Abstract
Chiropractic care is a common treatment sought by patients with headaches. As some patients may not benefit from this care, chiropractors must be aware of alternative management options. Botox has more recently become a common treatment for headaches. A case of a 45-year-old female with chronic headaches and neck pain is presented. After lengthy trials of chiropractic manipulation, trigger point therapy, and acupuncture, the patient was treated with Botox-A. She experienced pain relief following the initial treatment that lasted up to 3–4 months and has since undergone subsequent trials of Botox with the same results. No side effects were experienced. As more health care practitioners are recommending Botox, the need for a better understanding of the evidence and criteria for referral for Botox treatment is required. As such, chiropractors should consider this alternative approach to managing headaches when chiropractic management is unsuccessful.
Keywords: botox, chiropractic, manipulation, headache
Abstract
Les soins chiropratiques constituent un traitement fréquemment recherché par les patients souffrant de maux de tête. Puisque certains patients n’éprouvent aucun soulagement à la suite de ces soins, les chiropraticiens doivent connaître d’autres options de gestion. Le Botox est devenu récemment un traitement de plus en plus utilisé pour les maux de tête. On présente le cas d’une femme de 45 ans souffrant de migraines chroniques et de douleurs au cou. Après de longs traitements de manipulations chiropratiques, la méthode du point déclic et l’acupuncture, la patiente a été traitée au Botox-A. Elle a été soulagée de la douleur après le traitement initial, ce qui a duré pendant trois à quatre mois. Depuis, elle a reçu d’autres injections de Botox, avec des résultats à l’avenant. Elle n’a pas connu d’effets secondaires. Alors que de plus en plus de professionnels de la santé recommandent le Botox, il nous faut mieux comprendre le mécanisme et les critères nécessaires pour référer un patient pour un traitement au Botox, le cas échéant. À ce titre, les chiropraticiens devraient envisager cet autre traitement de gestion des maux de tête quand ils réalisent que leurs traitements ne donnent pas de résultats.
Introduction
Botulinum toxin is a neurotoxin that is made of a complex mixture of proteins containing botulinum neurotoxin and various non-toxic proteins. The neurotoxin Clostridium Botulinum is a spore-forming gram positive anaerobic bacterium that is made up of a light chain and a heavy chain linked by a single disulphide bond.1 The non-toxic proteins include a haemagglutinin complex and non-haemagglutinating proteins. The most serious symptom of botulism is paralysis, and it is this symptom that initially led scientists to evaluate its beneficial use in the treatment of many disorders of the human body.
The mechanism of Botox is complex. When botulinum neurotoxin is injected into a target tissue, a series of events occur, blocking the release of acetylcholine which result in flaccid paralysis.2,3
Botulinum toxin has been found to have no direct effects on the central nervous system (CNS), as it can not penetrate the blood-brain barrier, and although it can reach the CNS by retrograde axonal transport, this movement was found to be so slow that the botulinum toxin was likely to be inactivated before it reached the CNS.2 Some indirect effects on the CNS have, however, been identified. On the spinal level, botulinum toxin is capable of producing reflex inhibition of the muscle spindle organ. On the supraspinal level, studies have shown that botulinum toxin can normalize altered intracortical inhibition and altered somatosensory evoked potentials, however it fails to improve impaired activation of primary motor cortex.2
Botulinum neurotoxin exists in 7 different serotypes (A–G).2 Out of these serotypes, type A has been the most widely studied for therapeutic purposes and has also been found to be the most stable and potent serotype.3
Historically, botulinum neurotoxin-A (Botox-A) was utilized to treat strabismus, by inactivating specific ocular muscles. It became popular over the more recent years for its cosmetic use whereby injections of very low doses can effectively soften hyperactive facial lines.4 The toxin paralyzes the injected muscle such as the glabella or frontalis, which decreases the patient’s ability to frown or squint, and prevents the progressive hyperactivity of these lines over time.4
Botox has been utilized to treat a variety of other conditions such as low back pain, benign prostatic hypertrophy, bladder dysfunction and spasticity involved in Parkinson’s disease. It is considered to be the treatment of choice for cervical dystonia, which is characterized by abnormal involuntary contraction of the cervical and shoulder musculature resulting in abnormal postures of the head and significant muscle pain.5
The results of Botox treatment for painful conditions such as those mentioned above led researchers to evaluate its use for other painful disorders, such as headaches. Chronic daily headaches pertain to a group of headache disorders that are defined by the presence of headaches greater than 15 days per month for more than three months.6 These can include both tension-type headaches, cervicogenic headaches and migraine headaches, all of which can have a significant impact on physical, social and occupational functioning. Such headaches can be a challenge for health care practitioners as they are often perpetuated by the overuse of medications, a condition that is referred to as medication overuse headaches.7,8
Botox-A was utilized for the treatment of headaches in a case involving a 45 year old female with chronic headaches and neck pain.
Case Report
A 45-year-old female presented to a chiropractic office with ongoing neck pain and headaches. She reported that she had been experiencing ongoing neck pain since 1985 and had been receiving maintenance chiropractic treatment – on a monthly basis. She reported that the chiropractic treatment provided her with short term pain relief. Recently there was an exacerbation of the neck pain with increased headaches and general pain throughout the entire spinal region and the patient again, presented to a chiropractor for re-evaluation.
Her headaches at the time of presentation to the chiropractic office were on a daily basis and varied in intensity from 6–9/10. Aggravating factors included changes in weather and activity. She associated the headaches with neck stiffness, numbness and tingling into her hands. She did experience chronic neck stiffness. Factors at the time included taking tylenol #2, NSAIDs, and omega 3. She reported no nausea or vomiting. She did not report any photophobia. She did not report any food allergies. She was not performing any exercises, however, was very active. As her condition worsened, there was a decrease in sleep, she reported waking up with headaches and neck pain and having difficulty with short-term memory.
The assessment by the chiropractor resulted in a diagnosis of cervicogenic headaches and a myofascial pain syndrome of the cervical spine. She began receiving a course of chiropractic adjustments using both manual treatment and activator technique. She was also receiving some trigger-point therapy. She reported that the intensity of headaches would be relieved immediately after the chiropractic adjustment, however, within 2–3 days, the neck pain and shoulder pain returned. She then had an adverse reaction with neck adjustments which resulted in slight dizziness and a feeling of discomfort. After discussions with a chiropractor, the neck adjustments were discontinued. She continued to receive trigger-point therapy and manual treatment to the thoracic and lumbar spine for an additional 6–8 weeks. At this stage, her condition was worsening and she started feeling a significant amount of fatigue.
She went back to her family physician and was put on a series of medications. These included a calcium channel blocker to help the headaches and some anti-inflammatory medication. The patient also had a pre-existing thyroid condition and was taking Synthroid. She was then referred to an endocrinologist because of her ongoing symptoms. She was examined and diagnosed with a possibility of chronic fatigue syndrome or fibromyalgia.
Two weeks later, the patient was re-assessed by a chiropractor who recommended that she have a trial of acupuncture. Acupuncture was recommended as an alternative treatment option to manage her headaches. She began a course of acupuncture treatment which included needle acupuncture to her upper cervical spine as well as distal points for pain management. She reported that she had temporary benefit of up to a few hours. After the acupuncture for headaches, however, she was not getting any long-term benefits.
The patient was then referred to a neurologist who sent her for an MRI of her neck which was apparently normal. There was a query of possible multiple sclerosis which was further evaluated, however, it was noted that at this stage, she did not have multiple sclerosis.
In the year 2000, as the patient continued to regress and was now having extreme levels of pain and anxiety, her family physician sent her to a psychiatrist. She was assessed by the psychiatrist who prescribed antidepressant medication as well as a low dosage of amitriptyline to manage her headaches. This assisted her with her sleep patterns, however, did not significantly improve her headaches. She found temporary relief from the medications for the next 5 years. She attended the chiropractor occasionally over the five years, when the headache intensity and frequency increased.
In March 2005, on recommendation from her family physician and after reading information on the internet, the patient attended a pain clinic and was assessed by a medical physician. She was diagnosed with chronic post-traumatic headaches and chronic myofascial pain of the cervical spine. Discussions occurred regarding the possibility of Botox injections and after discussing the risks and benefits of Botox, the patient began a course of injections to her frontal and temporal regions as well as her suboccipital, neck and shoulder regions. She began having immediate relief and after a third dosage, the neck pain resolved completely.
Approximately 10 weeks post-injection, she started having some increased pain, and subsequently attended the pain clinic again. Approximately 4 months following her first series of injections, she received a second series of injections. At the beginning of the Botox injections, the patient began a headache diary. In reviewing the results of this diary, the patient was initially experiencing headaches daily which would last anywhere from 4–6 hours during the day. Pain was initially at a pain level of 8/10. After the second course of Botox the frequency decreased to 1 to 2 times per month, with the pain reduced to a mild stiffness rated at a 2–3/10. Based on the results of the Botox injections, she has been experiencing anywhere between 3–4 months of significant pain relief and by month 4, there has been a slow increase in symptoms.
According to her headache diary she has not experienced any side effects. She has also indicated that since receiving the Botox-A injections, she has had more energy for the first 2 months after the injection which lasts till about 4 months when she also begins to notice deterioration.
Discussion
Numerous studies have looked at the efficacy of Botox-A as a prophylactic treatment for headaches. In 2001, Schmitt et al published a double-blind, placebo-controlled study that showed only a slight benefit to patients suffering from chronic tension-type headaches following the first month of treatment with Botox-A injections.9 Studies produced since then, however, have indicated that benefits appear following 180 days, which equates to at least 3 treatments with Botox-A.10,11
It should be noted that in Schmitt’s study, only 20 units of Botox-A was injected per treatment and none of the injection sites included muscles in the neck.9 Other studies that revealed benefits with Botox-A, include Blumenfeld’s 2003 review study, whereby it was concluded that positive results were found when the mean Botox-A dose was 63.2 units, for a mean total treatment time of 8.6 months, during which patients received an average of 3.4 treatments.12
In 2005, the Cochrane Collaboration released a review on the effects of Botox-A injections on mechanical neck disorders. It examined studies that were published up to 2002 and concluded that there was moderate evidence indicating no benefit for Botox-A compared to saline intramuscular injections. The five studies examined in this review, however, looked only at the results of one treatment and the pain outcomes over four to eight weeks post treatment13. Most recently in 2005, the two largest randomly double-blind, placebo-controlled trials were published by Mathew et al and Silberstein et al. These studies used 702 and 571 patients respectively and conducted their studies over a period of 11 months. Both studies indicated a statistically significant decrease in the number of headache days per 30 day period when compared to a placebo group.10,11 Silberstein et al. looked at various dosages and concluded that the most benefits compared to placebo groups were found when the patient received 150 Units of Botox-A per treatment.10 This is significantly more than the 20 Units used in Schmitt’s 2001 study. Also of note is the fact that both of these studies utilized multiple injection sites in multiple muscles, including muscles of both the head and neck.10,11 All studies reported adverse effects to be transient and mild to moderate in severity.9,10,11,12,14 Overall the current research suggests that Botox-A treatment may be an effective, well tolerated alternative treatment for the management of chronic headache disorders.
Central sensitization of central trigeminovascular neurons has been determined to be an integral factor in the development, progression and maintenance of migraine-type headaches. Botox has been shown to inhibit this.5 Although the patient in this case report obtained relief through conservative management of her headaches, it was only up to two days. Alternatively, when she received the Botox-A treatments the relief lasted up to 4 months.
As indicated by its various uses, and as illustrated in the case report, the most therapeutic use of Botox-A is to decrease pain. This is accomplished through various methods. One method is by blocking the neuromuscular transmission. Blocking the release of acetylcholine stops the contraction of the muscle spindle, which in turn stops the pain-spasm-pain cycle and gives the patient relief from a painful posture.15
Another method is based on the evidence that Botox-A’s effect on the SNARE proteins also decreases the release of pain mediators including substance P, calcium gene-related peptide (CGRP), and glutamate.16 This may be accomplished directly by blocking both substance P from the trigeminal sensory afferent terminals, and the release of CGRP from autonomic vascular nerve terminals. It may also do this indirectly by inhibiting the release of glutamate, which stimulates the release of substance P and CGRP.17,18 These pain mediators are released from primary sensory afferent terminals in the injured area. They produce neurogenic inflammation and sensitized pain receptors, providing a feedback circuit for continuing inflammation and muscle pain, hyperalgesia and allodynia.17 By blocking their release peripherally an analgesic effect is created, however glutamate may also be blocked centrally.
A third method relates to a recent study which found that Botox-A may cause an analgesic effect without paralysis when it is conjugated with lectin and applied to dorsal root ganglion cells. Here it selectively affects the nociceptive sensory afferents, C fibers, and can attenuate nociceptive transmission in vitro and in vivo for at least 24 days.17 Botox-A can thus prevent pain facilitation by alleviating the painful muscle contraction, blocking the pain neurotransmitters and interrupting the nociceptive sensory afferents.
When administered for the treatment of headaches, Botox is released intramuscularly into various sites in the head and neck. The dosage is dependant on the size of muscle. Studies have shown effectiveness with as little as 25 units.19 According to one study, the clinical dose for migraine headaches is 25 to 100 units.6 As there is not yet a standardized methodology for the administration of Botox-A, the total dose administered should be individual, considering various factors such as the type of headache, severity of symptoms and body size.12 The patient in the above case report experienced relief with doses of 120 units.
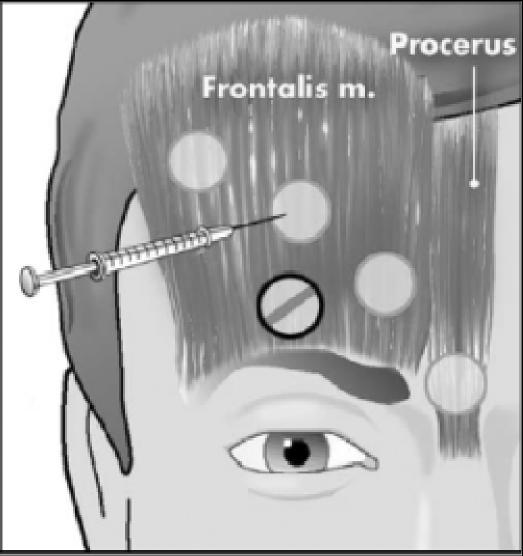
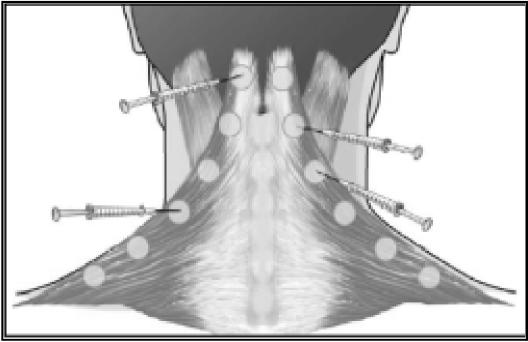
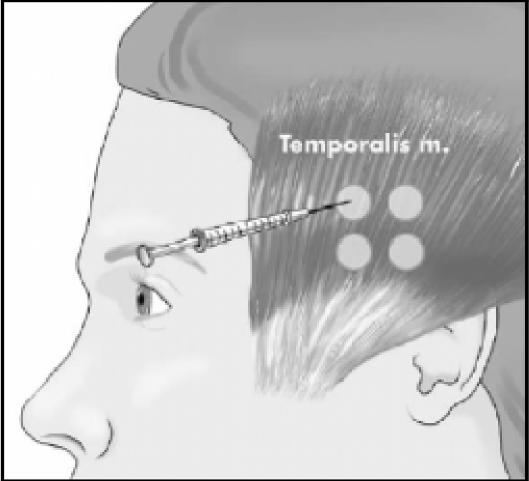
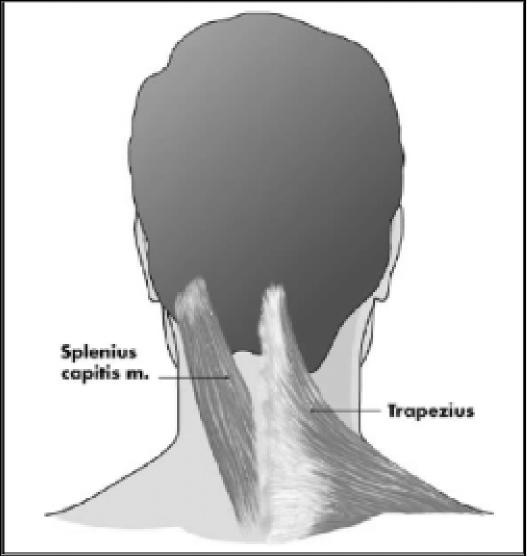
In disorders such as tension-type headaches, cervicogenic headaches and migraines it is difficult to determine specifically the involvement of hyperactive muscles.6 With respect to the occipital and cervical paraspinal regions (trapezius, splenius capitis, seminspinalis capitis), injection sites are more specific to subjective reports of pain, rather than the specific muscle. One to two injections per side are made, at a dose of 5 to 15 units per side. An additional 5 to 15 units should be injected into the trapezius muscle should it be involved.20 The frontalis muscle allows for greater dispersion of the toxin, therefore dosages range from 20 to 30 units. The temporalis muscle is commonly injected with 5 units in four sites for a total of 20 units.20 Figures 1 through 4 illustrate some common injection sites.14
Figure 1.
Anterior injection sites in procerus and frontalis muscles.
Figure 4.
Injection sites in trapezius and splenius capitis muscles. The number of injection sites and doses vary from patient to patient depending upon size, pain location, and palpable muscle spasm.
One study hypothesized that Botox-A injected into the head and neck musculature would prevent migraine and tension-type headaches from occurring. Out of 271 patients, 85.6% reported improvement in their headache symptoms following a series of Botox-A treatment.20 The patient in this case report received injections to her neck, shoulder, temporal, frontalis and suboccipital areas, utilizing the “follow-the-pain” approach.
There are three different types of injection protocols: the “fixed site” approach, the “follow-the-pain” approach and a combination of both. The fixed site approach is commonly utilized for migraine-type headaches, and involves fixed symmetrical injection sites with predetermined dosages. This is based on previous studies which show that patients injected unilaterally for a unilateral headache often develop headaches on the contralateral side.20 The follow-the-pain approach is more often utilized for the treatment of tension-type headaches, and involves using adjustable doses and sites, depending on the patient’s symptoms and location of pain.20 The combination of the fixed-site and follow-the-pain technique is utilized for patients who experience both migraines and tension-type headaches.
When botulinum toxin is injected into a striate muscle, paresis occurs after 2–5 days and lasts for 2–3 months before it gradually starts to wear off.2 The paresis produces a reduction in the diameter of the targeted hyperactive muscle, or it will normalize the diameter of a hypertrophic muscle. If botulinum toxin is administered over a prolonged period of time, however, true muscle atrophy can occur, both in the extrafusal and intrafusal muscle fibers within the muscle spindle organ.2 This is due to the presence of cholinergic neuromuscular junctions between the γ-motor neurons and the extrafusal fibers, and the α-motor neurons and the intrafusal fibers which form the muscle spindle organ.2 The botulinum toxin injection blocks the extrafusal and intrafusal release of acetylcholine, and effectively reduces the Ia and II afferent signal from the muscle spindle organs and the muscle tone by reflex inhibition without affecting muscle strength. This anti-dystonic effect, therefore, is caused not only by target muscle paresis, but also by spinal reflex inhibition.2
After one year of receiving injections every four months, the patient in this case report had not experienced any of these signs of atrophy.
The muscle being injected should be carefully chosen, however, as the injection of one muscle, for example the SCM which is an anterior neck flexor, not only causes paralysis of that muscle, but also causes temporary weakness. This can then potentially result in compensatory recruitment from other muscle groups, leading to altered posture and subsequent pain.
Immediately following injection and for 2–3 hours afterwards, it is common for bruising and redness to occur, however, avoiding injection into superficial veins will minimize the amount of bruising.20 Headache relief may take from 2 weeks up to several weeks to occur, and the effects of the Botox-A may diminish within 2 to 4 months, or 6–12 months.21 Patients are evaluated at 4 to 6 weeks following the injection to evaluate the need for further treatments. According to a study by Blumenfeld, some patients report a greater therapeutic benefit with repeated injections, whereby others show a diminished response with each subsequent treatment.20 As indicated above, the patient in this case report underwent one year of receiving Botox-A approximately every four months, and according to her headache diary, symptoms consistently increased by the fourth month.
According to the patient in the case report, immediate relief occurred after the first injection, and after a third dosage, her neck pain resolved completely. Approximately 10 weeks post-injection, she began having some increased pain, and subsequently attended the pain clinic again. Approximately 4 months following her first series of injections, she received a second series of injections. After the second course of Botox injections, the headache frequency decreased to approximately 1–2 times per month. After her second level of Botox injections, at approximately 4 months later, the patient began experiencing recurrent pain and in December 2005 had another course of Botox injections.
Although the patient in this case report did not experience any side effects, they can occur. Adverse effects are localized to the area of injury and may be related to an excessive dose, or the inadvertent injection to adjacent areas. Adverse effects with respect to facial injections may include transient ptosis, dry eyes, and bruising. Adverse effects with respect to neck musculature may include dyspnea, xerostomia, nausea, fatigue, blurry vision and photophobia.21 Expected effects of Botox-A injections include local muscle weakness, flu-like symptoms, and rash.23 According to a systematic review on the safety of Botox-A, the overall rate of adverse events from treatment with Botox-A was 25%, of which there were no severe or systemic events.21 Long term studies according to the systematic review indicated that no severe effects occurred. Another randomized double blind study indicated that 7 out of 33 participants noted transient symptoms such as arm heaviness, and contralateral pain. One of these participants noted a shift of neck pain to the midline which was successfully managed with a chiropractic adjustment.22
According to an 11-month randomized double-blind placebo-controlled study, Botox was shown to be an effective therapy for these types of headaches.6 Specifically, in the prevention and treatment of headaches, Botox was shown to be most effective in patients with recurring migraines that interfere with their activities of normal life despite other treatments, in patients with frequent headaches, and with those who do not tolerate or comply poorly with acute treatment.20
In this case report, the patient had been experiencing chronic headaches and neck pain, and with increased time, began experiencing symptoms of anxiety, sleep disturbance and short-term memory loss, all of which interfered with her activities of normal life. After attempting to relieve these symptoms through conservative treatment, it was the Botox-A injections that finally allowed her to resume her daily activities in a pain-free manner.
Given these findings, it would be beneficial for chiropractors to learn more about Botox-A, specifically, when it should be offered as an alternative treatment. A proposal for the development of guidelines is suggested, such that chiropractors can in the future determine suitable candidates for Botox-A treatment.
Summary
Botox-A is the serotype of a neurotoxin which, when combined with non-toxic proteins can be utilized for therapeutic purposes. It is injected into a target tissue and flaccid paralysis occurs, which decreases muscle hyper-activity and ultimately decreases pain within a short time frame. At the present time, Botox injection for pain management remains somewhat controversial, and more RCT studies as well as studies of the long term outcomes and outcome measures are necessary.
A case of a 45-year-old woman who was treated with Botox-A for chronic neck pain and headaches is presented. She was initially treated with conservative care including manipulation, acupuncture and trigger point therapy, however, this only provided short-term relief. Following Botox-A treatment she experienced pain relief immediately which lasted up to 4 months. She has been receiving Botox-A injections approximately every 4 months for one year and has noticed an increase in energy and overall improvements in her normal daily activities. This case illustrates the importance of having alternatives to conservative treatment for chiropractors. Development of a research proposal is suggested for the next steps in creating guidelines that can be utilized by chiropractors and other professionals in determining suitable candidates for this treatment alternative.
Figure 2.
Potential injection sites in temporalis muscle. Some patients received more or less depending upon pain location.
Figure 3.
Posterior view displaying splenius capitis and trapezius muscles.
Acknowledgments
The authors would like to thank Dr. Hanif Alibhai of MD Cosmetic and Laser Clinic for his guidance and his efforts in providing the resources for this report.
Figures 1–4 with kind permission of Springer Science and Business Media and Dr. Todd Troost.
References
- 1.Setler PE. Therapeutic uses of botulinum toxins: background and history. Clinical J Pain. 2002;18(6):S119–S124. doi: 10.1097/00002508-200211001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dressler D, Saberi FA. Botulinum toxin: mechanisms of action. European Neurology. 2005;53:3–9. doi: 10.1159/000083259. [DOI] [PubMed] [Google Scholar]
- 3.Bell MS, Vermeulen LC, Sperling KB. Pharmacotherapy with botulinum toxin: harnessing nature’s most potent neurotoxin. Pharmacotherapy. 2000;20(9):1079–1091. doi: 10.1592/phco.20.13.1079.35040. [DOI] [PubMed] [Google Scholar]
- 4.Lowe N. Cosmetic uses of botulinum toxins for lower aspects of the face and neck. Clinics in Dermatology. 2004;22(1):18–22. doi: 10.1016/j.clindermatol.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Lew M. Review of the FDA-approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clinical J Pain. 2002;18(6):S142–S46. doi: 10.1097/00002508-200211001-00005. [DOI] [PubMed] [Google Scholar]
- 6.Dodick D. Botulinum toxin Type A for the prophylaxis of chronic daily headache: subgroup analysis of patients not receiving other prophylactic medications: a randomized double-blind, placebo-controlled study. Headache. 2005;45(4):315–324. doi: 10.1111/j.1526-4610.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- 7.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd Edition. Cephalalgia. 2004;24 (suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 8.Limmroth V, Katsarva Z, Fritsche G, Przywara S, Diener H-C. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59(7):1011–1014. doi: 10.1212/wnl.59.7.1011. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt WJ, Slowey E, Fravi N, Weber S, Burgunder JM. Effect of botulinum toxin A injections in the treatment of chronic tension-type headache: a double blind, placebo-controlled trial. Headache. 2001;41:658–664. doi: 10.1046/j.1526-4610.2001.041007658.x. [DOI] [PubMed] [Google Scholar]
- 10.Siberstein SD, Stark SR, Lucas SM, Christie SN, DeGryse RE, Turkel CC. Botulinum toxin type A for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Mayo Clinic Proceedings. 2005;80(9):1126–1137. doi: 10.4065/80.9.1126. [DOI] [PubMed] [Google Scholar]
- 11.Mathew NT, Frishberg BM, Gawel M, Dimitrova R, Gibson J, Turkel C. Botulinum toxin type A (botox) for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Headache. 2005;45:293–307. doi: 10.1111/j.1526-4610.2005.05066.x. [DOI] [PubMed] [Google Scholar]
- 12.Blumenfeld A. Botulinum toxin type A as an effective prophylactic treatment in primary headache disorders. Headache. 2003;43(8):853–60. doi: 10.1046/j.1526-4610.2003.03163.x. [DOI] [PubMed] [Google Scholar]
- 13.Peloso P. Medicinal and injection therapies for mechanical neck disorders. Cervical Overview Group, Cochrane Database of Systematic Reviews. 2005;(2):CD000319. doi: 10.1002/14651858.CD000319.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Troost BT. Botulinum toxin type A in the management of headache: a review of the literature and personal experience. J Headache and Pain. 2004;5(1):15–22. [Google Scholar]
- 15.Arezzo JC. Possible mechanisms for the effects of botulinum toxin on pain. Clinical J Pain. 2002;18(6):S125–S132. doi: 10.1097/00002508-200211001-00003. [DOI] [PubMed] [Google Scholar]
- 16.Bentsianov B, Zalvan C, Blitzer A. Noncosmetic uses of botulinum toxin. Clinics in Dermatology. 2004;22(1):82–88. doi: 10.1016/j.clindermatol.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Sheean G. Botulinum toxin for the treatment of musculoskeletal pain and spasm. Current Pain and Headache Reports. 2002;6:460–469. doi: 10.1007/s11916-002-0065-y. [DOI] [PubMed] [Google Scholar]
- 18.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. NeuroToxicology. 2005;26:785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Evers S, Rahmann A, Vollmer-Hasse J, Husstedt I-W. Treatment of headache with botulinum toxin a – a review according to evidence-based medicine criteria. Cephalgia. 2002;22:699–710. doi: 10.1046/j.1468-2982.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 20.Blumenfeld A. Procedures for administering botulinum toxin Type A for migraine and tension-type headache. Headache. 2003;43(8):884–891. doi: 10.1046/j.1526-4610.2003.03167.x. [DOI] [PubMed] [Google Scholar]
- 21.Naumann M, Jankovic J. Safety of botulinum toxin Type A: A systematic review and meta-analysis. Current Medical Resident Opinion. 2004;20(7):981–990. doi: 10.1185/030079904125003962. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler AH, Gollkasian P, Gretz SS. A randomized double blind prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic paraspinal myofascial pain syndrome. Spine. 1998;23(15):1662–1666. doi: 10.1097/00007632-199808010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Cheshire WP. Botulinum toxin in the treatment of myofascial pain syndrome. Pain. 1994;59(1):65–9. doi: 10.1016/0304-3959(94)90048-5. [DOI] [PubMed] [Google Scholar]