Abstract
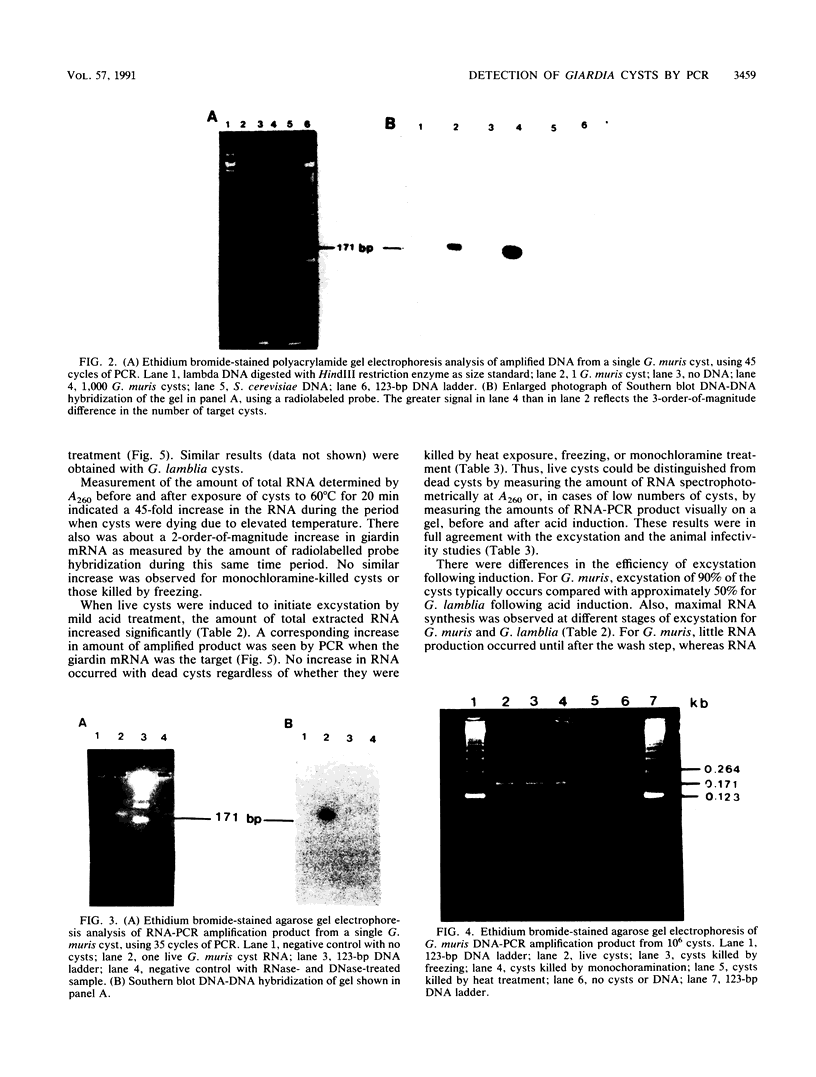
A method was developed for the detection of Giardia cysts by using the polymerase chain reaction (PCR) and the giardin gene as the target. DNA amplification by PCR, using giardin DNA as the target, resulted in detection of both live and dead cysts. When giardin mRNA was used as the target, the ability to amplify cDNA by PCR depended on the mode of killing. Cysts killed by freezing were not detected by PCR when giardin mRNA was the target. Cysts killed by heating or exposure to monochloramine, however, gave positive detection signals for both DNA and giardin mRNA targets. The amount of giardin mRNA and total RNA was significantly increased in live cysts following the induction of excystation. Cysts killed by freezing, heating, or exposure to monochloramine did not show a change in RNA content. The detection of the giardin gene by PCR permits a sensitive and specific diagnosis for Giardia spp. Discrimination between live and dead cysts can be made by measuring the amounts of RNA or PCR-amplified product from the giardin mRNA target before and after the induction of excystation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. A., Holberton D. V., Marshall J. Sequence of a giardin subunit cDNA from Giardia lamblia. Nucleic Acids Res. 1988 Jul 25;16(14B):7177–7177. doi: 10.1093/nar/16.14.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bej A. K., Mahbubani M. H., Miller R., DiCesare J. L., Haff L., Atlas R. M. Multiplex PCR amplification and immobilized capture probes for detection of bacterial pathogens and indicators in water. Mol Cell Probes. 1990 Oct;4(5):353–365. doi: 10.1016/0890-8508(90)90026-v. [DOI] [PubMed] [Google Scholar]
- Bej A. K., Steffan R. J., DiCesare J., Haff L., Atlas R. M. Detection of coliform bacteria in water by polymerase chain reaction and gene probes. Appl Environ Microbiol. 1990 Feb;56(2):307–314. doi: 10.1128/aem.56.2.307-314.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D., Hoff J. C. Inactivation of simian rotavirus SA11 by chlorine, chlorine dioxide, and monochloramine. Appl Environ Microbiol. 1984 Aug;48(2):317–323. doi: 10.1128/aem.48.2.317-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham A. K., Jarroll E. L., Jr, Meyer E. A., Radulescu S. Giardia sp.: physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp Parasitol. 1979 Apr;47(2):284–291. doi: 10.1016/0014-4894(79)90080-8. [DOI] [PubMed] [Google Scholar]
- Crossley R., Marshall J., Clark J. T., Holberton D. V. Immunocytochemical differentiation of microtubules in the cytoskeleton of Giardia lamblia using monoclonal antibodies to alpha-tubulin and polyclonal antibodies to associated low molecular weight proteins. J Cell Sci. 1986 Feb;80:233–252. doi: 10.1242/jcs.80.1.233. [DOI] [PubMed] [Google Scholar]
- Mahbubani M. H., Bej A. K., Miller R., Haff L., DiCesare J., Atlas R. M. Detection of Legionella with polymerase chain reaction and gene probe methods. Mol Cell Probes. 1990 Jun;4(3):175–187. doi: 10.1016/0890-8508(90)90051-z. [DOI] [PubMed] [Google Scholar]
- Meyer E. A., Jarroll E. L. Giardiasis. Am J Epidemiol. 1980 Jan;111(1):1–12. doi: 10.1093/oxfordjournals.aje.a112860. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Alonso R. A., Hein A., Caulfield J. P. Ultrastructural localization of giardins to the edges of disk microribbons of Giarida lamblia and the nucleotide and deduced protein sequence of alpha giardin. J Cell Biol. 1989 Nov;109(5):2323–2335. doi: 10.1083/jcb.109.5.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice E. W., Schaefer F. W., 3rd Improved in vitro excystation procedure for Giardia lamblia cysts. J Clin Microbiol. 1981 Dec;14(6):709–710. doi: 10.1128/jcm.14.6.709-710.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs J. L., Dupuis K. W., Nakamura K., Spath D. P. Detection of Giardia lamblia by immunofluorescence. Appl Environ Microbiol. 1983 Feb;45(2):698–700. doi: 10.1128/aem.45.2.698-700.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976 Jul;71(1):57–61. [PubMed] [Google Scholar]
- Sauch J. F. Use of immunofluorescence and phase-contrast microscopy for detection and identification of Giardia cysts in water samples. Appl Environ Microbiol. 1985 Dec;50(6):1434–1438. doi: 10.1128/aem.50.6.1434-1438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer F. W., 3rd, Rice E. W., Hoff J. C. Factors promoting in vitro excystation of Giardia muris cysts. Trans R Soc Trop Med Hyg. 1984;78(6):795–800. doi: 10.1016/0035-9203(84)90024-5. [DOI] [PubMed] [Google Scholar]
- Schupp D. G., Erlandsen S. L. Determination of Giardia muris cyst viability by differential interference contrast, phase, or brightfield microscopy. J Parasitol. 1987 Aug;73(4):723–729. [PubMed] [Google Scholar]
- Spaulding J. J., Pacha R. E., Clark G. W. Quantitation of Giardia cysts by membrane filtration. J Clin Microbiol. 1983 Sep;18(3):713–715. doi: 10.1128/jcm.18.3.713-715.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]