Abstract
Niemann-Pick type C (NPC) disease is associated with the accumulation of unesterified cholesterol in nearly all tissues and with progressive neurodegeneration. A murine model of this disease, the NPC mouse, was used to determine whether this sequestered cholesterol represented sterol carried in low density lipoprotein (LDL) and chylomicrons (CMs) taken up into the tissues through the coated-pit pathway. By 7 weeks of age, the sterol pool in the NPC mice had increased from 2,165 to 5,669 mg/kg body weight because of the daily sequestration of 67 mg of cholesterol per kg in the various organs. This was 7-fold greater than the rate of accumulation in control mice. The rate of LDL clearance in the NPC mouse was normal (523 ml/day per kg) and accounted for the uptake of 78 mg/day per kg of cholesterol in LDL whereas 8 mg/day per kg was taken up from CMs. Deletion of the LDL receptor in NPC mice altered the concentration of unesterified cholesterol in every organ in a manner consistent with the changes also observed in the rate of LDL cholesterol uptake in those tissues. Similarly, altering the flow of cholesterol to the liver through the CM pathway changed the concentration of unesterified cholesterol in that organ. Together, these observations strongly support the conclusion that, in NPC disease, it is cholesterol carried in LDL and CMs that is sequestered in the tissues and not sterol that is newly synthesized and carried in high density lipoprotein.
Niemann-Pick type C (NPC) disease is an autosomal recessive disorder characterized by a variable phenotype that includes hepatosplenomegaly, liver dysfunction, and progressive neurodegeneration (1). Many tissues from these patients have elevated concentrations of unesterified cholesterol, and neurons in the central nervous system contain polymorphous cytoplasmic bodies that react with filipin. In a series of seminal reports, it was shown that fibroblasts from these patients have a defect in the intracellular trafficking of cholesterol brought to the cell in low density lipoprotein (LDL-C) (2). After uptake through the coated-pit pathway, the sterol esters carried in this particle are hydrolyzed normally, but the unesterified cholesterol that is formed is sequestered in the lysosomal compartment. Such cholesterol apparently cannot reach the endoplasmic reticulum and regulate the rate of sterol synthesis or act as substrate for the esterifying enzyme acyl-CoA:cholesterol acyltransferase (2–5). This concept was further supported when the product of the mutated gene in this disease, NPC1 protein, was shown to have sequence similarities to several other molecules known to be active in the regulation of sterol metabolism (6). Furthermore, this protein was localized to vesicles that appeared to transiently interact with lysosomes to move unesterified cholesterol to other sites within the cell (7).
A similar mutation in NPC1 was described in a murine model of NPC disease, and these animals have provided important additional insights into the significance of this mutation in sterol metabolism in the whole animal (8). The mouse that is homozygous for this defect begins expanding its whole body pool of unesterified cholesterol in utero, and this expansion continues until the animal dies of neurological disease (9). Virtually every tissue in the body participates in this expansion, although the accumulation of cholesterol in the liver is quantitatively much greater than in the other organs. Despite these expanded tissue sterol pools, however, nearly all organs in these mice manifest elevated cholesterol synthesis so that the rate of whole animal sterol production is increased ≈50% (9). As in the human, these animals also show marked demyelination of the brain, rapid loss of cerebellar Purkinje cells, and polymorphous cytoplasmic bodies in various neurons (10).
This defect in NPC1 function provides a critically important tool for exploring several pathways in the intact animal that are essential for maintaining intracellular and whole animal cholesterol homeostasis during fetal development and postnatal growth. On the one hand, most cholesterol used for fetal tissue growth, including growth of the brain, comes from de novo synthesis (11–13). Such newly synthesized sterol is continuously shed from the peripheral organs into the plasma, incorporated into high density lipoprotein (HDL-C), and cleared from the vascular space by tissues like the adrenal and liver (14, 15). In the rodent, this uptake process involves a class B, type 1 scavenger receptor (16–18). On the other hand, cholesterol that is absorbed from the diet and carried in chylomicrons (CM-C) or secreted from the liver and carried in LDL is removed from the plasma by LDL receptors (LDLR) and LDLR related protein located in coated-pits (19, 20). Although the intracellular fate of HDL-C taken up by the class B, type 1 scavenger receptor is not yet fully understood, LDL-C and CM-C are known to be processed through the coated-pit pathway before being excreted or further metabolized by the cell (18, 21, 22). The current studies, therefore, were undertaken to determine whether the continuous accumulation of unesterified cholesterol that is the hallmark of NPC disease can be accounted for quantitatively by a block in the movement of cholesterol through the coated-pit pathway or, alternatively, whether a defect in another pathway must be evoked. This question was explored by determining the rate at which cholesterol is sequestered in the NPC mouse, by measuring the rates of tissue uptake of LDL-C and CM-C in the same animals, and by determining the effect of altering the rates of LDL-C and CM-C clearance on tissue unesterified cholesterol levels.
Materials and Methods
Animals and Diets.
BALB/c mice carrying the genetic mutation in NPC1 protein were transferred from the National Institutes of Health to our laboratories (8). The heterozygous NPC mice (NPC+/−) then were bred with animals homozygous for deletion of the LDLR (LDLR−/−) (9, 23, 24). Four groups were ultimately derived from these crosses and included mice that were NPC+/+/LDLR+/+, NPC−/−/LDLR+/+, NPC+/+/LDLR−/−, and NPC−/−/LDLR−/−. The genotypes of these various groups were established by using both PCR and Southern blot analysis (8, 9, 23, 24). After weaning, the animals were maintained on a pelleted, basal rodent diet (No. 7001, Harlan Teklad, Madison, WI) that had a cholesterol content of 0.016% (wt/wt) and a total lipid content of 5% (wt/wt) (9). In one experiment, animals were fed for 1 week a meal form of this basal diet, to which was added 0.4% (wt/wt) cholesterol. In most experiments, the animals were studied at 7 weeks of age. In one study, whole animal cholesterol pools were measured in 1-day-old pups. All experimental groups contained nearly equal numbers of males and females because there were no gender differences observed in any of these measurements, except for the concentration of cholesterol in the adrenals (9). In Figs. 3 and 4, therefore, data for the concentration of unesterified and esterified cholesterol in the adrenal were taken only from males. All experimental protocols were approved by the Institutional Animal Care and Research Advisory Committee.
Figure 3.
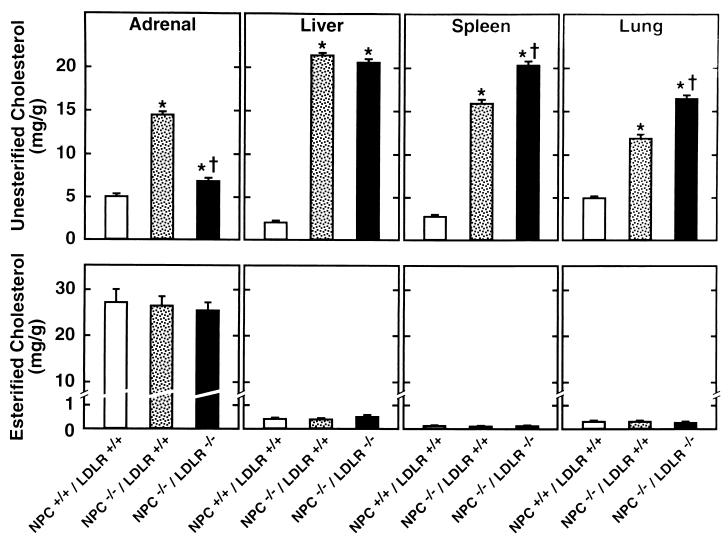
Tissue concentrations of unesterified (upper panels) and esterified (lower panels) cholesterol in NPC+/+/LDLR+/+, NPC−/−/LDLR+/+, and NPC−/−/LDLR−/− mice 7 weeks of age. There were 6–10 animals in each group. The asterisk (∗) identifies those values in the NPC−/−/LDLR+/+ and NPC−/−/LDLR−/− groups that were significantly different from those in the NPC+/+/LDLR+/+ animals. The dagger (†) indicates those values in the NPC−/−/LDLR−/− mice that were significantly different from those in the NPC−/−/LDLR+/+ groups.
Figure 4.
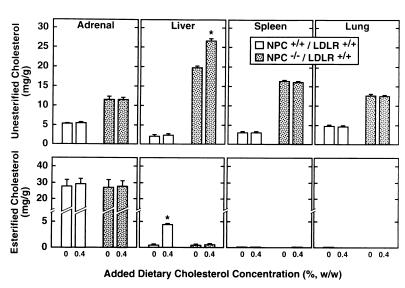
Tissue concentrations of unesterified (upper panels) and esterified (lower panels) cholesterol in NPC+/+/LDLR+/+ and NPC−/−/LDLR+/+ mice fed the basal diet and this same diet with additional amounts of cholesterol. At 6 weeks of age, half of the animals of each genotype were placed on the basal diet supplemented with 0.4% cholesterol while the remainder were maintained on the basal diet alone. One week later, the concentration of unesterified and esterified cholesterol was measured in various organs. There were 6–9 animals in each group. The asterisk (*) identifies those values found in the mice given additional dietary cholesterol that were significantly different from the corresponding values in those fed the basal diet alone.
Measurement of Intestinal Cholesterol Absorption.
Cholesterol absorption was measured by a fecal dual-isotope ratio method using [4-14C]cholesterol and [5, 6-3H]sitostanol as described (25). Stools were collected from each animal over a 72-hr period, and the ratio of 14C to 3H in each sample was determined.
Measurement of Plasma and Tissue Cholesterol Concentrations.
The total plasma cholesterol concentration was measured enzymatically (9). Plasma lipoproteins were isolated by using two procedures. First, these were separated by ultracentrifugation into three fractions having densities of <1.020, 1.020–1.063, and >1.063 g/ml. The cholesterol content in each of these fractions was quantitated by gas liquid chromatography using stigmastanol as an internal standard. Second, plasma lipoproteins also were separated by fast protein liquid chromatography (FPLC) using a Superose 6 column (26). Whole animal cholesterol pools were measured by saponifying the entire mouse and determining the content of cholesterol. In some experiments, tissues also were extracted in chloroform/methanol (2:1, vol/vol). The unesterified and esterified cholesterol was separated on Sep-Pak Vac RC cartridges (Waters), and the cholesterol was quantitated (9).
Measurement of LDL Clearance in Vivo.
The mice were anesthetized, and a catheter was inserted into a jugular vein. After awakening, each animal was given a bolus of 125I-tyramine cellobiose-labeled mouse LDL followed by a continuous infusion of the same preparation at a rate shown to maintain a constant specific activity in the plasma (24, 27, 28). Ten minutes before the termination of the 4-hr infusion, a bolus of 131I-labeled LDL was administered to each animal. The animals were exsanguinated 4 hr after beginning the infusion, and all of the major organs were removed. The remaining carcass was cut into small pieces. Tissue and plasma samples were assayed for their content of 125I and 131I. The rate of LDL uptake into each tissue was calculated as microliters of plasma cleared of its LDL content per hour per gram (μl/hr per g). Rates of clearance in the whole mouse were expressed as milliliters of plasma cleared each day of its LDL content per kilogram of body weight (ml/day per kg). By using the concentration of LDL-C in the plasma, and after correcting for small amounts of HDL-C contaminating the samples (29), the rate of LDL-C uptake into the individual organs and the whole animal was calculated. In the case of the individual tissues, these rates were expressed as micrograms of LDL-C taken up each hour per gram of tissue (μg/hr per g) while the whole animal data were calculated as milligrams of LDL-C transported into all tissues each day per kilogram of body weight (mg/day per kg).
Measurement of Rates of Cholesterol Synthesis and Sterol Excretion in Vivo.
The rates of cholesterol synthesis in the whole animal and the rates of cholesterol excretion in the feces as neutral and acidic sterols were measured as described (9). In both cases, the data were expressed as the milligrams of cholesterol synthesized or excreted each day per kilogram of body weight (mg/day per kg).
Calculations.
All data are presented as mean values ± 1 SEM. The Student’s unpaired t test was used to compare the various sets of data for significance at the P < 0.05 level. In the figures and tables, an asterisk or a dagger indicate that a particular value was significantly different from the appropriate control value.
Results
Cholesterol Pools in Mice Lacking NPC1 Function.
As summarized in Table 1, the 7-week-old NPC−/−/LDLR+/+ animals used in this study had marginally lower body and brain weights and slightly elevated liver weights compared with the NPC+/+/LDLR+/+ mice. However, after correcting for body weight, brain weight was similar in the mutant (22 g/kg body weight) and control (21 g/kg) animals whereas liver weight was increased 36% (76 g/kg vs. 56 g/kg). The characteristic finding with this mutation, however, was revealed in the size of the whole animal cholesterol pool, which was significantly elevated, even in the 1-day-old pups (2,453 mg/kg vs. 1,725 mg/kg). This pool expanded essentially as a linear function of age and reached a value 262% higher (5,669 mg/kg) than seen in the control mice (2,165 mg/kg) at 7 weeks. Over this period, therefore, the sterol pool expanded by only 9.2 mg/day per kg in the NPC+/+/LDLR+/+ animals but by 67 mg/day per kg in the NPC−/−/LDLR+/+ mice. At the same time, net absorption of cholesterol from the diet in this latter group was only 8 mg/day per kg and, therefore, by itself could not have accounted for this very high rate of sterol sequestration.
Table 1.
Animal and organ weights, cholesterol pool sizes, and rates of dietary cholesterol absorption in the NPC+/+/LDLR+/+ and NPC−/−/LDLR+/+ mice used in these studies
| NPC+/+/LDLR+/+ | NPC−/−/LDLR+/+ | |
|---|---|---|
| Weight of whole animal, g | 21.3 ± 0.3 | 17.4 ± 0.3* |
| Weight of liver, g | 1.20 ± 0.03 | 1.33 ± 0.037* |
| Weight of brain, g | 0.44 ± 0.01 | 0.38 ± 0.03* |
| Cholesterol pool at 1 day, mg/kg | 1725 ± 22 | 2453 ± 35* |
| Cholesterol pool at 7 weeks, mg/kg | 2165 ± 47 | 5669 ± 173* |
| Cholesterol sequestration, mg/day per kg | 9.2 | 67.0 |
| Cholesterol absorption, mg/day per kg | 14 ± 2 | 8 ± 2* |
Except for the values of the cholesterol pool at 1 day of age, these measurements were carried out in 7-week-old mice. The whole animal cholesterol pool represents milligrams of total cholesterol per kilogram of body weight while the sequestration rate equals milligrams of cholesterol retained in the mouse each day per kilogram of body weight. There were 80–83 (weights), 5–12 (pools), or 10–11 (absorption) animals in each group. An asterisk (*) identifies those values in the NPC−/−/LDLR+/+ mice that were significantly different from those in the control NPC+/+/LDLR+/+ animals.
Rates of LDL Clearance in the NPC Mouse.
To determine whether the uptake and trapping of LDL-C could account for most of this cholesterol accumulation, LDL clearance rates were measured in the NPC+/+/LDLR+/+ and NPC−/−/LDLR+/+ animals. In addition, to quantitate the magnitude of the LDLR-independent component of this LDL clearance process, similar measurements were carried out in NPC+/+/LDLR−/− and NPC−/−/LDLR−/− mice. As shown in Table 2, LDL clearance was nearly identical in the NPC+/+/LDLR+/+ (555 ml/day per kg) and NPC−/−/LDLR+/+ (523 ml/day per kg) animals and, in both cases, ≈80% of this clearance took place in the liver. In contrast, in the two groups of animals lacking LDLR activity, LDL clearance was markedly reduced (59–63 ml/day per kg), and the liver accounted for only ≈40% of this uptake. Thus, the rate of LDL clearance, and the tissue distribution of this transport activity, was determined entirely by the presence or absence of LDLR function and was not influenced by whether the animal was NPC+/+ or NPC−/−.
Table 2.
Whole animal LDL clearance rates in control mice and animals lacking functional NPC1 and/or LDLR activity
| LDL transport parameter | NPC+/+/LDLR+/+ | NPC−/−/LDLR+/+ | NPC+/+/LDLR−/− | NPC−/−/LDLR−/− |
|---|---|---|---|---|
| Clearance in whole animal, ml/day per kg | 555 ± 34 | 523 ± 59 | 63 ± 12* | 59 ± 11* |
| Clearance in liver, ml/day per kg | 452 ± 32 | 418 ± 46 | 25 ± 4* | 22 ± 8* |
| Clearance in extrahepatic tissues, ml/day per kg | 103 ± 7 | 105 ± 8 | 38 ± 7* | 37 ± 9* |
Mice of four different genotypes were used in these studies, and all were 7 weeks of age. There were 6–11 mice in each group. An asterisk identifies those values that were significantly different from the corresponding values in the control NPC+/+/LDLR+/+ animals. There were no significant differences between values in the NPC+/+/LDLR+/+ and NPC−/−/LDLR+/+ animals or between the NPC+/+/LDLR−/− and NPC−/−/LDLR−/− groups.
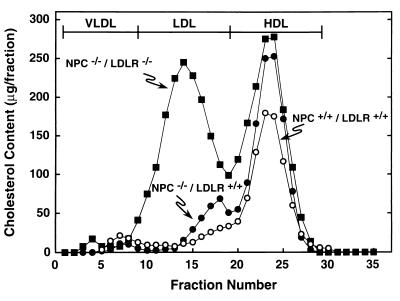
The distribution of plasma cholesterol in the NPC+/+/LDLR+/+, NPC−/−/LDLR+/+, and NPC−/−/LDLR−/− mice is shown in Fig. 1. Because LDL clearance in the NPC+/+/LDLR−/− mice was indistinguishable from that in the NPC−/−/LDLR−/− group, this genotype is not shown. The plasma cholesterol concentration was slightly higher in the NPC−/−/LDLR+/+ mice (146 ± 4 mg/dl) than in the control animals (107 ± 3 mg/dl), and this was attributable to small elevations of both LDL-C and HDL-C. With deletion of LDLR activity, however, the total plasma cholesterol concentration rose to 267 ± 7 mg/dl, and this was attributable largely to a 14-fold increase in the concentration of LDL-C. When LDL from the critical NPC−/−/LDLR+/+ group was isolated and purified to remove small amounts of apoE-containing HDL-C, the concentration of LDL-C equaled 15 ± 2 mg/dl. By using this value and the rate of LDL clearance in these mice (Table 2), the amount of LDL-C taken up into the tissues of the NPC mouse was calculated to equal 78 mg/day per kg. Thus, the amount of cholesterol cleared into the tissues through the coated-pit pathway as LDL-C (78 mg/day per kg) and as dietary cholesterol (8 mg/day per kg) totaled ≈86 mg/day per kg and could fully account for the daily sequestration of the 67 mg/day per kg seen in the NPC mouse (Table 1).
Figure 1.
Distribution of the plasma cholesterol in the major lipoprotein fractions of the NPC+/+/LDLR+/+, NPC−/−/LDLR+/+, and NPC−/−/LDLR−/− mice. The animals used in this experiment were 7 weeks of age. Plasma was pooled from 8–10 animals in each group, and the lipoproteins were separated by FPLC.
Effects of Eliminating LDLR Activity on Tissue Cholesterol Concentrations.
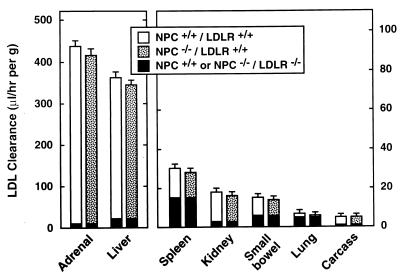
To further support this conclusion, the next experiment explored the effect of eliminating LDLR activity on the rates of LDL-C uptake and levels of unesterified cholesterol in the various organs of the NPC mouse. As shown in Fig. 2, the rates of total LDL clearance by individual organs varied 80-fold, from ≈410 μl/hr per g in the adrenal to only ≈5 μl/hr per g in tissues of the residual carcass, in the NPC+/+/LDLR+/+ and NPC−/−/ LDLR+/+ groups. Furthermore, there were no significant differences in the rates of clearance in any organ of these two genotypes. LDL clearance in each tissue was markedly reduced in the NPC+/+/LDLR−/− and NPC−/−/LDLR−/− mice (the black portion of each column in Fig. 2), and, again, there were no significant differences in these rates in any organ in these two genotypes. However, the relative importance of this LDLR-independent component of LDL clearance varied markedly among the different organs. In the adrenal, for example, it accounted for only ≈2% of total clearance, but in the spleen equaled ≈50% of LDL-C uptake. From these clearance values and the concentrations of LDL-C in each experimental group, the changes that occurred in tissue LDL-C uptake could be calculated when LDLR activity was abrogated in the NPC−/− mice. Thus, in the adrenal LDL-C, uptake equaled 61 μg/hr per g in the NPC−/−/LDLR+/+ mice and declined to 16 μg/hr per g in the NPC−/−/LDLR−/− group. In the liver, however, nearly equal amounts of LDL-C were taken up in the NPC−/− animals when LDLR activity was present (48 μg/hr per g) or absent (42 μg/hr per g). Because the LDLR-independent component represented a significantly higher percentage of total LDL clearance in the remaining extrahepatic organs, the calculated rate of LDL-C uptake in these tissues was always greater in the NPC−/−/LDLR−/− mice than in the NPC−/−/LDLR+/+ animals.
Figure 2.
Total and LDLR-independent LDL clearance in all organs of control mice and animals lacking functional NPC1 and/or LDLR activity. Mice of the four different genotypes (Table 2) were used in these studies and were 7 weeks of age. The height of each bar represents the rate of total LDL clearance per gram of tissue while the lower, black part of each column represents the LDLR-independent component of this clearance that was measured in the NPC+/+/LDLR−/− and NPC−/−/LDLR−/− animals. The “carcass” represents all residual tissues in the animal after removal of the other, identified organs. There were 6–11 mice in each group. There were no significant differences among the values in any organ.
Fig. 3 shows the concentration of unesterified and esterified cholesterol in the organs of these same three genotypes. As previously described (9), deletion of NPC1 activity raised the concentration of unesterified cholesterol nearly 10-fold in the liver and 2- to 5-fold in the other organs. However, when LDLR activity then was abrogated, as in the NPC−/−/LDLR−/− mice, these levels of unesterified cholesterol were markedly reduced in the adrenal, were virtually unchanged in the liver, and were increased in the spleen, lung, and other (not shown in Fig. 3) extrahepatic organs. Thus, deletion of LDLR activity in the homozygous NPC mice changed the levels of sequestered unesterified cholesterol in a manner that was consistent with the observed changes in LDL-C uptake in those same organs. Fig. 3 also illustrates that the pool of esterified cholesterol in organs like the liver, spleen, and lung was very low and unaffected by the genotype of the animals. It is noteworthy, however, that the large pool of cholesteryl esters typically found in the adrenal was maintained and this level was unaffected by deletion of either NPC1 or LDLR activity.
Effect of Increasing Dietary Sterol Input on Tissue Cholesterol Concentrations.
In another experiment, the amount of cholesterol added to the diet was increased to 0.4% in animals 6 weeks of age. One week later, the concentration of unesterified and esterified cholesterol was measured in these same organs, as shown in Fig. 4. As is apparent in the NPC+/+/LDLR+/+ mice, the increased dietary sterol load significantly raised the concentration of esterified, but not unesterified, cholesterol in the liver. In contrast, in the NPC−/−/LDLR+/+ animals, there was a significant increase in the concentration of unesterified, but not esterified, cholesterol in this organ. There were no changes in the concentration of cholesterol in any other organ in either genotype. This finding again was consistent with the thesis that changing the rate of delivery of cholesterol, in this case CM-C, through the coated-pit pathway altered the level of unesterified cholesterol in the appropriate tissue of the NPC mice.
Rates of Fecal Sterol Excretion.
Finally, measurements of sterol balance were carried out to determine whether the flow of cholesterol from the sites of synthesis through the HDL pathway to the liver for excretion as fecal neutral and acidic sterols was intact in the NPC mice. The rates of cholesterol synthesis in the NPC−/−/LDLR+/+ mice (169 ± 10 mg/day per kg) was significantly higher than in the control animals (117 ± 6 mg/day per kg). Importantly, this difference in sterol synthesis, the majority of which occurred in the extrahepatic compartment, closely reflected the rates of fecal sterol excretion in the NPC+/+/LDLR+/+ (93 ± 5 mg/day per kg) and NPC−/−/LDLR+/+ (150 ± 7 mg/day per kg) animals. Thus, HDL-C apparently was not only delivered normally to the adrenal (Fig. 3) but also was cleared normally by the liver and excreted through the neutral and acidic pathways into the feces.
Discussion
Although regulation of cholesterol homeostasis is more complex in the whole animal than in the isolated cell, these studies provide strong support for the concept that the unesterified sterol that accumulates in the tissues of the NPC animal, as in the NPC fibroblast, is derived from lipoprotein-cholesterol taken up through the coated-pit pathway and sequestered in the lysosomal compartment (2). In vivo, LDL-C and CM-C, but not HDL-C, are primarily cleared from the plasma through this pathway so that the concentration of cholesterol in the various organs should reflect the rates of uptake of these two particles. Four lines of evidence derived from these studies support this conclusion. First, in NPC−/−/LDLR+/+ animals 7 weeks of age, the concentration of sterol is elevated 10-fold in the liver, lesser amounts in organs like lung, spleen, and kidney, and barely at all in muscle (Fig. 3 and ref. 9). This profile of cholesterol accumulation closely reflects the relative rates at which sterol carried in LDL and CMs is taken up by these same tissues in the mouse (Fig. 2), as well as in the rat, hamster, rabbit, and primate (24, 27, 28, 30, 31). Second, when the receptor-dependent component of LDL transport is abrogated, the amount of LDL-C cleared from the plasma in mice nearly doubles, and the extrahepatic tissues become relatively more important in this clearance process (Table 2 and ref. 24). Similar changes in LDL-C transport occur in rabbits and humans lacking LDLR activity (31, 32). These same alterations in LDL-C uptake also were found in the NPC−/−/LDLR−/− mice (Table 2 and Fig. 2) and were closely correlated with changes in the level of sequestered unesterified cholesterol in the different organs (Fig. 3). Third, clearance of CM-C takes place nearly entirely in the liver. Increasing the amount of cholesterol passing through this pathway elevated the amount of unesterified cholesterol trapped in the liver whereas the concentrations in the remaining organs of the NPC mice were unchanged (Fig. 4). Finally, the amount of cholesterol carried in LDL and CMs, and cleared from the plasma each day, was quantified in these studies to equal 86 mg per kg. The uptake of this amount of sterol, therefore, could fully account for the daily accumulation of the 67 mg per kg observed in the NPC animals (Table 1).
If, as these experiments indicate, nearly all of the cholesterol entering the coated-pit pathway is sequestered within cells in a pool that is metabolically inaccessible, an important question can be raised as to how fetal and neonatal development proceeds essentially normally in these mutant mice. In utero, nearly all of the cholesterol that is required for cell division and tissue growth, including in the central nervous system, comes from de novo synthesis (11–13). As the concentration of LDL-C is very low and as no dietary cholesterol absorption is taking place, there is relatively little sterol passing through the coated-pit pathway in the fetus (33). Thus, the cholesterol pool in the 1-day-old NPC mice is only modestly elevated (Table 1). With increased intake of dietary cholesterol and triacylglycerol in the neonate, however, more sterol passes through the CM and LDL pathways, and the rate of unesterified cholesterol accumulation accelerates. Importantly, the increase in the rate of cholesterol synthesis observed in the mutant mice essentially equals the rate at which these animals sequester sterol (9). Presumably, the cells of each organ simply replace the sterol usually acquired through the coated-pit pathway with newly synthesized cholesterol. As such newly synthesized sterol normally is continuously transported from the peripheral organs to the adrenal and liver, this finding further implies that the HDL/class B, type 1 scavenger receptor pathway is intact in the NPC mice. This possibility is supported by two observations. First, the pool of cholesteryl esters uniquely is maintained in the adrenal and is unaffected by deletion of either NPC1 or LDLR activity (Fig. 3). In the rodent in vivo, this gland expresses the class B, type 1 scavenger receptor at high levels and uses predominately HDL-C to maintain its ester pools (18, 34, 35). Second, the excretion of cholesterol from the NPC mice as fecal neutral and acidic sterols closely reflects the increased rate of synthesis seen in these same animals. Together, these data imply that the mechanisms for the centripetal movement of cholesterol from the sites of synthesis in the extrahepatic organs to the sites of metabolism and excretion by the endocrine glands and liver are intact in the NPC mice. A similar conclusion has been reached in humans with NPC disease (36).
This model suggests that, in NPC disease, cholesterol homeostasis in cells is maintained by increasing the rate of local synthesis whereas cell dysfunction and death ultimately may be related to the accumulation of cholesterol and other lipids within the lysosomal compartment. Such a paradigm also could explain the changes typically found in the central nervous system of the NPC animal. Although lipoproteins such as LDL and CM do not reach the central nervous system, a source for cholesterol and apolipoprotein E have been shown to be required for nerve growth (37). It has been postulated, therefore, that during constant remodeling of nerve cells there may be recycling of cholesterol within the brain that involves one or more lipoprotein receptors and apolipoprotein E (38, 39). If this recycling process also involves the coated-pit pathway, then it could account for the time-dependent appearance of polymorphous cytoplasmic bodies in nerve cells and the neurodegeneration typical of NPC disease. Whether this formulation proves to be correct requires more detailed studies of cholesterol metabolism in the cells of the brain and spinal cord. Nevertheless, the observation that brain development and myelination depend entirely on de novo synthesis while this putative accumulation of unesterified cholesterol and other lipids occurs later during remodeling of the brain fits well with the natural history of NPC disease.
Acknowledgments
The authors thank Brian Jefferson, Jeffrey Graven, and Elizabeth Moore for their excellent technical assistance and Merikay Presley for preparation of the manuscript. This work was supported by U.S. Public Health Service Research Grant R37 HL 09610 and Training Grant T32 DK 07745 and by a grant from the Moss Heart Fund.
Abbreviations
- NPC
Niemann-Pick type C
- LDL-C
cholesterol in low density lipoprotein
- HDL-C
cholesterol in high density lipoprotein
- CM-C
cholesterol in chylomicrons
- LDLR
LDL receptors
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pentchev P G, Vanier M T, Suzuki K, Patterson M C. In: The Metabolic and Molecular Bases of Inherited Diseases. Scriver C R, Beaudet A L, Sly W S, Valle D, Stanbury J B, Wyngaarden J B, Fredrickson D S, editors. Vol. 2. New York: McGraw–Hill; 1995. pp. 2625–2639. [Google Scholar]
- 2.Pentchev P G, Comly M E, Kruth H S, Vanier M T, Wenger D A, Patel S, Brady R O. Proc Natl Acad Sci USA. 1985;82:8247–8251. doi: 10.1073/pnas.82.23.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pentchev P G, Boothe A D, Kruth H S, Weintroub H, Stivers J, Brady R O. J Biol Chem. 1984;259:5784–5791. [PubMed] [Google Scholar]
- 4.Liscum L, Faust J R. J Biol Chem. 1987;262:17002–17008. [PubMed] [Google Scholar]
- 5.Sokol J, Blanchette-Mackie E J, Kruth H S, Dwyer N K, Amende L M, Butler J D, Robinson E, Patel S, Brady R O, Comly M E, et al. J Biol Chem. 1988;263:3411–3417. [PubMed] [Google Scholar]
- 6.Carstea E D, Morris J A, Coleman K G, Loftus S K, Zhang D, Cummings C, Gu J, Rosenfeld M A, Pavan W J, Krizman D B, et al. Science. 1997;277:228–231. [Google Scholar]
- 7.Neufeld E B, Wastney M, Patel S, Suresh S, Cooney A M, Dwyer N K, Roff C F, Ohno K, Morris J A, Carstea E D, et al. J Biol Chem. 1999;274:9627–9635. doi: 10.1074/jbc.274.14.9627. [DOI] [PubMed] [Google Scholar]
- 8.Loftus S K, Morris J A, Carstea E D, Gu J Z, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld M A, Tagle D A, et al. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 9.Xie C, Turley S D, Pentchev P G, Dietschy J M. Am J Physiol. 1999;276:E336–E344. doi: 10.1152/ajpendo.1999.276.2.E336. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka J, Nakumura H, Miyawaki S. J Neuropathol Exp Neurol. 1988;47:291–300. doi: 10.1097/00005072-198805000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Turley S D, Burns D K, Rosenfeld C R, Dietschy J M. J Lipid Res. 1996;37:1953–1961. [PubMed] [Google Scholar]
- 12.Jurevics H A, Kidwai F Z, Morell P. J Lipid Res. 1997;38:723–733. [PubMed] [Google Scholar]
- 13.Turley S D, Dietschy J M. Nutri Metab Cardiovasc Dis. 1997;7:195–201. [Google Scholar]
- 14.Osono Y, Woollett L A, Marotti K R, Melchior G W, Dietschy J M. Proc Natl Acad Sci USA. 1996;93:4114–4119. doi: 10.1073/pnas.93.9.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolley C D, Woollett L A, Turley S D, Dietschy J M. J Lipid Res. 1998;39:2143–2149. [PubMed] [Google Scholar]
- 16.Acton S, Rigotti A, Landschulz K T, Xu S, Hobbs H H, Krieger M. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 17.Landschulz K T, Pathak R K, Rigotti A, Krieger M, Hobbs H H. J Clin Invest. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stangl H, Cao G, Wyne K L, Hobbs H H. J Biol Chem. 1998;273:31002–31008. doi: 10.1074/jbc.273.47.31002. [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi S, Herz J, Maeda N, Goldstein J L, Brown M S. Proc Natl Acad Sci USA. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Véniant M M, Zlot C H, Walzem R L, Pierotti V, Driscoll R, Dichek D, Herz J, Young S G. J Clin Invest. 1998;102:1559–1568. doi: 10.1172/JCI4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein J L, Dana S E, Faust J R, Beaudet A L, Brown M S. J Biol Chem. 1975;250:8487–8495. [PubMed] [Google Scholar]
- 22.Brown M S, Goldstein J L. Proc Natl Acad Sci USA. 1979;76:3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibashi S, Brown M S, Goldstein J L, Gerard R D, Hammer R E, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osono Y, Woollett L A, Herz J, Dietschy J M. J Clin Invest. 1995;95:1124–1132. doi: 10.1172/JCI117760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turley S D, Daggy B P, Dietschy J M. Gastroenterology. 1994;107:444–452. doi: 10.1016/0016-5085(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 26.Woollett L A, Spady D K. J Clin Invest. 1997;99:1704–1713. doi: 10.1172/JCI119334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spady D K, Bilheimer D W, Dietschy J M. Proc Natl Acad Sci USA. 1983;80:3499–3503. doi: 10.1073/pnas.80.11.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turley S D, Spady D K, Dietschy J M. J Lipid Res. 1995;36:67–79. [PubMed] [Google Scholar]
- 29.Weisgraber K H, Mahley R W. J Lipid Res. 1980;21:316–325. [PubMed] [Google Scholar]
- 30.Spady D K, Turley S D, Dietschy J M. J Clin Invest. 1985;76:1113–1122. doi: 10.1172/JCI112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spady D K, Huettinger M, Bilheimer D W, Dietschy J M. J Lipid Res. 1987;28:32–41. [PubMed] [Google Scholar]
- 32.Dietschy J M, Turley S D, Spady D K. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 33.Cavender C P, Turley S D, Dietschy J M. Am J Physiol. 1995;269:E331–E340. doi: 10.1152/ajpendo.1995.269.2.E331. [DOI] [PubMed] [Google Scholar]
- 34.Andersen J M, Dietschy J M. J Biol Chem. 1978;253:9024–9032. [PubMed] [Google Scholar]
- 35.Spady D K, Woollett L A, Meidell R S, Hobbs H H. J Lipid Res. 1998;39:1483–1492. [PubMed] [Google Scholar]
- 36.Shamburek R D, Pentchev P G, Sech L A, Blanchette-Mackie J, Carstea E D, VandenBroek J M, Cooper P S, Neufeld E B, Phair R D, Brewer H B, Jr, et al. J Lipid Res. 1997;38:2422–2435. [PubMed] [Google Scholar]
- 37.Handelmann G E, Boyles J K, Weisgraber K H, Mahley R W, Pitas R E. J Lipid Res. 1992;33:1677–1688. [PubMed] [Google Scholar]
- 38.Pitas R E, Boyles J K, Lee S H, Foss D, Mahley R W. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 39.Weisgraber K H, Roses A D, Strittmatter W J. Curr Opin Lipidol. 1994;5:110–116. doi: 10.1097/00041433-199404000-00007. [DOI] [PubMed] [Google Scholar]