Abstract
Cannabinoid agonists exert complex actions on modulatory neurotransmitters involved in attention and cognition. Previous studies have demonstrated that acute systemic administration of the synthetic cannabinoid agonist, WIN 55,212-2, increases norepinephrine efflux in the rat frontal cortex. In an effort to elucidate whether cannabinoid (CB1) receptors are positioned to presynaptically modulate norepinephrine release in the frontal cortex, immunocytochemical detection of the CB1 receptor and the catecholamine-synthesizing enzyme dopamine-β-hydroxylase (DβH) was performed using confocal immunofluorescence microscopy and immunoelectron microscopy in rat brain. Fluorescence microscopy analysis of dually-labeled tissue sections from the frontal cortex indicated that individual axonal processes exhibited both CB1 receptor and DßH immunoreactivities. Ultrastructural analysis confirmed that one-third of axon terminals containing CB1 immunolabeling also exhibited DβH labeling. Cortical neurons were also found to be targeted by separately labeled CB1- and DβH-containing axon terminals. In conclusion, the present neuroanatomical data suggest that cortical norepinephrine release may be modulated, in part, by CB1 receptors that are presynaptically distributed on noradrenergic axon terminals.
Keywords: locus coeruleus, cognition, electron microscopy, confocal microscopy
Introduction
Behavioral studies indicate that acute cannabinoid administration impairs attention, vigilance and cognitive processing (Casswell and Marks 1973; Chait 1992). Long-term heavy cannabis users show impairments in attention that endure beyond the period of intoxication and worsen with increasing years of regular cannabis administration (Solowij, Stephens et al. 2002). Studies examining the effects of cannabinoids on attention, utilizing a combination of performance and event-related potential measures, have shown that chronic cannabis use affects information processing (Kempel, Lampe et al. 2003). Specifically, cannabis consumers are unable to filter out extraneous information and there is an inability to effectively focus attention and reject irrelevant information (Solowij, Michie et al. 1995). These data suggest a failure to habituate to irrelevant stimuli and may represent a faulty gating mechanism or inefficient information processing strategies (Solowij, Michie et al. 1995). Noradrenergic projections from the locus coeruleus (LC) to the frontal cortex participate in regulating attention and cognitive processing (Robbins 1984) and vigilance (Aston-Jones 1985). The finding that cannabis use impairs the ability to effectively focus attention and reject irrelevant information suggests an impact of cannabinoids on the noradrenergic coeruleo-cortical pathway (Solowij, Michie et al. 1995).
Long-term cannabis use has also been related to several affective disorders including anxiety, depression, cognitive impairment, and psychosis (Troisi, Pasini et al. 1998; Arnold, Topple et al. 2001; Degenhardt, Hall et al. 2001; Berrendero and Maldonado 2002; Manzanares, Uriguen et al. 2004). One longitudinal study reported that daily use of cannabis by teenagers was associated with a two-fold increased risk for depression and anxiety during their adult years (Patton, Coffey et al. 2002). Upregulation of the CB1 receptor has been reported in the prefrontal cortex of depressed suicide victims (Hungund, Vinod et al. 2004).
Brain noradrenergic systems regulate many of the same behavioral dimensions that are affected in depression and anxiety disorders (Morilak and Frazer 2004). Several lines of evidence point to dysfunction in the noradrenergic system in these conditions (for review see (Leonard 1997)). The question then arises as to how cannabinoids intersect with noradrenergic circuits and anatomical substrates underlying interactions between the two systems. Our laboratory has previously shown that systemic administration of a synthetic cannabinoid agonist causes an increase in extracellular norepinephrine release in the frontal cortex (Oropeza, Page et al. 2005). However, our previous study did not examine anatomical sites of CB1 receptor modulation. Therefore, in the present study, we examined the distribution of CB1 receptors with respect to noradrenergic axon terminals in the rat frontal cortex (Pettit, Harrison et al. 1998; Tsou, Brown et al. 1998) to examine the possibility that CB1 receptors are located presynaptically in this brain region.
Results
Immunoperoxidase detection of the CB1 receptor was conducted in the rat frontal cortex using light microscopic analysis (Fig. 1). The distribution of the CB1 receptor was consistent with previous studies examining this receptor in the frontal cortex (Tsou, Brown et al. 1998). CB1 immunoreactivity was localized to varicose processes and occasionally to cell bodies here (Fig. 1). Immunoperoxidase labeling of CB1 was most abundant in layers II and III as well as in layer VI. Within these layers, using higher magnification, immunoperoxidase labeling indicative of the CB1 receptor was associated primarily with processes resembling presynaptic cellular profiles (Fig. 1).
Figure 1.
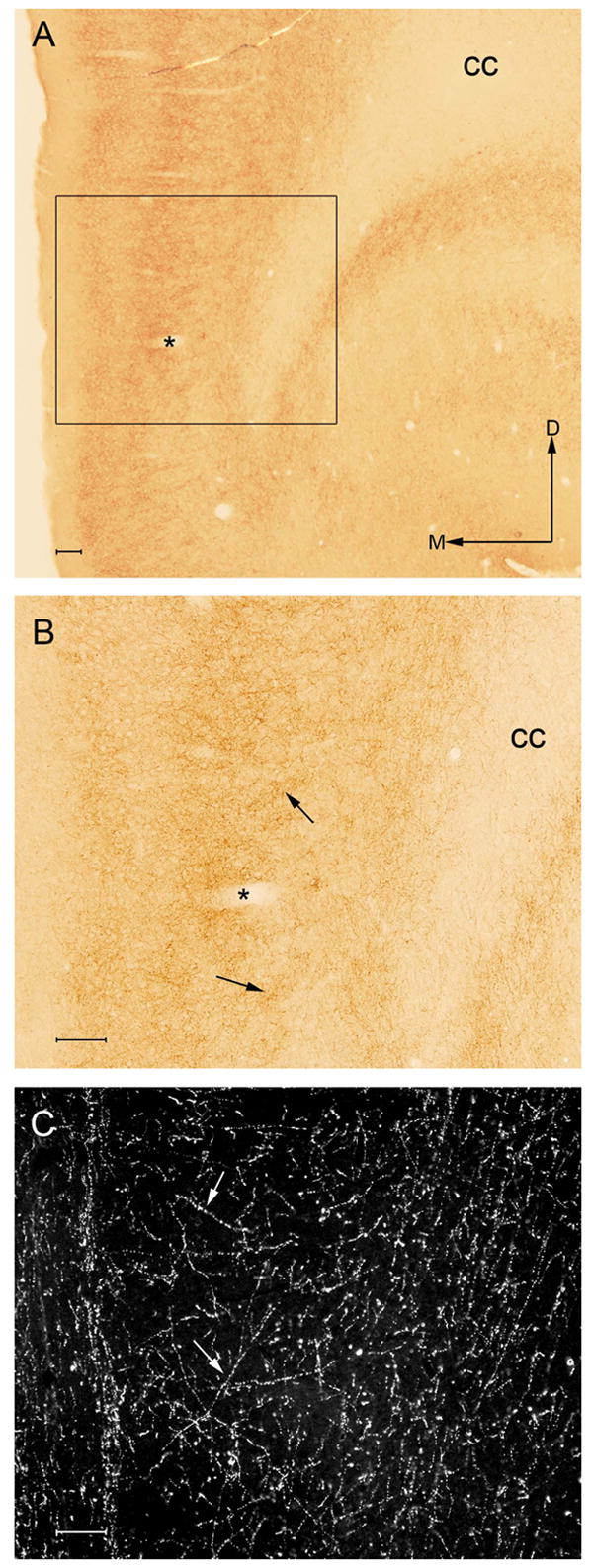
A. Low magnification brightfield photomicrograph showing immunoperoxidase labeling for the CB1 receptor in the rat frontal cortex. The region denoted within the black box is shown at higher magnification in panel B. The asterisks denotes a blood vessel that can be identified at higher magnification in panel B. The frontal cortical area indicated by the black box, defined as the infralimbic cortex, was the region sampled for ultrastructural analysis. Arrows indicate dorsal (D) and medial (M) orientation of the tissue section. Bar = 100 μm. B. High magnification image of the same section shown in panel A showing CB1 immunoperoxidase labeling in the frontal cortex. CB1 receptor labeling is localized abundantly to varicose processes in layers II, III and VI of the rat frontal cortex. Arrows indicate labeled fibers and processes. In panels A and B, cc indicated the corpus callosum. C. High magnification darkfield illuminated photomicrograph showing the noradrenergic synthesizing enzyme dopamine-β-hydroxylase (DßH) in varicose processes in a separate section of tissue through the same region illustrated in panel A. Localization of DßH was conducted using immunoperoxidase detection. White arrows depict DßH-labeled beaded processes in the frontal cortex. Bar for B and C = 100 μm.
To test the hypothesis that noradrenergic axon terminals contain CB1 receptors, dual immunocytochemical detection of the CB1 receptor and the catecholamine-synthesizing enzyme DßH was performed in the same section of tissue through the frontal cortex using immunofluorescence detection in one series of tissue sections as well as immunoelectron microscopy. As previously described by others (Lapierre, Beaudet et al. 1973), immunoperoxidase labeling for DßH was abundant in the infralimbic area of the frontal cortex (Fig. 1C). In these regions, DßH labeling was identified as a dense plexus of thin and beaded fibers.
Dual immunofluorescence localization of CB1 (identified with a fluorescein isothiocyanate tagged secondary antibody) and DßH (identified with a tetramethyl rhodamine isothiocyanate tagged secondary antibody) indicated abundant labeling of both antigens in the frontal cortex (Fig. 2A-F). In addition, numerous fibers exhibited both fluorophore labels indicating co-existence between CB1 and DßH immunoreactivities using confocal microscopy (Fig. 2A-F). Image analysis of projection images from confocal fluorescence z stacks demonstrated the proportion of CB1 labeled fibers that co-expressed DßH to be 32.4% (±3.8%) (Fig. 2G). Intensity of labeling for the CB1 receptor is represented on the y-axis (Ch2-T2), and intensity of labeling for DßH is shown on the x-axis (Ch3-T1). Colors in the histogram represent pixel frequency of intensity values (Fig. 2G). Areas in yellow denote co-localization of both fluorophores in common cellular profiles.
Figure 2.
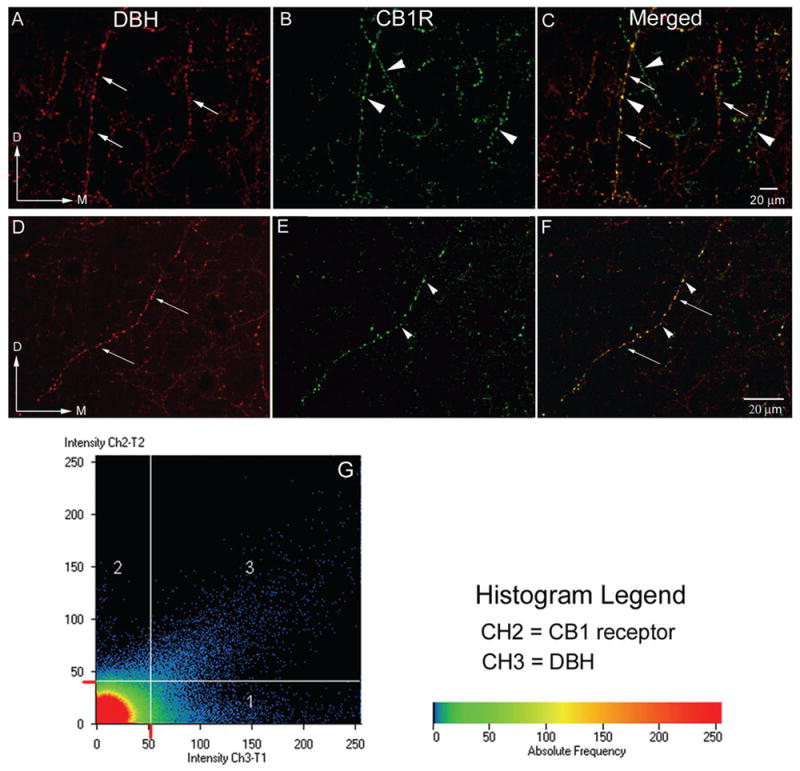
A–F: Confocal microscopy photomicrographs showing sections processed for the immunocytochemical detection of the catecholamine synthesizing enzyme, DßH (panel A, D) and CB1 receptors (panel B, E) in coronal sections from the infralimbic area of the frontal cortex. Abundant labeling in varicose processes was present in the frontal cortex for both antigens. Processes containing DßH alone can be seen at the long white arrows while fibers containing CB1 alone are shown at arrowheads. Panels C and F are merged images. Yellow labeling denotes co-localization of both CB1 and DßH in the same process. Magnification A–C: 20x, D–F: 40x oil G. Histogram analysis demonstrating the co-localization of DßH (red) and CB1 receptor (green). Intensity of labeling for DßH is seen on the x-axis (CH2-T1) and intensity of labeling for the CB1 receptor is on the y-axis (CH3-T2). Colors in the histogram represent pixel frequency and intensity.
At the ultrastructural level, CB1 receptor labeling in the frontal cortex was frequently localized to axon terminals and occasionally to somatodendritic processes (Fig. 3). Two methods of detection were used for each antigen. In one series, the CB1 receptor was detected using a peroxidase reaction product and was combined with gold-silver labeling of DßH (Fig. 3 A, B, D). In another series, the CB1 receptor was detected using immunogold-silver labeling while DßH was visualized using immunoperoxidase labeling (Fig. 3 C). Irrespective of the markers used, similar percentages of co-existence were identified.
Figure 3.
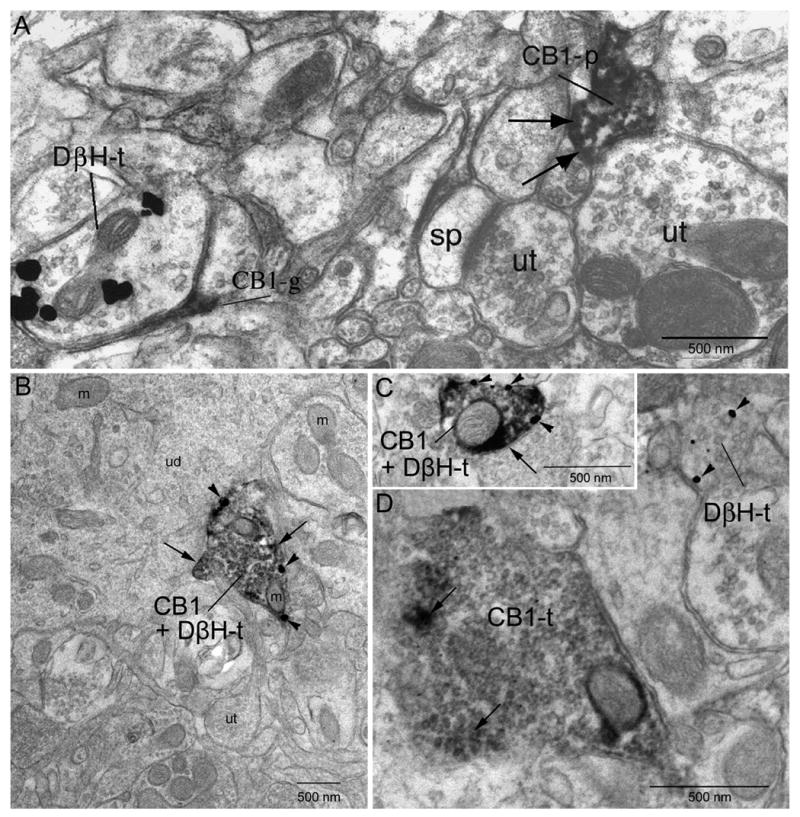
Electron micrographs showing localization of CB1 and DßH in axon terminals (t) in the frontal cortex. A. Dense peroxidase labeling for CB1 can be identified in a cellular process (CB1-p) denoted at black arrows. The CB1-p is apposed to an unlabeled terminal (ut). Another ut nearby contacts a spine (sp). The DßH-t is apposed by a profile resembling a glial process that is immunoreactive for CB1. B. Co-localization of gold-silver labeling for DßH (arrowheads) and peroxidase labeling (indicated by long black arrows) for CB1 in the same axon terminal. The DßH + CB1 terminal is apposed to an unlabeled dendrite (ud). Several unlabeled terminals can be seen in the neuropil. C. Reversal of immunolabels indicates similar patterns of immunolabeling. Gold-silver labeling for the CB1 receptor (arrowheads) and peroxidase labeling for DßH (long black arrows) can be seen in the same terminal. Gold-silver labeling for the CB1 receptor shows localization (CB1-t) to the plasma membrane of the axon terminal. D. A CB1 receptor immunoperoxidase-labeled terminal and a DßH immunogold-labeled terminal converge (DßH-t) onto a common dendrite. m: mitochondria. ut: unlabeled terminal. sp: spine.
CB1 receptor labeling using peroxidase detection was identified along the plasma membrane of axon terminals as well as within the axoplasm. This is consistent with the known tendency of the peroxidase reaction product to diffuse within cellular profiles. Axon terminals exhibiting CB1 receptor immunoreactivity often contained abundant small (30–40 nm) clear, spherical vesicles (Fig. 3D). In cases in which the synaptic specializations were distinguishable, the majority (69%) were of the symmetric or inhibitory type (Gray’s Type 2, Fig. 3D). Only 14% of CB1 receptor labeled axon terminals formed asymmetric synapses. The synaptic specialization of the remaining 17% of synaptic contacts were not able to be classified and therefore were termed “undefined”. The morphological differentiation of the DßH-labeled axon terminals was consistent with previously published studies in the frontal cortex (Fig. 3A) (Branchereau, Van Bockstaele et al. 1996).
CB1 receptor and DßH labeling were often identified in similar portions of the neuropil (Fig. 3A). Of 224 CB1 receptor labeled axon terminals, 31% exhibited DßH immunoreactivity (Fig. 3B, 4) confirming the results obtained using immunofluorescence detection. The majority of axon terminals exhibiting both CB1 and DßH were in direct contact with dendrites, although a few dually labeled axon terminals were apposed to other axon terminals. In some instances, CB1 and DßH-labeled axon terminals converged onto unlabeled dendrites (Fig. 3D, 4). Appositions between CB1 labeled terminals and CB1 labeled dendrites were also observed (8%, Fig. 4). Finally, axo-axonal contacts between CB1 labeled terminals and DßH labeled or unlabeled terminals were observed in 13% of profiles (Figure 4).
Figure 4.
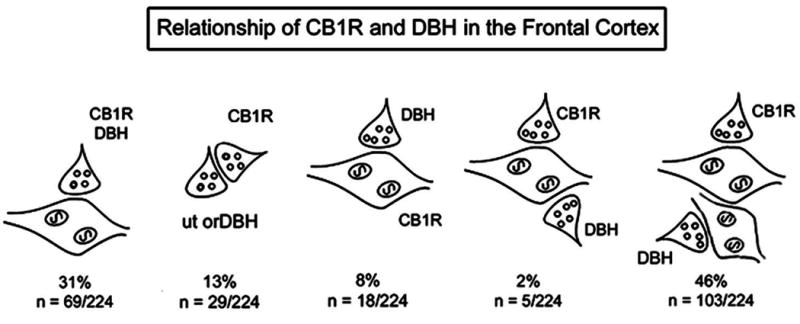
Schematic illustration depicting cellular associations of CB1 labeled terminals (CB1R) with dopamine-β-hydroxylase labeled terminals, dendrites, and unlabeled axon terminals. Percentages reflect associations of at least one-fourth of the plasmalemma of CB1 labeled profiles that are directly apposed to different cellular elements in the microenvironment.
Discussion
Previous studies from our laboratory have demonstrated that acute systemic cannabinoid administration causes an increase in extracellular norepinephrine efflux in the frontal cortex (Oropeza, Page et al. 2005). The present study extends these findings by showing that norepinephrine release in the frontal cortex may be modulated, in part, by activation of cannabinoid receptors that are localized to cortical noradrenergic axon terminals.
Our study is in agreement with previously published studies showing that CB1 receptors are localized to presynaptic axon terminals in the frontal cortex (Herkenham, Groen et al. 1991; Marsicano and Lutz 1999; Egertova and Elphick 2000). Presynaptic distribution of CB1 receptors has also been reported in other brain regions, including the hippocampal formation (Hoffman, Riegel et al. 2003), caudate putamen (Rodriguez, Mackie et al. 2001) and amygdala (Katona, Rancz et al. 2001). In addition, previous investigations support a role for CB1 receptors in the modulation of noradrenergic nerve terminals. For example, Poddar et al. (1980) showed that Δ9-tetrahydrocannabinol causes an increase in norepinephrine-containing synaptosomes in the striatum and hypothalamus. In the peripheral nervous system, CB1 receptors have been reported to be localized to noradrenergic terminals where they are also involved in the modulation of norepinephrine release (Ishac, Jiang et al. 1996; Vizi, Katona et al. 2001).
Our study focuses on the coeruleo-cortical pathway as a target of cannabinoid modulation of noradrenergic neurons. However, this is not out of disregard for other brain regions where cannabinoid receptors and noradrenergic cells may overlap (e.g. the nucleus of the solitary tract). The LC is the source of the majority of noradrenergic projections throughout the neuroaxis including the frontal cortex, hippocampus and amygdala as well as the cerebellum and spinal cord. Efferents from the lateral tegmentum (A1, A2, A5 and A7 cell groups) form the ventral noradrenergic bundle and have less extensive projections. They provide predominant innervation of the hypothalamus and also innervate areas of the septum and the extended amygdala nuclei including the bed nucleus of the stria terminalis (Card and Moore 1984). Further studies are therefore required to address potential differences in CB1 receptor modulation of distinct noradrenergic circuits.
Autoradiographic techniques have demonstrated the presence of CB1 receptor in the LC (Herkenham, Groen et al. 1991) and a low density of CB1 receptor mRNA in these neurons (Matsuda, Bonner et al. 1993; Tsou, Brown et al. 1998). Recent electrophysiological studies have shown that cannabinoid agonists activate LC neurons (Mendiguren and Pineda 2006; Muntoni, Pillolla et al. 2006). However, few studies have focused on the distribution of CB1 receptors in the frontal cortex with respect to norepinephrine containing terminals. The frontal cortex plays an important role in processes related to cognition, mood control, attention and motor performance (Goodwin 1997). This brain region receives a strong modulatory input from monoaminergic transmitters and many affective disorders are thought to reflect disruptions in the regulation of these neurotransmitters (Le Moal and Simon 1991; Arnsten 1997).
Modulation of the coeruleo-cortical pathway via CB1 receptor activation may contribute to changes in attention, cognition and anxiety commonly observed following cannabinoid exposure as this circuit is involved in modulating these behaviors (Jouvet 1969; Aston-Jones, Foote et al. 1984; Aston-Jones, Shaver et al. 1984; Valentino, Foote et al. 1993). Activity of noradrenergic neurons correlates with levels of cortical arousal and attention to internal and external stimuli (Aston-Jones and Bloom 1981; Foote, Bloom et al. 1983; Aston-Jones, Foote et al. 1984). Dysfunction of this system is thought to be involved in the development of affective disorders such as anxiety and depression. Cannabinoid receptors have been implicated in the regulation of anxiety-like behaviors (Berrendero and Maldonado, 2002; Chaperon and Thiebot, 1999; Degenhardt et al., 2001; Manzanares et al., 2004). For example, cannabinoid administration causes both anxiogenic and anxiolytic responses in animal studies (Rodriguez de Fonseca, Rubio et al. 1996).
Considerable interest exists in understanding the role of cannabinoid receptors and endocannabinoids in affective and cognitive disorders. In an attempt to explore the therapeutic possibilities that agonists or antagonists may offer, research into the actions of the cannabinoid antagonist SR 141716A (rimonabant) has provided some interesting findings (Tzavara, Davis et al. 2003). SR 141716A reduces the perception of the rewarding value of positive reinforcers, attenuates morphine induced place preference and withdrawal (Mas-Nieto, Pommier et al. 2001), reduces alcohol intake at high doses (2, 5 and 10 mg/kg) (Colombo, Agabio et al. 1998), reduces cocaine craving (De Vries, Shaham et al. 2001) and suppresses food intake (Chaperon and Thiebot 1999). SR 141716A increases arousal (Santucci, Storme et al. 1996) and enhances spatial memory in the radial arm maze (Lichtman 2000). However, although these data point to potential therapeutic approaches, interactions between the cannabinoid system and monoaminergic transmitters is only recently beginning to emerge. A more detailed analysis of the distribution of CB1 receptors in the LC using immunoelectron microscopy is also warranted. Similarly, direct infusion of cannabinoid agonists into the LC is necessary to elucidate the contribution of LC neurons in the modulation of norepinephrine release in the LC-frontal cortex pathway.
For many modulatory neurotransmitters, CB1 receptor activation has been associated with decreases in neurotransmitter release. However, in vivo microdialysis studies have shown that systemic administration of cannabinoid agonists can increase neurotransmitter efflux as well. For example, cannabinoid agonists increase extracellular dopamine (Tanda, Pontieri et al. 1997; Malone and Taylor 1999), glutamate (Ferraro, Tomasini et al. 2001) and acetylcholine (Acquas, Pisanu et al. 2000) efflux in the frontal cortex (Pistis, Ferraro et al. 2002). Our previous findings add to this growing literature by showing that systemic administration of a synthetic CB1 agonist stimulates norepinephrine release. Recently, promiscuous G protein coupling has been recognized for CB1 receptors (Lauckner, Hille et al. 2005). Glass and Felder (Glass and Felder 1997) demonstrated a novel pathway in which the CB1 receptor couples to a Gs protein, indicating that CB1 receptor function may be more complex than the simple Gi/o linkage described previously. Based on these studies, understanding the coupling of CB1 receptors to various G proteins is essential to understanding mechanisms of action of exogenous or endogenous cannabinoid signaling with noradrenergic neurons. In noradrenergic axon terminals, increased coupling of Gs to CB1 receptors may be a potential mechanism underlying increased catecholamine release in the frontal cortex. Future studies are required to test this hypothesis.
In summary, the present study provides neuroatomical evidence that cannabinoid receptors are presynaptically distributed in the frontal cortex where they are localized to a subset of noradrenergic axon terminals. These results suggest that presynaptic cannabinoid receptors may be positioned to influence cortical norepinephrine release by virtue of their localization to presynaptic cellular profiles.
Experimental Procedure
Subjects
Adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 250–300 g were housed 2–3 per cage on a 12-hour light schedule in a temperature-controlled (20ºC) colony room. Rats were given ad libitum access to standard rat chow and water. The Thomas Jefferson University Institutional Animal Care and Use Committee (IACUC) approved the care and use of animals and all studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Tissue fixation
Ten adult male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 250–300 g) were used in the present study. Rats were anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused through the ascending aorta with heparin (1000 units/ml) followed by acrolein (Electron Microscopy Sciences, Hatfield, PA) (3.75% in 2% paraformaldehyde) and 2% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 M phosphate buffer (PB, pH 7.4). The brain was removed and forty-micron thick coronal sections were cut through the rostrocaudal extent of the frontal cortex (Paxinos and Watson 1986)] and the rostral pons [plates 55 through 58 from the Paxinos and Watson rat brain atlas (Paxinos and Watson 1986)] using a Vibratome Series 1000 and collected into chilled 0.1 M PB.
Antibody specificity and control experiments
The polyclonal antiserum directed against the CB1 receptor was generated using the N-terminal 77 amino acid residues of the cloned CB1 receptor and fused to glutathione S-transferase (GST) as the antigen. The specificity of the antibody was previously determined by staining AtT20 cells that had been transfected with the CB1 receptor (Tsou, Brown et al. 1998). Antibody staining was specific as non-transfected cells showed an absence of immunostaining (Tsou, Brown et al. 1998). In the present study, cell homogenates obtained from the frontal cortex, LC and cerebellum were used to further analyze specificity. Western blot analysis using the CB1 receptor antibody showed a band at a molecular weight of 63 kDa consistent with the predicted size of the CB1 receptor (data not shown). Preabsorption of the antiserum (1:1000) with the immunizing protein (4 μg/ml) resulted in the absence of immunolabeling in tissue samples (data not shown). Specificity controls were also conducted for the DβH antiserum that was raised in mouse against purified bovine DβH. Specifically, preabsorption with the blocking peptide (Alpha Diagnostics, San Antonio, TX) resulted in an absence of immunolabeling in tissue sections from the frontal cortex. Control sections were also processed where one of the primary antisea was omitted. To evaluate possible cross-reactivity of secondary antibodies with the primary antisera in the dual labeling experiments, some sections were processed for dual labeling with omission of one of the primary antisera.
Immunofluorescence labeling
Free-floating sections of the frontal cortex were treated with a 1% sodium borohydride solution for thirty minutes. They were rinsed in 0.1M PB and 0.1M tris-saline buffer (TS; pH 7.6). Sections were then blocked in 0.5% bovine serum albumin (BSA)/0.25% Triton X-100 in 0.1M TS for 30 minutes and rinsed extensively in 0.1M TS. Sections were subsequently processed for CB1 receptor and DßH labeling as described below.
Sections were incubated in a primary antibody cocktail of rabbit anti-CB1 [1:1,000] and mouse anti-DßH (Chemicon International, Temecula, CA) [1:1,000] prepared in 0.1% BSA/0.25% Triton X-100 in 0.1M TS and incubated overnight on a rotary shaker at room temperature. Sections were subsequently washed in 0.1M TS and then incubated in a secondary antibody cocktail containing fluorescein isothiocyanate (FITC) goat anti-rabbit [1:50] (Jackson ImmunoResearch Laboratories, Inc.) and tetramethyl rhodamine isothiocyanate (TRITC) goat anti-mouse [1:50] (Jackson ImmunoResearch Laboratories, Inc.; West Grove, PA) prepared in 0.1% BSA/0.25% Triton X-100 in 0.1M TS for 2 hours in the dark on a rotary shaker.
At the conclusion of the incubation, sections were washed and mounted onto gelatinized slides. The sections were then allowed to dry in complete darkness. Slides were dehydrated in a series of alcohols, soaked in xylene, and coverslipped using DPX (Sigma-Aldrich, St. Louis, MO). A Zeiss 510 LSM Meta confocal microscope (Oberkochen, Germany) was used to visualize the immunofluorescence labeling and images were generated using a Zeiss LSM510 META confocal microscope. Z-stacks were collected, and projection images were created from the Z-stack. Histograms showing pixel frequency of intensity values were created using Zeiss LSM5 Image Examiner software. Data analysis of dual fluorescence co-localization is presented in Figure 2G. Intensity of labeling for CB1 can be seen on the y axis, and intensity of labeling for DβH can be seen on the x axis. Colors in the histogram represent pixel frequency of intensity values.
Light and electron microscopy immunocytochemistry
Free-floating sections of the frontal cortex were treated with a 1% sodium borohydride solution for thirty minutes. They were rinsed with 0.1 M PB and later washed in 0.1M TS three times for ten minutes. The tissue was blocked in 0.5 % BSA in 0.1M TS for an hour and then washed for ten minutes, three times. The sections were incubated overnight in a cocktail containing the rabbit-polyclonal antibody directed against the CB1 receptor at a dilution of 1:1000 in 0.1% BSA in 0.1M TS. The sections were washed in 0.1 M TS (three times, 10 minutes each). They were incubated in biotinylated goat-anti rabbit IgG (Vector Laboratories, Burlingame, CA) for 30 minutes, followed by rinsing with 0.1 M TS (three times, 10 minutes each). The tissue was incubated with an avidin-biotin complex solution (ABC) (Vector Laboratories) for 30 minutes and then washed again for ten minutes, three times in 0.1 M TS. A peroxidase color reaction was obtained by exposing the tissue sections to a solution containing 22 mg of 3,3-diaminobenzidine tetrahydrochloride (DAB) (Sigma-Aldrich, St. Louis, MO) in a 0.1 M TS solution containing 0.05% hydrogen peroxide for 6 min. The reaction was stopped with 0.1 M TS. Three, five minute rinses with 0.1 M PB followed. The sections were mounted onto gelatinized glass slides from a 0.05 M PB solution. The slides were dehydrated through a graded series of alcohols and cleared in xylene before being coverslipped with Permount. Sections were visualized using a Leica DMRBE microscope (Wetzlar, Germany) and images were acquired using SPOT Advanced software (Diagnostics Instruments, Inc., Sterling Heights, MI).
For dual labeling experiments, tissue sections were processed using primary antibodies directed against the CB1 receptor and DßH. The CB1 receptor was visualized using immunoperoxidase detection (described above) or immunogold-silver labeling. When the CB1 receptor was labeled with peroxidase dectection, DßH was identified using immunogold-silver labeling. To this end, sections were rinsed extensively in 0.1M TS and 0.01M phosphate buffered saline (PBS; pH 7.4). Sections were incubated in a 0.2% gelatin/0.8% BSA buffer for 10 minutes. This was followed by incubation with goat anti-mouse IgG conjugated to 1 nm gold particles (Amersham Corp., Bioscience Corp, Piscataway, NJ) at room temperature for two hours. Sections were rinsed in buffer containing the same concentrations of gelatin and BSA as above and subsequently rinsed with 0.01M PBS. Sections were then incubated in 2% glutaraldehyde (Electron Microscopy Sciences) in 0.01M PBS for 10 minutes followed by washes in 0.01M PBS and 0.2M sodium citrate buffer (pH 7.4). A silver enhancement kit (Amersham Bioscience Corp, Piscataway, NJ) was used for silver intensification of the gold particles. The optimal silver enhancement times were determined empirically for each experiment and ranged from 11–12 minutes. Following intensification, tissue was rinsed in 0.1M PB and incubated in 2% osmium tetroxide in 0.1M PB for one hour, washed in 0.1M PB, dehydrated, and flat embedded in Epon 812 (Electron Microscopy Sciences). Thin sections of approximately 50–100 nm in thickness were cut with a diamond knife (Diatome-U.S., Fort Washington, PA) using an LKB ultramicrotome. Thin sections were collected on copper mesh grids. Sections were examined with a Morgagni 268 electron microscope (FEI Company, Eindhoven, The Netherlands) and micrographs were prepared exclusively from regions of tissue near the interface between tissue and plastic. Detection of each antigen was reversed in a separate series of tissue sections where the CB1 receptor was detected using immunogold-silver labeling and DßH was identified using a peroxidase reaction product using a similar protocol described above.
Data analysis
Quantitative evaluation of immunoreactive elements was applied only to the outer 1–3 μm of the epon-tissue interface where penetration of antisera is optimal. For dual labeling, only micrographs containing both peroxidase and gold-silver markers were used for the tissue analysis to ensure that the absence of one marker did not result from uneven penetration of markers (Leranth and Pickel 1989). Examination of serial sections was used to determine synaptic associations of axon terminals not always apparent in single sections. Furthermore, to ensure specificity in the pre-embedding gold-silver labeling method, selective gold-silver labeled profiles were identified by the presence, in single thin sections, of at least three times more gold particles within cytoplasmic compartments as compared to the neuropil. Whenever possible, the more lightly labeled cellular profiles were confirmed by detection of gold-silver particles in at least two adjacent thin sections. A profile containing a small number of gold-particles (e.g. two gold particles) that was unlabeled in adjacent thin sections was designated as lacking detectable immunoreactivity.
The region selected for analysis included the infralimbic region of the frontal cortex. Adequate preservation of ultrastructural morphology was one of the criteria imposed when selecting tissue sections to be used for ultrastructural analysis. Fifteen sections from nine animals were used for the analysis. Grids containing 5–10 thin sections each were collected from plastic-embedded sections of the frontal cortex from each animal. All cellular profiles exhibiting immunolabeling for the CB1 receptor and DßH-labeling in the same field were captured using a digital camera and stored electronically for subsequent analysis.
Classification of cellular elements was based on characterizations described in Peters et al. (Peters 1991). Proximal dendrites were identified by the presence of endoplasmic reticulum and a diameter of greater than 0.7 μm. Axon terminals were distinguished from unmyelinated axons based on synaptic vesicle presence and a diameter of greater than 0.1 μm. Synaptic terminals were characterized by a junctional complex, a restricted zone of parallel membrane apposition with slight enlargement of the intercellular space and/or associated postsynaptic thickening. Asymmetric and symmetric synapses were defined as follows (Peters 1991): asymmetric synapses (Gray’s Type I) were documented as those contacts by a presynaptic terminal onto a postsynaptic dendrite or axon with thick postsynaptic densities. An additional characteristic of asymmetric synapses was a synaptic cleft of 20 nm wide between presynaptic and postsynaptic structures; symmetric synapses (Gray’s Type II) had thinner, less prominent post-synaptic densities with narrower synaptic clefts (approximately 12 nm) than that observed at asymmetric synapses. Appositions (non-synaptic contacts) were defined by closely spaced parallel plasma membranes of the axon of interest and the membranes of dendrites, other axon terminals, myelinated, and unmyelinated axons.
The similar gray levels of the peroxidase reaction product and silver-intensified gold particles are occasionally difficult to discern in the same axon terminal. To circumvent this potential problem, several methods were used to visualize CB1 and DßH immunoreactivities in the frontal cortex. First, both immunofluorescence and immunoelectron microscopy detection were employed. Second, tissue sections were processed where the detection methods were reversed. In such experiments, peroxidase labeling was used to identify DβH and gold-silver labeling was used to identify CB1 (Fig. 3C).
Acknowledgments
Supported by NIDA DA 020129 and DA15395. V.O. was supported by a Minority Student Supplement to DA09082 from NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Pisanu A, et al. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur J Pharmacol. 2000;401(2):79–85. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Topple AN, et al. The distribution of cannabinoid-induced Fos expression in rat brain: differences between the Lewis and Wistar strain. Brain Res. 2001;921(1–2):240–55. doi: 10.1016/s0006-8993(01)03127-4. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11(2):151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. Behavioral functions of locus coeruleus derived from cellular attributes. Physiol Psychol. 1985;13:118–126. [Google Scholar]
- Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1(8):887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, et al. Anatomy and physiology of locus coeruleus neurons: functional implications. In: Ziegler M, Lake CR, editors. Norepinephrine (Frontiers of Clinical Neuroscience) Vol. 2. Baltimore: Williams and Wilkins; 1984. pp. 92–116. [Google Scholar]
- Aston-Jones G, Shaver R, et al. Cortically projecting nucleus basalis neurons in rat are physiologically heterogeneous. Neurosci Lett. 1984;46(1):19–24. doi: 10.1016/0304-3940(84)90192-7. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163(1):111–7. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Van Bockstaele EJ, et al. Pyramidal neurons in rat prefrontal cortex show a complex synaptic response to single electrical stimulation of the locus coeruleus region: evidence for antidromic activation and GABAergic inhibition using in vivo intracellular recording and electron microscopy. Synapse. 1996;22(4):313–31. doi: 10.1002/(SICI)1098-2396(199604)22:4<313::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13(2):415–31. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- Casswell S, Marks D. Cannabis induced impairment of performance of a divided attention task. Nature. 1973;241(5384):60–1. doi: 10.1038/241060b0. [DOI] [PubMed] [Google Scholar]
- Chait LD, Pierri J. Effects of smoked marijuana on human performance: A critical review. In: Murphy L, Bartke A, editors. Marijuana/Cannabinoids: Neurobilogy and Neurophysiology. Boca Raton, FL: CRC Press; 1992. pp. 387–423. [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13(3):243–81. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, et al. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33(2):126–30. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7(10):1151–4. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, et al. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96(11):1603–14. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422(2):159–71. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, et al. Cannabinoid receptor agonist WIN 55,212-2 inhibits rat cortical dialysate gamma-aminobutyric acid levels. J Neurosci Res. 2001;66(2):298–302. doi: 10.1002/jnr.1224. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, et al. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63(3):844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17(14):5327–33. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM. Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol. 1997;11(2):115–22. doi: 10.1177/026988119701100204. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Groen BG, et al. Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res. 1991;552(2):301–10. doi: 10.1016/0006-8993(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Riegel AC, et al. Functional localization of cannabinoid receptors and endogenous cannabinoid production in distinct neuron populations of the hippocampus. Eur J Neurosci. 2003;18(3):524–34. doi: 10.1046/j.1460-9568.2003.02773.x. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Vinod KY, et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9(2):184–90. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, et al. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118(8):2023–8. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163(862):32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21(23):9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempel P, Lampe K, et al. Auditory-evoked potentials and selective attention: different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48(2):95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- Lapierre Y, Beaudet A, et al. Noradrenergic axon terminals in the cerebral cortex of rat. II. Quantitative data revealed by light and electron microscope radioautography of the frontal cortex. Brain Res. 1973;63:175–82. doi: 10.1016/0006-8993(73)90086-3. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Hille B, et al. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci U S A. 2005;102(52):19144–9. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71(1):155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The role of noradrenaline in depression: a review. J Psychopharmacol (Oxf) 1997;11(4):S39–47. Suppl. [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. New York: Plenum Publishing; 1989. [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404(1–2):175–9. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol. 1999;128(1):21–6. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Uriguen L, et al. Role of endocannabinoid system in mental diseases. Neurotox Res. 2004;6(3):213–24. doi: 10.1007/BF03033223. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11(12):4213–25. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mas-Nieto M, Pommier B, et al. Reduction of opioid dependence by the CB(1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol. 2001;132(8):1809–16. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, et al. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327(4):535–50. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur J Pharmacol. 2006;534(1–3):83–8. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7(2):193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, et al. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23(9):2385–94. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, et al. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046(1–2):45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, et al. Cannabis use and mental health in young people: cohort study. Bmj. 2002;325(7374):1195–8. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Peters A. The Fine Structure of the Nervous System. 1991. [Google Scholar]
- Pettit DA, Harrison MP, et al. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51(3):391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, et al. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948(1–2):155–8. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Cortical noradrenaline, attention and arousal. Psychol Med. 1984;14(1):13–21. doi: 10.1017/s0033291700003032. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Rubio P, et al. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276(1):56–64. [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, et al. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21(3):823–33. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci V, Storme JJ, et al. Arousal-enhancing properties of the CB1 cannabinoid receptor antagonist SR 141716A in rats as assessed by electroencephalographic spectral and sleep-waking cycle analysis. Life Sci. 1996;58(6):PL103–10. doi: 10.1016/0024-3205(95)02319-4. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, et al. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry. 1995;37(10):731–9. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens R, et al. Does marijuana use cause long-term cognitive deficits? Jama. 2002;287(20):2653–4. author reply 2654. [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, et al. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276(5321):2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Troisi A, Pasini A, et al. Psychiatric symptoms in male cannabis users not using other illicit drugs. Addiction. 1998;93(4):487–92. doi: 10.1046/j.1360-0443.1998.9344874.x. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, et al. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, et al. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138(4):544–53. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, et al. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993;697:173–88. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Katona I, et al. Evidence for presynaptic cannabinoid CB(1) receptor-mediated inhibition of noradrenaline release in the guinea pig lung. Eur J Pharmacol. 2001;431(2):237–44. doi: 10.1016/s0014-2999(01)01413-3. [DOI] [PubMed] [Google Scholar]


