected subsequent electrical synaptogenesis in a cell-specific manner.
Table 1.
Presence of chemical synaptic transmission from neuron 110 at specific cell-cell contacts of three-cell neuronal networks.
| Presynaptic | Postsynaptic | Third Cell | Actual† | Predicted†† | χ2 |
|---|---|---|---|---|---|
| 5d 110c | 5d 110 | 1d 110 | 0.25 (n=8) | 0.17 (n=6) | 0.273 |
| 5d 110c * | 5d 19 | 1d 110 | 0.20 (n=10) | 0.95 (n=21) | < 0.001 |
| 5d 110c | 5d 110 | 1d 19 | 0.33 (n=6) | 0.17 (n=6) | 0.273 |
| 5d 110c * | 5d 19 | 1d 19 | 0.13 (n=16) | 0.95 (n=21) | < 0.001 |
| 5d 110c | 1d 110 | 5d 110 | 0.50 (n=6) | 0.50 (n=8) | 0.480 |
| 5d 110c | 1d 19 | 5d 110 | 0.50 (n=6) | 0.25 (n=10) | 0.057 |
| 5d 110c * | 1d 110 | 5d 19 | 0.10 (n=10) | 0.50 (n=8) | < 0.004 |
| 5d 110c * | 1d 19 | 5d 19 | 0.06 (n=16) | 0.25 (n=10) | < 0.02 |
| 5d 110 | 5d 110c | 1d 110 | 0.18 (n=6) | 0.18 (n=6) | 1.00 |
| 5d 110 * | 5d 19c | 1d 110 | 0.50 (n=14) | 0.95 (n=21) | < 0.004 |
| 5d 110 | 5d 110c | 1d 19 | 0.10 (n=10) | 0.17 (n=6) | 0.074 |
| 5d 110 * | 5d 19c | 1d 19 | 0.25 (n=8) | 0.95 (n=21) | < 0.004 |
| 1d 110 | 5d 110c | 5d 110 | 0.17 (n=6) | 0.11 (n=9) | 0.289 |
| 1d 110 | 5d 19c | 5d 110 | 0.71 (n=14) | 0.80 (n=10) | 0.114 |
| 1d 110 | 5d 110c | 5d 19 | 0.10 (n=10) | 0.11 (n=9) | 0.724 |
| 1d 110 * | 5d 19c | 5d 19 | 0.00 (n=14) | 0.80 (n=10) | < 0.004 |
Actual values equal the fraction of contacts in three-cell networks with cholinergic transmission detected.
Predict values equal the fraction of contacts among appropriately matched two-cell networks with cholinergic transmission detected.
cindicates the central neuron of a three-cell network.
indicates a value significantly different from predicted two-cell networks.
Electrical connectivity in three-cell networks
The presence of electrical and chemical synaptic communication was assessed in neuronal networks using simultaneous electrophysiological recordings from three neurons while injecting one with current (Fig. 3D). We first determined whether electrical coupling was maintained or eliminated at the 5d synaptic connections. Coupling between homotypic neurons was relatively strong, with ECC values between 0.17 ± 0.04 and 0.50 ± 0.12 (Fig. 4A and D), where 110-110 synapses were significantly stronger than other homotypic connections after 5 days (ANOVA, p<0.5). In contrast, coupling at all four heterotypic 5d contacts was weak, with no significant differences between groups (ANOVA, p=0.48). As predicted from two-cell studies, homotypic electrical connections were maintained, but coupling at heterotypic contacts was diminished.
Since two Helisoma motoneurons contacting for 24h possessed strong electrical coupling regardless of identity, we predicted that similar contacts in three-cell networks would possess equally strong coupling. Instead, 6 of 8 1d contacts possessed weak coupling with ECC values less than 0.1. A comparison of all 8 groups indicated that coupling at the two other 1d contacts was significantly stronger (Fig. 4A and D; ANOVA, p<0.0001). In both cases where coupling was strong, the central neuron was a 19 that had been in contact with a neuron 110 for 5 days (Fig. 4D). On the other hand, when a central neuron 110 had contacted a 19 for 5 days, electrical synapses formed with any new contact were weak (Fig. 4A). Similarly, when a central 19 had contacted another 19 for 5 days, it did not form strong new connections. Thus, for neuron 19, electrical synaptogenic capabilities were more strongly affected by its existing network connectivity, rather than the neuronal identity of a new synaptic partner (Fig. 4E and F). For central neuron 110, although the identity of its partner was again not critical, the timing of its synapse formation was a major factor determining its synaptogenic outcomes and the existing connections did not alter new synaptic outcomes (Fig. 4B and C).
Chemical connectivity within three-cell networks
Neuron 19 does not form chemical synapses with other buccal neurons in culture (Haydon and Zoran, 1991; Zoran and Poyer, 1996) and did not form chemical synaptic connections with neuron 110 in either two-cell or three-cell configurations. Therefore, in this study, all chemical synaptic connectivity refers to cholinergic neurotransmission of neuron 110. When neuron 110 was plated into contact with a homotypic neuronal pair, no chemical synapses were detected after 24 hours, even though electrical coupling was weak at these new contacts (Table 1). For example, chemical neurotransmission was not present at contacts formed by a new neuron 110 with the central 19 of a homotypic pair (0 of 14 preparations; Fig. 5A). In contrast, heterotypic pairs possessed weak electrical coupling. When a new 110 was added to form a three-cell network, strong chemical synapses formed with the central 19 (10 of 14 preparations; Fig. 5B; Table 1). Thus, electrical connectivity in a neural circuit affected subsequent chemical synaptogenesis within that network.
Figure 5.
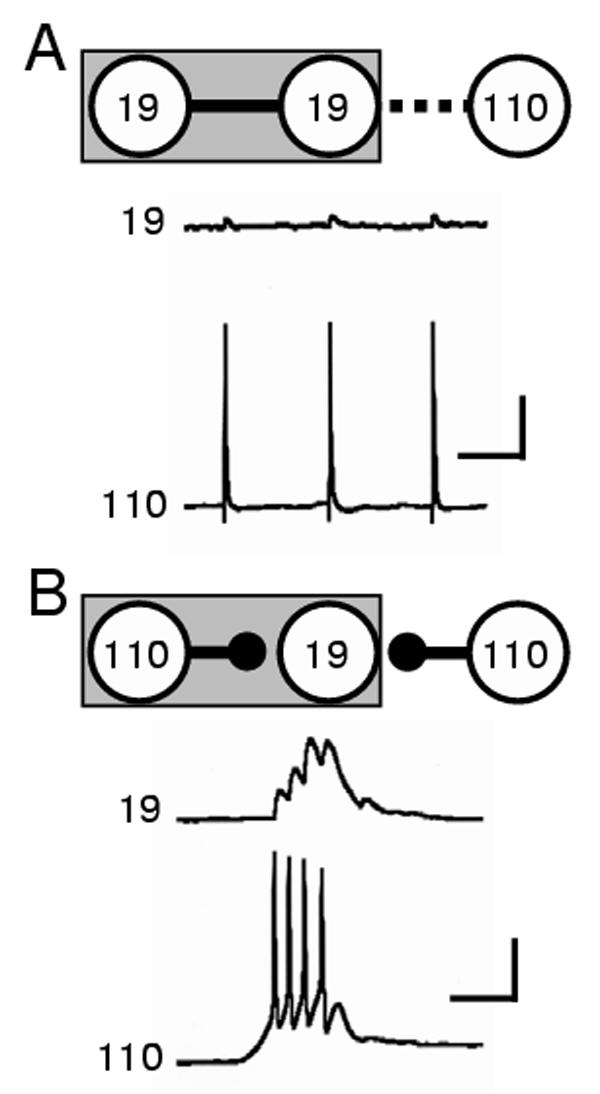
Chemical synapse formation between specific neurons is network dependent. (A) Schematic and sample traces illustrating one example of synaptogenesis in a 3-cell network: a 5d 19-19 contact possessed strong electrical coupling (solid line in schematic) and no chemical synaptic transmission, while only weak electrical coupling (dashed line) and no chemical synaptic transmission was recorded at the 1d contact of the central 19 and 110 (record traces). Scale bars equal 20mV and 1 sec. (B) By 5 days in culture, the strength of electrical connections between central neuron 19 and neuron 110 had diminished and strong chemical synaptic transmission was present (solid circle head). Strong chemical connections were also formed at the 1d contact between neurons 19 and 110 in this three-cell configuration (record traces). Scale bars equal 20mV and 1 sec.
Developmental window for transient electrical synapse formation
The differences in synaptogenic outcomes between two-cell and three-cell networks might have been caused by factors related to “age” in culture. Cells that had been plated into culture for 5 days were “older” than cells newly plated and might therefore have altered synaptogenic capabilities. We therefore conducted experiments examining the impact of age. Neurons were isolated for 4 days, one into adhesive culture conditions permitting neurite outgrowth and the other into non-adhesive conditions limiting outgrowth (Fig. 6A). On day 4, the latter was paired with the former for 24h and their connectivity was examined. Only weak electrical synaptic connections formed between these 5 day old neurons with 1d contacts. No significant differences in coupling were observed across groups (ANOVA, p=0.8; n = 28; Fig. 6B). In addition, chemical synaptic transmission was detected in only 3.6% of these preparations (data not shown).
Figure 6.
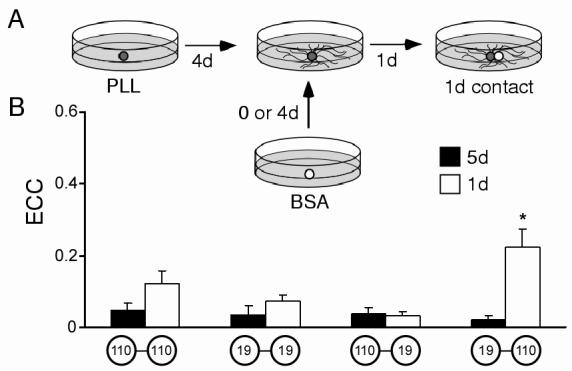
Effects of cell age on electrical synapse formation. (A) Schematic of the experimental paradigm. Cells were plated onto PLL-coated dishes and allowed to extend processes (dark spheres). On d4, cells were contacted by new somata (white sphere) that had been cultured in separate non-adhesive conditions for either 0 or 4 days. After 24 hours of contact, synaptic connectivity was assessed. (B) Histogram illustrating ECC values for “equally-aged” contacts (5d, solid bars) and “differently-aged” contacts (1d, open bars). 5d pairs were not significantly different (ANOVA, p=0.8; from left to right, 110-110, n=7; 19-19, n=6; 110-19, n=9; 19-110, n=8.) The 1d contact between a 5d 19 and a 1d 110 was significantly different from all other groups (ANOVA (post-hoc LSD), *, p<0.002; 110-110, n=8; 19-19, n=8; 110-19, n=10; 19-110, n=10).
The effects of “differently-aged” partners on synaptic formation were also examined by plating a “younger” (0d) neuron with an older (4d) neuron for 24h. In particular, when a 0d 110 was paired with a 4d 19, connections 24h later possessed stronger electrical coupling than that of any other pair configuration (ANOVA, p<0.002; Fig. 6B). As in three-cell networks, 5d19s formed stronger electrical connections. These younger 110 neurons did not form chemical connections at homotypic contacts (0 of 8 pairs), but exhibited strong synaptic transmission at heterotypic contacts (8 of 10 pairs). These experiments indicate that synaptogenesis between Helisoma neurons is constrained to a specific developmental period and that synaptogenic capabilities are limited in a cell-specific fashion.
Finally, we examined the prevalence of chemical synaptogenesis in each of 16 possible configurations of three-cell networks (Table I). The actual incidence of contacts with chemical neurotransmission was compared statistically to predicted values from appropriate two-cell experiments. Although some synaptogenic outcomes could be explained by differences in cell age, seven connections in the three-cell networks were significantly different than their counterparts in two-cell networks. Thus, the effects of extant network connectivity on subsequent chemical synaptogenesis were not adequately explained by differences in cell age or duration of contact, but instead indicate that previous synaptic history influences future neural network formation.
DISCUSSION
The predominant form of new synaptic connectivity among specific networks of Helisoma motoneurons following injury is transient electrical coupling (Bulloch et al., 1984; Haydon et al., 1987; Haydon and Kater, 1988). Among the motoneurons studied here, the strength of electrical coupling was inversely related to that of chemical synaptic transmission, such that over 4-5 days electrical coupling coefficients declined as chemical synapses emerged. The time course of this developmental sequence was similar to that seen during nerve regeneration in vivo (Szabo et al., 2004). We therefore hypothesized that synaptogenic shifts and transitions in neuronal communication could influence the outcomes of neuronal network formation and, to investigate this idea, we examined synapse formation in three-cell neuronal networks formed in cell culture.
Chemical synaptogenesis was absent at connections newly formed within strongly coupled neuronal networks. However, in networks where electrical synapses were transient and gap junctional uncoupling had occurred, new chemical synaptogenesis was pervasive. For example, a neuron 110 making initial contact with a neuron 19 did not form cholinergic synapses with this acetylcholine-sensitive target if that 19 was strongly coupled to another neuron (Fig. 7). Yet, if the 19 had progressed through transient electrical coupling with a neuron 110, chemical synaptogenesis at new contacts was not only possible, but was prevalent (Table I, Fig. 7). Thus, gap junctional coupling, at least in simple networks of Helisoma motoneurons, directly or indirectly, shapes ongoing synaptogenesis at newly established cell-cell contacts.
Figure 7.
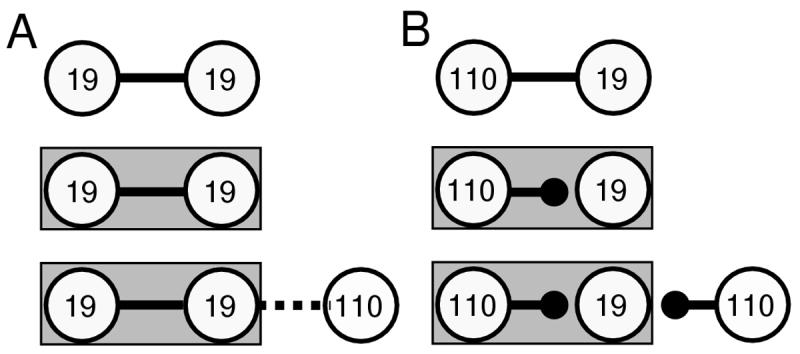
Summary of key observations of chemical synapse formation in three-cell networks. (A) Electrical coupling was strong (solid line) at homotypic 19-19 contacts following 24h of contact (top diagram). At day 4 (middle diagram), strong electrical coupling was maintained. At newly formed 1d connections with a neuron 110 (bottom diagram), only weak electrical coupling (dashed line) was observed between neurons 19 and 110. (B) Heterotypic 1d contacts possessed strong electrical coupling (top diagram). However, by day 4 uncoupling had occurred and strong chemical connections were present (middle diagram). At newly formed 1d connections on day 5 (bottom diagram), neuron 110 formed strong chemical connections with central neuron 19. Note that the new neuron 110 is forming very different kinds of synaptic connections, strong chemical in B and weak electrical in A, with the same postsynaptic neuron 19. The only difference in the postsynaptic neuron is its other existing network connectivity.
A rapid developmental switch from gap junction-mediated signaling to NMDA-dependent communication exists during the construction of cortical columnar networks (Dupont et al., 2006). Similar to synaptogenesis during the development of many nervous systems (Warner et al., 1984; Peinado et al., 1993a; Chang and Balice-Gordon, 2000), regenerating neural networks often exhibit a progression from transient electrical to chemical communication (Hadley et al., 1985; Allen and Warner, 1991; Chang et al., 2000; Personius et al., 2001; Curtin et al., 2002). The regulatory mechanisms that underlie synaptogenic progressions from electrical to chemical neurotransmission are not well understood. Among mouse hypothalamic neurons, NMDA receptor activation mediates gap junctional uncoupling via a calcium-cAMP response element binding protein (CREB)-dependent downregulation of Cx36 gene expression (Arumagam et al, 2005). Also, agrin, a protein known for its ability to cluster acetylcholine receptors at vertebrate neuromuscular junctions, mediates the dual modulation of electrical and chemical intercellular communication during the critical period at developing splanchnic nerve cholinergic synapses (Martin et al., 2005).
Synchronized activities between postnatal mammalian neurons prior to and during the onset of chemical synaptic connectivity have been suggested to contribute to the formation of cortical network domains (Peinado et al., 1993a, b; Kandler and Katz, 1995). Gap junctions are thought to mediate this synchronized activity, via either electrical or biochemical signaling (Kandler and Katz, 1998). Although central pattern generator inputs are not present in vitro, some endogenous spiking activity exists in these motoneuronal networks. Thus, cell-cell signaling mediated by synchronized activities where gap junctional conduits are strong may constitute the molecular mechanism by which existing network connections influence synapse formation. Regardless of the mechanism involved, it seems clear that gap junctional coupling of Helisoma networks, or a cellular process associated with it, are involved in regulating ongoing chemical synaptogenesis.
The transient electrical synapses formed between Helisoma motoneurons 19 and 110 are replaced by inhibitory chemical synapses (Szabo et al., 2004). This rapid developmental switch from gap junctional coupling to inhibitory neurotransmission represents a fundamental shift in network communication: neurons can fire in synchrony at one developmental time-point and inhibit each other at the next. It is becoming clear that interplay between electrical and chemical modes of neural communication have important impacts on neural network formation. The formation of specific neural circuits in leech embryos involves the development of inhibitory, GABAergic connections that shape pre-existing electrical synaptic connections (Marin-Burgin et al., 2005; Marin-Burgin et al., 2006). Interestingly, most electrical synapses in the juvenile neocortex on mammals couple inhibitory networks of GABA-ergic interneurons (Galarreta and Hestrin, 1999; Gibson et al., 1999; Hestrin and Galarreta, 2005). Among astrocytes, there also exists an inverse relationship between gap junctional coupling and chemical signaling such that purinergic signaling mechanisms mediated by ATP release are enhanced among astrocytes cultured from Cx43 knockout mice (Suadicani et al., 2003). When gap junctional coupling among cultured chick diencephalic astrocytes is reduced by the neurohormone melatonin, the spread of calcium waves are enhanced (Peters et al., 2005). Thus, the interplay between gap junctional and paracrine signaling modes may be a pervasive phenomenon.
The formation of electrical coupling between Helisoma neurons is thought to require mutual neurite elongation (Hadley and Kater, 1983; Hadley et al., 1983; Hadley et al., 1985). Since Helisoma neurons start slowing their process outgrowth by d4 or d5 in culture, a time similar to that of electrical uncoupling, the process of electrical synapse formation may not only be age-dependent, but also growth-dependent. Here we observed that age- and growth-dependent effects on synapse formation were ameliorated in a cell-specific manner. For example, an older neuron 110 rarely formed a strong electrical connection with a younger neuronal partner. Neuron 19, on the other hand, was capable of overcoming age-dependent restrictions on electrical synapse formation, but not when strongly coupled to other network partners. Thus, the potential for new synapse formation among Helisoma neurons depends on both the identity of the neurons involved, as well as the extent of their electrical connectivity. In developing leech embryos, intrinsic abilities to extend axons that project bilaterally are inhibited in an age-dependent fashion that is likely mediated by gap-junctional communication (Wolszon et al., 1995). Still, anesthetic treatment that blocks synaptogenesis among cultured Lymnaea neurons had no effect on neurite extension, indicating that synapse formation and neuritic outgrowth can be dissociated (Woodall et al., 2003).
In summary, Helisoma motoneurons possess the ability to form and maintain strong electrical synaptic connections at homotypic contacts, while at heterotypic contacts electrical coupling initially forms, and is then eliminated after several days. Electrical connectivity in three-cell neuronal networks affects subsequent chemical synaptogenesis and is different from that formed between two neurons of similar age and identity. Thus, several general principles for simple neuronal network formation in vitro emerged from these studies. 1) Actively growing neurons forming initial contacts are relatively unconstrained in the formation of electrical synaptic connections. 2) New connections formed with established networks have little impact on existing electrical connections. 3) Some aspects of synaptogenic potential are restricted to discrete developmental windows. 4) Synaptogenic outcomes are dependent on existing network connectivity. Thus, network-dependent regulatory mechanisms, as well as cell-intrinsic processes, mediate synapse formation within simple neural networks.
Acknowledgments
This work was supported by NSF Grant IBN 9421372 (MJZ) and NINDS Grant PO1 NS-39546 (MJZ). We thank Alberto Pereda for critical reading of this manuscript and helpful discussion and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen F, Warner A. Gap junctional communication during neuromuscular junction formation. Neuron. 1991;6:101–111. doi: 10.1016/0896-6273(91)90125-j. [DOI] [PubMed] [Google Scholar]
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8:1720–1726. doi: 10.1038/nn1588. [DOI] [PubMed] [Google Scholar]
- Bem T, Le Feuvre Y, Simmers J, Meyrand P. Electrical coupling can prevent expression of adult-like properties in an embryonic neural circuit. J Neurophysiol. 2002;87:538–47. doi: 10.1152/jn.00372.2001. [DOI] [PubMed] [Google Scholar]
- Bennett MVL. Electrical transmission: a functional analysis and comparison to chemical transmission. In: Kandel ER, editor. Handbook of physiology. Amer Phys Society; Bethesda, MD: 1977. pp. 357–416. [Google Scholar]
- Bennett MVL, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Bentley D, Caudy M. Pioneer axons lose directed growth after selective killing of guidepost cells. Nature. 1983;304:62–65. doi: 10.1038/304062a0. [DOI] [PubMed] [Google Scholar]
- Bulloch AG, Kater SB, Miller HR. Stability of new electrical connections between adult Helisoma neurons is influenced by preexisting neuronal interactions. J Neurophysiol. 1984;52:1094–1105. doi: 10.1152/jn.1984.52.6.1094. [DOI] [PubMed] [Google Scholar]
- Chang Q, Balice-Gordon RJ. Gap junctional communication among developing and injured motor neurons. Brain Res Brain Res Rev. 2000;32:242–249. doi: 10.1016/s0165-0173(99)00085-5. [DOI] [PubMed] [Google Scholar]
- Chang Q, Pereda A, Pinter MJ, Balice-Gordon RJ. Nerve injury induces gap junctional coupling among axotomized adult motor neurons. J Neurosci. 2000;20:674–684. doi: 10.1523/JNEUROSCI.20-02-00674.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin KD, Zhang Z, Wyman RJ. Gap junction proteins expressed during development are required for adult neural function in the Drosophila optic lamina. J Neurosci. 2002;22:7088–96. doi: 10.1523/JNEUROSCI.22-16-07088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;404:72–5. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–9. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Hadley RD, Bodnar DA, Kater SB. Formation of electrical synapses between isolated, cultured Helisoma neurons requires mutual neurite elongation. J Neurosci. 1985;5:3145–3153. doi: 10.1523/JNEUROSCI.05-12-03145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RD, Kater SB. Competence to form electrical connections is restricted to growing neurites in the snail, Helisoma. J Neurosci. 1983;3:924–932. doi: 10.1523/JNEUROSCI.03-05-00924.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RD, Kater SB, Cohan CS. Electrical synapse formation depends on interaction of mutually growing neurites. Science. 1983;221:466–468. doi: 10.1126/science.6867723. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28(6):304–9. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Haydon PG. The formation of chemical synapses between cell-cultured neuronal somata. J Neurosci. 1988;8:1032–1038. doi: 10.1523/JNEUROSCI.08-03-01032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Kater SB. The differential regulation of formation of chemical and electrical connections in Helisoma. J Neurobiol. 1988;19:636–655. doi: 10.1002/neu.480190706. [DOI] [PubMed] [Google Scholar]
- Haydon PG, McCobb DP, Kater SB. The regulation of neurite outgrowth, growth cone motility, and electrical synaptogenesis by serotonin. J Neurobiol. 1987;18:197–215. doi: 10.1002/neu.480180206. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Zoran MJ. Chemical synapses in cell culture. In: Chad J, Wheal H, editors. Cellular neurobiology: a practical approach. Oxford University Press; New York: 1991. pp. 57–71. [Google Scholar]
- Kandler K, Katz LC. Neuronal coupling and uncoupling in the developing nervous system. Curr Opin Neurobiol. 1995;5:98–105. doi: 10.1016/0959-4388(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Nicholls J. Steps in the development of chemical and electrical synapses by pairs of identified leech neurons in culture. Proc R Soc Lond B Biol Sci. 1989;236:253–68. doi: 10.1098/rspb.1989.0023. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A, Eisenhart FJ, Baca SM, Kristan WB, Jr, French KA. Sequential development of electrical and chemical synaptic connections generates a specific behavioral circuit in the leech. J Neurosci. 2005;25:2478–89. doi: 10.1523/JNEUROSCI.4787-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin A, Eisenhart FJ, Kristan WB, Jr, French KA. Embryonic electrical connections appear to pre-figure a behavioral circuit in the leech CNS. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:123–33. doi: 10.1007/s00359-005-0055-8. [DOI] [PubMed] [Google Scholar]
- Martin AO, Alonso G, Guerineau NC. Agrin mediates a rapid switch from electrical coupling to chemical neurotransmission during synaptogenesis. J Cell Biol. 2005;169:503–514. doi: 10.1083/jcb.200411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Diaz E, Moran LB, Navarrete R. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J Physiol. 2002;544:757–64. doi: 10.1113/jphysiol.2002.028159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro RJ, Yuste R. Gap junctions in developing neocortex: a review. Brain Res Brain Res Rev. 2004;47:216–226. doi: 10.1016/j.brainresrev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Zoran MJ. Electrical synapse formation disrupts calcium-dependent exocytosis, but not vesicle mobilization. Synapse. 2005;56:154–165. doi: 10.1002/syn.20139. [DOI] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993a;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex. 1993b;3:488–498. doi: 10.1093/cercor/3.5.488. [DOI] [PubMed] [Google Scholar]
- Peinado A. Immature neocortical neurons exist as extensive syncitial networks linked by dendrodendritic electrical connections. J Neurophysiol. 2001;85:620–9. doi: 10.1152/jn.2001.85.2.620. [DOI] [PubMed] [Google Scholar]
- Personius K, Chang Q, Bittman K, Panzer J, Balice-Gordon R. Gap junctional communication among motor and other neurons shapes patterns of neural activity and synaptic connectivity during development. Cell Commun Adhes. 2001;8:329–333. doi: 10.3109/15419060109080748. [DOI] [PubMed] [Google Scholar]
- Peters JL, Cassone VM, Zoran MJ. Melatonin modulates intercellular communication among cultured chick astrocytes. Brain Res. 2005;1031:10–19. doi: 10.1016/j.brainres.2004.09.064. [DOI] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, De Pina-Benabou MH, Urban-Maldonado M, Spray DC, Scemes E. Acute downregulation of Cx43 alters P2Y receptor expression levels in mouse spinal cord astrocytes. Glia. 2003;42:160–71. doi: 10.1002/glia.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo TM, Faber DS, Zoran MJ. Transient electrical coupling delays the onset of chemical neurotransmission at developing synapses. J Neurosci. 2004;24:112–120. doi: 10.1523/JNEUROSCI.4336-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Navarrete R. Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat lumbar spinal cord. J Physiol (Lond) 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner AE, Guthrie SC, Gilula NB. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984;311:127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- Wolszon LR, Passani MB, Macagno ER. Interactions during a critical period inhibit bilateral projections in embryonic neurons. J Neurosci. 1995;15:1506–15. doi: 10.1523/JNEUROSCI.15-02-01506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall AJ, Naruo H, Prince DJ, Feng ZP, Winlow W, Takasaki M, Syed NI. Anesthetic treatment blocks synaptogenesis but not neuronal regeneration of cultured Lymnaea neurons. J Neurophysiol. 2003;90:2232–9. doi: 10.1152/jn.00347.2003. [DOI] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- Zoran M, Poyer J. Cellular mechanisms governing synapse formation: lessons from identified neurons in culture. Invert Neurosci. 1996;2:1–8. [Google Scholar]


