Abstract
Previous studies have shown that corticotropin-releasing factor (CRF), an integral mediator of the stress response, and opioids regulate the activity of the locus-coeruleus-norepinephrine (LC-NE) system during stress in a reciprocal manner. Furthermore, repeated opiate exposure sensitizes noradrenergic neurons to CRF. Previous studies have shown that μORs are prominently distributed within somatodendritic processes of catecholaminergic neurons in the LC and axon terminals containing opioid peptides and CRF converge within the LC. To further examine cellular sites for interactions between CRF receptor type 1 (CRFr) and μOR, immunofluorescence and electron microscopic analysis of the rat LC was conducted. Triple immunofluorescence showed prominent co-localization of the CRFr and μOR in noradrenergic somata in the LC. Ultrastructural analysis confirmed dual localization of CRFr and μOR in common dendritic processes in the LC. Semi-quantitative analysis showed that of the dendrites exhibiting CRFr immunolabeling, 57% expressed μOR immunoreactivity. These data provide ultrastructural evidence that CRFr and μOR are co-localized in LC neurons, a cellular substrate that may underlie opiate-induced sensitization of brain noradrenergic neurons to CRF.
Keywords: corticotropin-releasing factor receptor 1, μ-opioid receptor, immunocytochemistry, electron microscopy
Corticotropin-releasing factor (CRF), the hypothalamic neurohormone that initiates release of adrenocorticotropin in response to stress [20] also serves as a neurotransmitter that activates neurons of the noradrenergic nucleus locus coeruleus (LC). CRF administration increases the spontaneous discharge rate of LC neurons [21]. Microinfusion of CRF receptor antagonists into the LC abrogates increases in LC discharge activity elicited by both intracerebroventricularly (icv) administered CRF [6] and certain stimuli [24]. Likewise, hypotensive stress-induced activation of LC neurons is blocked by microinfusion of CRF directly into the LC [24]. Taken together, these data suggest that CRF release in the LC increases activity of noradrenergic neurons. In addition, CRF potently activates forebrain electroencephalographic (EEG) activity [7]. Consistent with physiological findings, ultrastructural studies demonstrated that CRF-immunoreactive terminals form synaptic specializations with LC dendrites [31].
The LC is densely innervated by processes exhibiting endogenous opioid peptides [8, 28, 29]. In vitro studies have shown that the LC contains a high concentration of μ-opioid receptors (μOR) [11, 28, 29]. Ultrastructural studies have demonstrated that μOrs are prominently distributed on somatodendritic processes in the LC [28] and are specifically localized to the plasma membrane of dendrites that are targeted by the enkephalin family of opioid peptides [29].
Endogenous opioids and CRF interact [28, 34] in the LC. The CRF-opioid convergence in the LC-norepinephrine (LC-NE) system is shown to be involved in the neural circuitry underlying stress responses [22] and opiate actions [12]. We have shown that chronic but not acute morphine treatment selectively sensitized LC-NE system to CRF [34]. CRF produced a near maximal-activation of LC neurons of rats chronically treated with morphine compared to vehicle-treated rats. Moreover, the chronic opiate-induced LC sensitization altered the behavioral repertoire in response to swim stress, a form of environmental stress [34]. In light of these findings and the expression of CRFr and μOR immunoreactivities in the LC in independent studies [15, 29, 34], we hypothesized that CRFr and μOR are co-localized in the same dendritic processes in LC neurons.
In an effort to further elucidate cellular substrates for interactions between CRF receptor type 1 (CRFr) and μOR, the present study used combined immunofluorescence and dual immunoelectron microscopy. Triple immunocytochemical labeling for CRFr, μOR and tyrosine hydroxylase (TH) was conducted on the same section of tissue. Immunoelectron microscopy combined methods of immunoperoxidase and immunogold-silver detection for labeling CRFr and μOR, respectively. The present data suggest potential anatomical substrates for LC’s involvement in opiate-induced sensitization of the brain NE system in stress.
Adult male Sprague-Dawley rats (220–250 g; Harlan Sprague-Dawley, Inc., Indianapolis, IN) were used. The procedures in this study were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University and conformed to NIH guidelines. All efforts were made to utilize only the number of animals necessary to produce reliable scientific data, and attempts were made to minimize animal distress.
Using immunohistochemical analysis, the characterization and specificity of the goat antiserum against CRFr and the rabbit antiserum against μOR have been previously described [15, 29, 34]. The goat polyclonal CRFr was raised against the C-terminus of CRFr that recognizes both CRFr1 and CRFr2 [15, 34]. The rabbit polyclonal μOR was raised against a glutaraldehyde conjugate of the C-terminal 18 amino acids rat μOR and keyhole limpet hemocyanin that specifically recognizes immunocytochemical labeling for μOR in Western blotting, immunoprecipitation and light microscopic studies [18, 28, 29]. Likewise, using immunodot-blot analysis, the antiserum recognizes amino acid sequences within μOR, however, it does not recognize either sequence of δ- and κ-opioid receptors [4]. Mouse antiserum against TH specifically recognizes the catecholamine synthesizing enzyme [34].
Perfusion, sectioning and immunohistochemical protocols for tissue sections intended for immunofluorescence have been previously described [15, 16]. Every fourth coronal tissue section (30 μm thick) of LC from four rats were incubated in goat anti-CRFr (C-20; 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-μ-OR antiserum (1:2,500; Chemicon, Temecula, CA) and mouse anti-TH (1:1,000; Immunostar Inc., Hudson, WI) for 15–18 h in 0.1 M tris-buffered saline (TBS; pH 7.6) with 0.1% bovine serum albumin (BSA) and 0.25% Triton X-100. Subsequently, tissue sections were incubated in a cocktail containing fluorescein isothiocyanate-conjugated donkey anti-rabbit (1:100; Jackson Immunoresearch, West Grove, PA), rhodamine isothiocyanate-conjugated donkey anti-goat (1:100; Jackson Immunoresearch) and Cy5-conjugaated donkey anti-mouse (1:100; Jackson Immunoresearch) secondary antibodies for 2 h in the dark at room temperature. The sections were mounted using DPX (Sigma-Aldrich Inc., St. Louis, MO, USA) and observed under a confocal microscope (Zeiss LSM 510 Meta, Carl Zeiss Inc., Thornwood, NY, USA).
Perfusion, sectioning and immunohistochemical protocols for tissue sections intended for electron microscopy have been previously described [18, 29]. Forty micron thick coronal tissue sections were incubated in goat anti-CRFr (C-20; 1:1,000; Santa Cruz Biotechnology) and rabbit anti-μ-OR (1:2,500; Chemicon) in 0.1 M TBS with 0.1% BSA for 15–18 h at room temperature. For labeling CRFr, sections were processed using a previously described avidin-biotin immunoperoxidase protocol For labeling μ-OR, tissue sections were incubated in anti-rabbit IgG conjugated to 1nm gold particles (Amersham Biosciences, Piscataway, NJ) and intensified using a silver enhancement kit (Amersham Biosciences). Some sections were processed for reverse immunolabeling to ensure that labeling was not biased due to immunolabeling techniques. Sections from LC were examined using an electron microscope (Morgagni, Fei Company, Hillsboro,OR, USA). Digital images were captured using the AMT advantage HR/HR-B CCD camera system (Advance Microscopy Techniques Corp., Danvers, MA, USA). All figures for immunofluorescence and electron microscopy were assembled and adjusted for brightness and contrast in Adobe Photoshop.
For immunofluorescence, the percentage of TH-immunoreactive perikarya showing co-localization with CRFr and μOR were obtained from four rats. Every fourth section (120 μm intervals) through the rostro-caudal segment of the LC was collected and used for analysis. Cell counts included only tissues taken at approximately −10.30 mm from bregma, through approximately − 9.3 mm from bregma. This resulted in 8 sections per animal. Cell counts were then taken unilaterally and were represented as mean ± SEM of the numbers of cells found per animal. Data for electron microscopy were taken from four rats with optimal immunocytochemical labeling and preservation of ultrastructural morphology. The quantitative approach used in the present study is well established and has been described previously [28–31]. The cellular elements were identified based on the description of Peters and colleagues [13]. Asymmetric and symmetric synapses were identified based on the description of Gray [9]. A nonsynaptic contact or apposition was defined as an axon terminal plasma membrane juxtaposed to that of a dendrite or soma devoid of recognizable membrane specializations and no intervening glial processes.
Spurious gold-silver labeling for μOR that was not associated with cellular membranes was negligible. Hence, μOR-labeled profiles containing two or more gold particles were classified as immunolabeled and included in the counts. CRFr-labeled profiles exhibited an intense labeling and were clearly distinguishable from immunogold-silver labeling for μOR. A total of 451 and 485 μOR- and CRFr-labeled profiles, respectively, were identified.
Controlled conditions were necessary to ensure the reproducibility of the quantitative analysis of the co-localization of the neurotransmitters and types of synaptic associations formed by immunoreactive processes. Thus, tissue sections were collected near the tissue-Epon interface because we have observed that this procedure minimizes artifacts that may be associated with the incomplete penetration of antiserum [31] and enables both markers to be clearly detectable in all sections used for analysis [3].
Using immunofluorescence labeling, immunoreactivities for CRFr, μOR and TH were identified in the same tissue section through the LC (Fig. 1A-D). CRFr immunoreactivity was present within LC dendrites and perikarya, however, none was evident within nuclei (Fig. 1B, D). Similarly, μOR immunoreactivity was found distributed within LC dendrites and perikarya with no apparent immunofluorescence labeling in the nuclei (Fig. 1A, D) as previously reported [28, 29]. TH immunoreactivity in cell bodies was evident in the LC specifically in the core (Fig. 1A, D), the area containing mainly noradrenergic somata [2]. Within the core of the LC, CRFr and μOR are both co-localized within common TH-labeled neurons (Fig. 1D). Of the 418 ± 16.17 TH-labeled perikarya analyzed, approximately 38% (157.75 ± 8.46) contained both CRFr and μOR.
Figure 1.
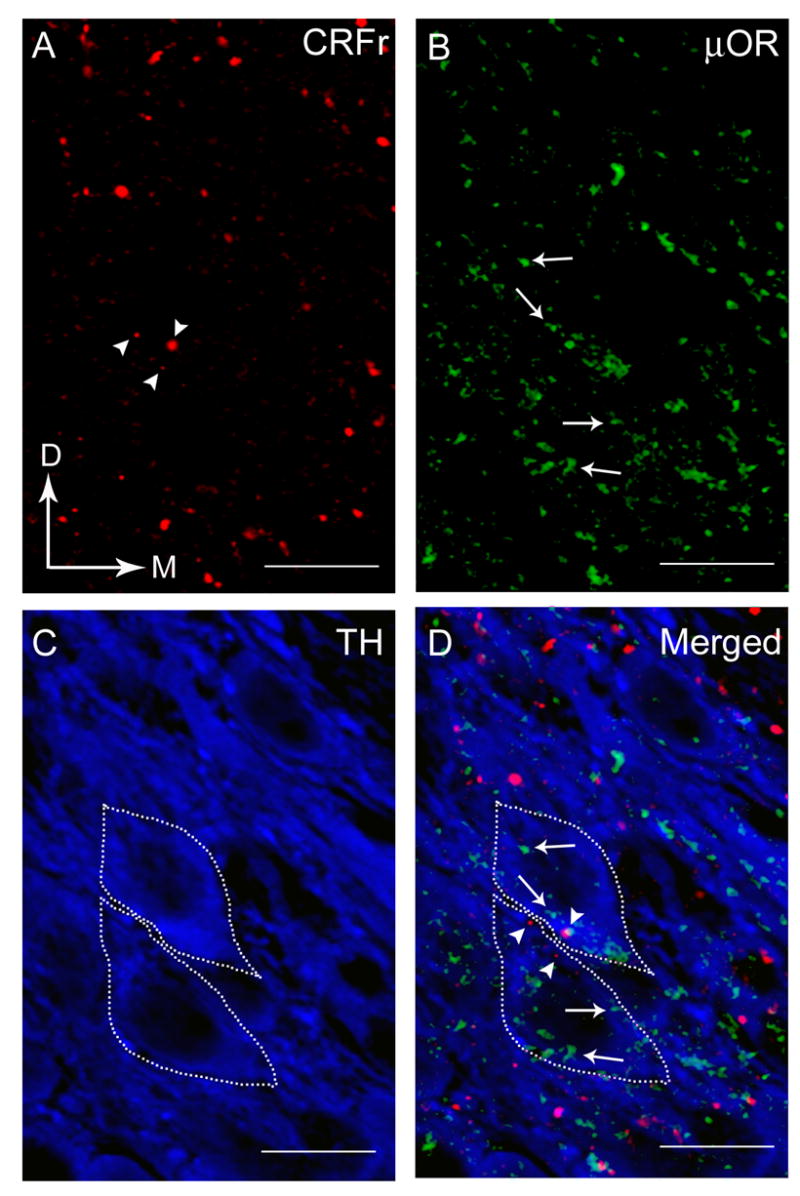
Triple-immunofluorescence labeling in the LC showing CRFr (red), μOR (green) and TH (blue). A. CRFr immunoreactivity was detected using TRITC-tagged secondary antibody. Arrowheads point to CRFr -labeled process. Arrows indicate dorsal (D) and medial (M) orientation of the sections. B. μOR immunoreactivity was detected using FITC-tagged secondary antibody. Arrows point to μOR-labeled process. C. TH immunoreactivity was detected using Cy5-tagged secondary antibody. Dashed lines are the boundaries of each TH-labeled perikaryon containing both CRFr and μOR immunoreactivities. D. Photomicrograph showing merged images CRFr (red), μOR (green) and TH (blue). Dashed lines are the boundaries of each TH-labeled perikaryon containing both CRFr (arrowheads) and μOR (arrows) immunoreactivities. Scale bars, 100 μm.
Using electron microscopy, immunocytochemical labeling for CRFr and μOR was prominently distributed within the LC (Fig. 2). Peroxidase labeling for CRFr was represented by an electron dense, and diffuse reaction product within cytoplasmic compartments of perikarya and dendrites (Fig. 2). Most frequently CRFr was found in dendritic processes and was associated with the plasmalemma (Fig. 2A-B, D).
Figure 2.

Electron photomicrographs showing peroxidase labeling for CRFr and immunogold-silver labeling for μOR in the LC. A. A peroxidase-labeled CRFr dendrite (arrows) and an immunogold-labeled (arrowheads) μOR dendrite in the same field. B. Peroxidase labeling can be seen in a CRFr-labeled dendrite (arrows) that also contains immunogold-labeled (arrowheads) μOR (CRFr+μOR). An unlabeled terminal (ut) that contains dense core vesicles (dcv) forms a synapse with the CRFr+μOR dendrite. C–D. Dendrites that contain CRFr (arrows) and μOR (arrowheads) forming synapses with an unlabeled axon terminal (ut). Dense core vesicles (dcv) are found in unlabeled terminal (ut). Scale bars, 0.5μm.
Immunogold-silver labeling for μOR was identified in perikarya and dendrites (Fig. 2). Similar to CRFr, the plasmalemmal distribution of μOR immunoreactivity was evident (Fig. 2A-C). μOR immunoreactivity was also associated with the cytoplasmic component of somatic and dendritic plasma membranes (Fig. 2A-C). In addition, μOR immunoreactivity was associated with the cytoplasmic surfaces of extrasynaptic plasma membranes of dendrites and perikarya (Fig. 2A-B). Gold-silver labeling was found distributed along the extrasynaptic portion of the dendritic membrane as previously described [29]. Extrasynaptic labeling was defined by the presence of gold-silver particles along any portion of the plasma membrane of the dendrites, whether or not synaptic input was seen within the section examined [29].
Although immunoreactivities for CRFr and μOR were observed in separate dendrites in the LC, numerous dendrites containing CRFr also co-localized μOR (Figs. 2, 3). Of the 485 total dendrites exhibiting CRFr immunoreactivity, 57% (n = 276) also exhibited μOR. While of the 451 total dendrites exhibiting μOR immunoreactivity, 61% (n = 276) also exhibited CRFr immunoreactivity.
Figure 3.
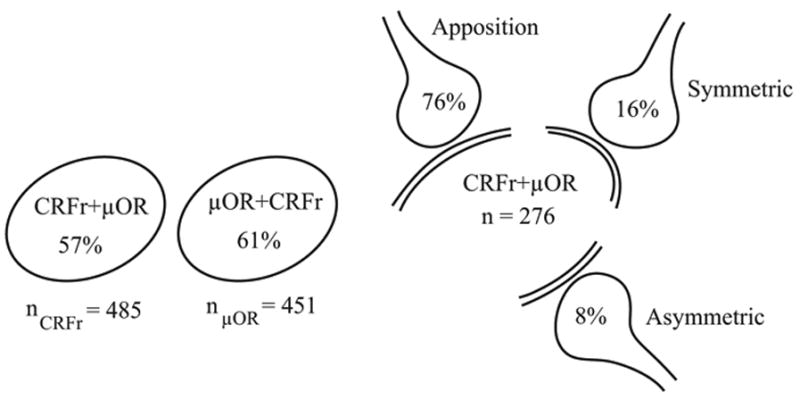
Schematics depicting percentages of dual-labeled CRFr+μOR dendrites with respect to the individual CRFr (n = 485) and μOR (n = 451) dendritic profiles analyzed. Also shown are the percentages reflecting synaptic associations of unlabeled terminals to dual-labeled CRFr+μOR dendrites.
Numerous unlabeled axon terminals were directly apposed to dendrites showing both CRFr and μOR immunoreactivities (Figs. 2B, 3). These unlabeled axon terminals did not form clearly defined synaptic specializations with the dual-labeled dendritic processes in the plane of section analyzed. Of the 276 total dendrites showing co-localization of CRFr and μOR, 76% (n = 209) were apposed by unlabeled axon terminals (Fig. 3). Approximately 16% (45/276) of dual-labeled dendrites received symmetric synapses (Figs. 2B, D, 3) from unlabeled axon terminals. The unlabeled axon terminals contained copious small clear spherical vesicles which were distributed throughout the axoplasm (Fig. 2). Often, these axon terminals contained one or more large dense core vesicles (Fig. 2B, D). Axon terminals forming synaptic contacts with dendrites exhibiting co-localization of CRFr- and μOR, also formed asymmetric synapses (n = 22; Fig. 3).
The results of the present study provide ultrastructural evidence that CRFr and μOR are co-localized in dendritic processes in the LC. Frequently, these dendrites receive heteregeneous synapses from unlabeled axon terminals. CRFr immunoreactivity was commonly associated within the cytoplasmic compartments of perikarya and dendrites as well as with the plasmalemma. Correspondingly, μOR immunoreactivity was often localized to perikarya and dendrites and found distributed along the somatic and dendritic plasma membranes. Furthermore, immunofluorescence revealed that CRFr and μOR are co-localized within noradrenergic neurons in the LC. Considering the previous investigations showing that CRF and opioids regulate the activity of noradrenergic LC neurons in a reciprocal manner [12, 21, 22, 33], our data suggest that CRFr and μOR are strategically poised within noradrenergic neurons to mediate opiate-induced sensitization of the brain NE system as well as responsiveness to stress.
The co-localization of CRFr and μOR in dendritic processes suggests that these two receptors may function principally in a postsynaptic fashion. The present study does not unequivocally establish whether dendrites exhibiting co-localization for CRFr and μOR are catecholaminergic using EM. However, our immunofluorescence results revealed that CRFr and μOR are co-localized within the same TH-labeled neurons in the LC suggesting that at ultrastructural level the dendritic processes exhibiting co-localization for CRFr and μOR are likely to be catecholaminergic. In addition, our ultrastructural analysis was restricted to portions of the neuropil known to be enriched in catecholaminergic perikarya and dendrites. Independent ultrastructural studies have demonstrated that about half of the μOR-labeled dendrites in LC contained TH [28] and about a quarter of CRF-labeled axon terminals contacted TH-labeled dendrites [31]. In the present study, immunofluorescence microscopy revealed that 38% of catecholaminergic perikarya in the LC co-localized CRFr and μOR while ultrastructural analysis showed that more than half of CRFr-labeled dendrites also exhibited μOR. Taken together, these results suggest that the co-localized CRFr and μOR are expressed within a subset of catecholaminergic dendrites.
The axon terminals contained both small clear vesicles and dense core vesicles, a characteristic of opioid- and CRF-containing axon terminals in the LC [31] and has been described in the dorsal raphe nucleus [23] the nucleus of solitary tract [14] and the area postrema [1]. The dense core vesicles in unlabeled axon terminals contacting dendrites containing both CRFr and μOR are often clustered at the perimeter of the axon terminals suggesting that the receptive sites for the CRF and opioid peptide may be more complex because release may occur from dense core vesicles at extrasynaptic sites [35]. Nevertheless, unlabeled axon terminals formed symmetric or asymmetric synapse with dendritic processes containing both CRFr and μOR. Asymmetric synapses have been correlated with excitatory transmission whereas symmetric synapses have been correlated with inhibitory transmission [9, 13]. Previous anatomical studies have shown that CRF and enkephalin afferents form asymmetric and symmetric synapses, respectively with catecholaminergic LC dendrites [27, 31] suggesting that the unlabeled axon terminals in the present study may represent CRF or ENK terminals that may activate or inhibit catecholaminergic dendrites containing both CRFr and μOR during stress.
The LC-NE system has been shown to be activated by a myriad of stressors. CRF and opioids are important mediators of the LC-NE system during stress [5]. Moreover, it is an integral site of CRF and opioid convergence that plays a vital role in the cognitive component of the stress response [25]. Ultrastructural studies have shown that LC is targeted by CRF [30, 31] and endogenous opioids [26, 29]. Both of these pathways have been implicated in both the stress response [22] and opiate actions [12]. Interestingly, the sensitivity of the LC-NE system to stress has been known to be regulated in part by CRF and opioids in a reciprocal fashion [5]. Recent evidence showing convergence of CRF- and ENK-immunoreactive axon terminals onto LC dendrites [19] support the reciprocal regulation of LC-NE system during stress.
The present ultrastructural study suggests mechanisms by which CRF and opioids may act to reciprocally affect LC neuronal activity. It is possible that CRF and opioids which are co-released from the same axon terminals could directly affect LC neuronal activity through synaptic contacts with the common LC dendrites. In addition, CRF and opioids could be co-released from separate axon terminals, and converge onto common LC dendrites. We have shown that chronic but not acute morphine sensitized LC-NE neurons selectively to CRF [34]. Consequently, this translates to enhanced activation by a physiological stressor. Hyperactivity in the behavioral manifestation to environmental stress ensues. The integrity of the CRF-opioid balance in the LC is important and particularly relevant to opiate-seeking behavior for individuals that are chronically taking opiates [10, 17]. Thus, the strategic co-localization of CRFr and μOR in LC dendrites may underlie the continued opiate-seeking behavior in an effort to attenuate the hypersensitivity of the LC-NE system to stress.
Acknowledgments
Supported by National Institutes of Health grant DA 09082 to EVB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong DM, Pickel VM, Joh TH, Reis DJ, Miller RJ. Immunocytochemical localization of catecholamine synthesizing enzymes and neuropeptides in area postrema and medial nucleus tractus solitarius of rat brain. J Comp Neurol. 1980;196:505–517. doi: 10.1002/cne.901960312. [DOI] [PubMed] [Google Scholar]
- 2.Bajic D, Proudfit H, Van Bockstaele EJ. Periaqueductal gray neurons monosynaptically innervate extranuclear noradrenergic dendrites in the rat pericoerulear region. J Comp Neurol. 2000;427:649–662. doi: 10.1002/1096-9861(20001127)427:4<649::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng PY, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural localization of mu-opioid receptors in the superficial layers of the rat cervical spinal cord: extrasynaptic localization and proximity to Leu5-enkephalin. Brain Res. 1996;731:141–154. doi: 10.1016/0006-8993(96)00492-1. [DOI] [PubMed] [Google Scholar]
- 5.Curtis AL, Bello NT, Valentino RJ. Evidence for functional release of endogenous opioids in the locus coeruleus during stress termination. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-13-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis AL, Grigoriadis DE, Page ME, Rivier J, Valentino RJ. Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J Pharmacol Exp Ther. 1994;268:359–365. [PubMed] [Google Scholar]
- 7.Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- 8.Drolet G, Van Bockstaele EJ, Aston-Jones G. Robust enkephalin innervation of the locus coeruleus from the rostral medulla. J Neurosci. 1992;12:3162–3174. doi: 10.1523/JNEUROSCI.12-08-03162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray EG. Axosomatic and axo-dendritic synapses of the cerebral cortex: an electron microscopic study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- 10.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 11.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 12.Nestler EJ, Alreja M, Aghajania GK. Molecular and cellular mechanisms of opiate actions: studies in the rat locus coeruleus. Brain Res Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 13.Peters A, Palay SL, Webster Hd. The Fine Structure of the Nervous System. Oxford University Press; 1991. [Google Scholar]
- 14.Pickel VM. Immunocytochemical localization of neuronal antigens: tyrosine hydroxylase, substance P, [Met5]-enkephalin. Fed Proc. 1979;38:2374–2380. [PubMed] [Google Scholar]
- 15.Reyes BAS, Glaser JD, Magtoto R, Van Bockstaele EJ. Proopiomelanocortin co-localizes with corticotropin-releasing factor in axon terminals of the noradrenergic nucleus locus coeruleus. Eur J Neurosci. 2006;23:2067–2077. doi: 10.1111/j.1460-9568.2006.04744.x. [DOI] [PubMed] [Google Scholar]
- 16.Reyes BAS, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 17.Schluger JH, Bart G, Green M, Ho A, Kreek MJ. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology. 2003;28:985–94. doi: 10.1038/sj.npp.1300156. [DOI] [PubMed] [Google Scholar]
- 18.Surratt CK, Johnson PS, Moriwaki A, Seidleck BK, Blaschak CJ, Wang JB, Uhl GR. Mu opiate receptor: charged transmembrane domain amino acids are critical for agonist recognition and intrinsic activity. J Biol Chem. 1994;32:20548–20553. [PubMed] [Google Scholar]
- 19.Tjoumakaris SI, Rudoy C, Peoples J, Valentino RJ, Van Bockstaele EJ. Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J Comp Neurol. 2003;466:445–456. doi: 10.1002/cne.10893. [DOI] [PubMed] [Google Scholar]
- 20.Vale WW, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticortropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 21.Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:636–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- 22.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann NY Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- 23.Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- 24.Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- 25.Valentino RJ, Van Bockstaele EJ. Opposing regulation of the locus coeruleus by corticotropin releasing factor and opioids: potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology. 2001;158:331–342. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- 26.Van Bockstaele EJ, Chan J. Electron microscopic evidence for coexistence of leucine5-enkephalin and gamma-aminobutyric acid in a subpopulation of axon terminals in the rat locus coeruleus region. Brain Res. 1997;746:171–82. doi: 10.1016/s0006-8993(96)01194-8. [DOI] [PubMed] [Google Scholar]
- 27.Van Bockstaele EJ, Colago EEO, Pickel VM. Enkephalin terminals form inhibitory-type synapses on neurons in the rat nucleus locus coeruleus that project to the medial prefrontal cortex. Neuroscience. 1996;71:429–42. doi: 10.1016/0306-4522(95)00432-7. [DOI] [PubMed] [Google Scholar]
- 28.Van Bockstaele EJ, Colago EEO, Cheng P, Moriwaki A, Uhl GR, Pickel VM. Ultrastructural evidence for prominent distribution of the mu-opioid receptor at extrasynaptic sites on noradrenergic dendrites in the rat nucleus locus coeruleus. J Neurosci. 1996;16:5037–5048. doi: 10.1523/JNEUROSCI.16-16-05037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Bockstaele EJ, Colago EEO, Moriwaki A, Uhl GR. Mu-opioid receptor is located on the plasma membrane of dendrites that receive asymmetric synapses from axon terminals containing leucine-enkephalin in the rat nucleus locus coeruleus. J Comp Neurol. 1996;376:65–74. doi: 10.1002/(SICI)1096-9861(19961202)376:1<65::AID-CNE4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 30.Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrin. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Bockstaele EJ, Colago EEO, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents int he rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Van Bockstaele EJ, Pickel VM. Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol. 1993;334:603–617. doi: 10.1002/cne.903340408. [DOI] [PubMed] [Google Scholar]
- 33.Williams JT, North RA. Opiate-receptor interactions on single locus coeruleus neurones. Mol Pharmacol. 1984;26:489–497. [PubMed] [Google Scholar]
- 34.Xu G, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ. Chronic morphine sensitizes the brain norepinephrine system to stress. J Neurosci. 2004;24:8193–8197. doi: 10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu PC, Thureson-Klein A, Klein RL. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience. 1986;19:43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]


