Abstract
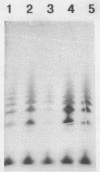
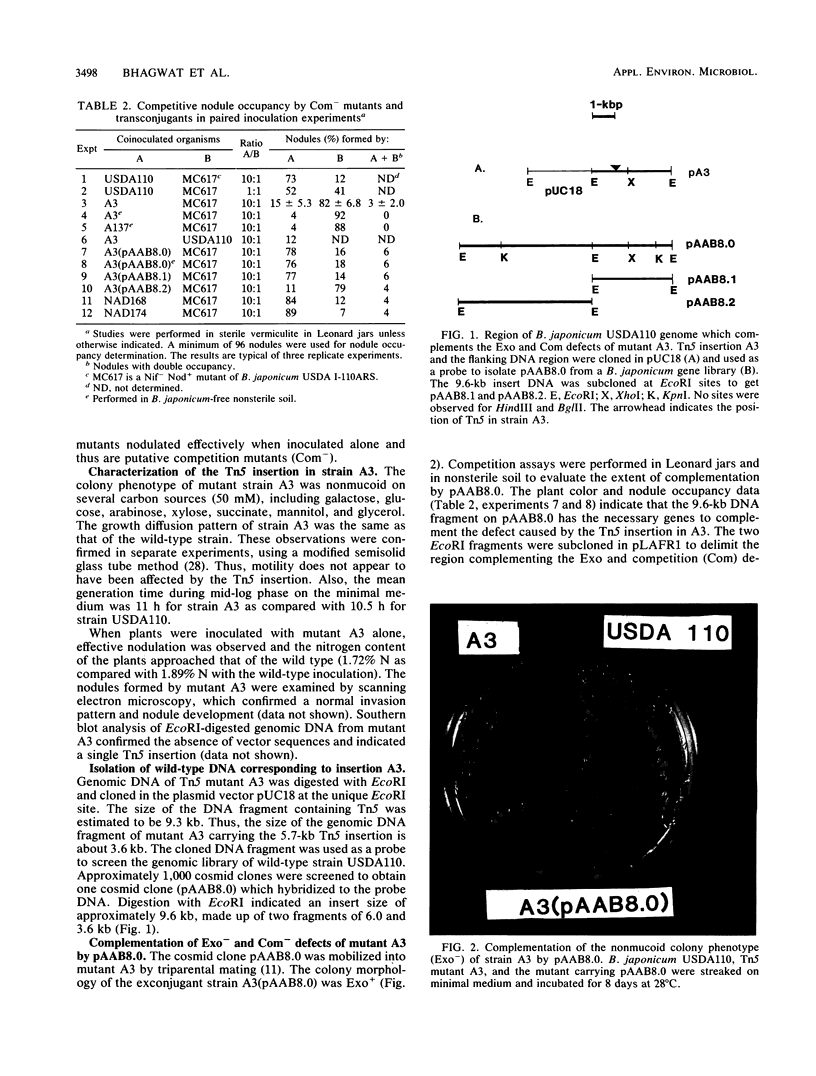
Tn5 mutagenesis was coupled with a competition assay to isolate mutants of Bradyrhizobium japonicum defective in competitive nodulation. A double selection procedure was used, screening first for altered extracellular polysaccharide production (nonmucoid colony morphology) and then for decreased competitive ability. One mutant, which was examined in detail, was deficient in acidic polysaccharide and lipopolysaccharide production. The wild-type DNA region corresponding to the Tn5 insertion was isolated, mapped, and cloned. A 3.6-kb region, not identified previously as functioning in symbiosis, contained the gene(s) necessary for complementation of the mutation. The mutant was motile, grew normally on minimal medium, and formed nodules on soybean plants which fixed almost as much nitrogen as the wild type during symbiosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagwat A. A., Thomas J. Legume-Rhizobium interactions: cowpea root exudate elicits faster nodulation response by Rhizobium species. Appl Environ Microbiol. 1982 Apr;43(4):800–805. doi: 10.1128/aem.43.4.800-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. A., Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973 Sep;4(3):248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. O., Evans I. J., Johnston A. W. Identification of nodX, a gene that allows Rhizobium leguminosarum biovar viciae strain TOM to nodulate Afghanistan peas. Mol Gen Genet. 1988 Jun;212(3):531–535. doi: 10.1007/BF00330860. [DOI] [PubMed] [Google Scholar]
- Deshmane N., Stacey G. Identification of Bradyrhizobium nod genes involved in host-specific nodulation. J Bacteriol. 1989 Jun;171(6):3324–3330. doi: 10.1128/jb.171.6.3324-3330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold R., Noel K. D. Rhizobium leguminosarum exopolysaccharide mutants: biochemical and genetic analyses and symbiotic behavior on three hosts. J Bacteriol. 1989 Sep;171(9):4821–4830. doi: 10.1128/jb.171.9.4821-4830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic S. P., Chen H., Batley M., Redmond J. W., Rolfe B. G. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J Bacteriol. 1987 Jan;169(1):53–60. doi: 10.1128/jb.169.1.53-60.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling D. N., Broughton W. J. Competition for nodulation of legumes. Annu Rev Microbiol. 1986;40:131–157. doi: 10.1146/annurev.mi.40.100186.001023. [DOI] [PubMed] [Google Scholar]
- Dylan T., Helinski D. R., Ditta G. S. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1----2)-glucan. J Bacteriol. 1990 Mar;172(3):1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Nagpal P., Helinski D. R., Ditta G. S. Symbiotic pseudorevertants of Rhizobium meliloti ndv mutants. J Bacteriol. 1990 Mar;172(3):1409–1417. doi: 10.1128/jb.172.3.1409-1417.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J., Walker G. C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989 Feb 24;56(4):661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- Gray J. X., Rolfe B. G. Exopolysaccharide production in Rhizobium and its role in invasion. Mol Microbiol. 1990 Sep;4(9):1425–1431. doi: 10.1111/j.1365-2958.1990.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Göttfert M., Lamb J. W., Gasser R., Semenza J., Hennecke H. Mutational analysis of the Bradyrhizobium japonicum common nod genes and further nod box-linked genomic DNA regions. Mol Gen Genet. 1989 Feb;215(3):407–415. doi: 10.1007/BF00427037. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis: detection of a protein factor in soybean root exudate which is involved in the nodulation process. Plant Physiol. 1984 Jan;74(1):84–89. doi: 10.1104/pp.74.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Keyser H. H., Cregan P. B. Nodulation and Competition for Nodulation of Selected Soybean Genotypes among Bradyrhizobium japonicum Serogroup 123 Isolates. Appl Environ Microbiol. 1987 Nov;53(11):2631–2635. doi: 10.1128/aem.53.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Kuykendall L. D., Shah K. S., Keister D. L. Induction of Symbiotically Defective Auxotrophic Mutants of Rhizobium fredii HH303 by Transposon Mutagenesis. Appl Environ Microbiol. 1988 Feb;54(2):423–427. doi: 10.1128/aem.54.2.423-427.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Influence of Environmental Factors on Interstrain Competition in Rhizobium japonicum. Appl Environ Microbiol. 1985 May;49(5):1128–1133. doi: 10.1128/aem.49.5.1128-1133.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Liu Ruilong, Tran Van Mai, Schmidt E. L. Nodulating Competitiveness of a Nonmotile Tn7 Mutant of Bradyrhizobium japonicum in Nonsterile Soil. Appl Environ Microbiol. 1989 Aug;55(8):1895–1900. doi: 10.1128/aem.55.8.1895-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium genetics. Annu Rev Genet. 1989;23:483–506. doi: 10.1146/annurev.ge.23.120189.002411. [DOI] [PubMed] [Google Scholar]
- Maier R. C., Norris H. J. Glassy cell carcinoma of the cervix. Obstet Gynecol. 1982 Aug;60(2):219–224. [PubMed] [Google Scholar]
- McDermoti T. R., Graham P. H. Competitive Ability and Efficiency in Nodule Formation of Strains of Bradyrhizobium japonicum. Appl Environ Microbiol. 1990 Oct;56(10):3035–3039. doi: 10.1128/aem.56.10.3035-3039.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin T. J., Merlo A. O., Satola S. W., Johansen E. Isolation of competition-defective mutants of Rhizobium fredii. J Bacteriol. 1987 Jan;169(1):410–413. doi: 10.1128/jb.169.1.410-413.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S. Improvement of Rhizobium inoculants. Appl Environ Microbiol. 1989 Apr;55(4):862–865. doi: 10.1128/aem.55.4.862-865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Cregan P. B., Gottfert M., Sharma A., Gerhold D., Rodriguez-Quinones F., Keyser H. H., Hennecke H., Stacey G. The Bradyrhizobium japonicum nolA gene and its involvement in the genotype-specific nodulation of soybeans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):637–641. doi: 10.1073/pnas.88.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B., Keyser H. H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl Environ Microbiol. 1987 Nov;53(11):2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., So J. S., Roth L. E., Lakshmi SK B., Carlson R. W. A lipopolysaccharide mutant of Bradyrhizobium japonicum that uncouples plant from bacterial differentiation. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):332–340. doi: 10.1094/mpmi-4-332. [DOI] [PubMed] [Google Scholar]
- Triplett E. W. Construction of a Symbiotically Effective Strain of Rhizobium leguminosarum bv. trifolii with Increased Nodulation Competitiveness. Appl Environ Microbiol. 1990 Jan;56(1):98–103. doi: 10.1128/aem.56.1.98-103.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- van Berkum P. Evidence for a Third Uptake Hydrogenase Phenotype among the Soybean Bradyrhizobia. Appl Environ Microbiol. 1990 Dec;56(12):3835–3841. doi: 10.1128/aem.56.12.3835-3841.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]