Abstract
CVD 909 is a novel live attenuated S. Typhi oral vaccine candidate derived from strain CVD 908-htrA which constitutively expresses Vi. Herein we investigated whether the genetic manipulations involved in modifying CVD 908-htrA altered its ability to induce potent T-cell immune responses (CMI) after a single dose (5 subjects) and, in a separate trial, whether a second dose (8 subjects) further enhanced its immunogenicity. In these clinical trials we observed that CVD 909 immunization elicits a wide array of CMI, including cytotoxic T cells (CTL), IFN-γ, TNF-α and IL-10 (but not IL-2, IL-4 or IL-5) production, and proliferation to S. Typhi antigens. However, the administration of a second dose did not result in increases in CMI. These results suggest that the genetic manipulations to constitutively express Vi did not adversely affect the ability of CVD 909 to elicit a wide array of CMI responses. These observations add impetus for the continuing evaluation of CVD 909 as a typhoid vaccine candidate.
Keywords: T cells, Bacteria, Vaccination
1. Introduction
Typhoid fever remains an important public health priority, particularly in developing countries. It is estimated that about 21,650,000 episodes of typhoid fever and 216,500 deaths occurred in 2000 [1]. The appearance of antibiotic resistant strains of Salmonella enterica serovar Typhi (S. Typhi), the causative agent of typhoid fever, has added new urgency for the development of improved typhoid vaccines [2,3].
Currently licensed typhoid vaccines in the USA include the purified Vi polysaccharide parenteral vaccine and the live oral attenuated Vi negative galE mutant, S. Typhi strain Ty21a vaccine (henceforth Ty21a vaccine). Each of these vaccines mediates protection by distinct mechanisms. The purified Vi polysaccharide vaccine is T cell independent and mediates protection by eliciting anti-Vi antibody responses [4]. In contrast, the Ty21a vaccine likely mediates protection by eliciting antibodies to S. Typhi LPS, flagella and other antigens, as well as through a wide array of T-cell immune responses (CMI) at the systemic and mucosal levels [5–11]. However, each of these vaccines has drawbacks. Vi polysaccharide is a parenteral vaccine that cannot stimulate CMI and fails to elicit responses to purified polysaccharides in children under 2 years of age [12,13]. Concerning Ty21a , this vaccine does not elicit anti-Vi antibodies (because it does not make Vi capsule) and is modestly immunogenic, requiring three or four doses to evoke protective immunity in ~47% to 96%, depending on the number of doses and formulations used and the time of surveillance [12,14,15].
To overcome these limitations, we and others have recently engineered new attenuated typhoid candidate vaccine strains that are as well-tolerated as Ty21a yet more immunogenic, with the goal of eliciting protective immunity with just a single oral dose [2]. One of these strains, CVD 908-htrA, has been attenuated by deletions in genes encoding enzymes required for the biosynthesis of aromatic amino acids (aroC and aroD) and htrA, a gene encoding a heat shock protein [16]. CVD 908-htrA has been shown to be well tolerated and high immunogenic in Phase a 1 and 2 clinical trials [17–19]. After a single dose, we observed a wide array of CMI responses, including the secretion of interferon (IFN)-□ and the killing of S. Typhi-infected cells (cytotoxicity), as well as, anti-S. Typhi LPS antibody responses in serum, and anti-LPS IgG and IgA antibody secreting cells (ASC) [17,19,20]. Disappointingly, CVD 908-htrA, which does not express Vi constitutively, did not induce an immune response against Vi. This lack of Vi responsiveness could be the result of low expression of the Vi capsule, which is likely to be down regulated in vivo once the bacteria gain their intracellular niche in the gut associated lymphoid tissue [21]. To render the Vi expression constitutive, promoter PtviA in serovar Typhi vaccine CVD 908-htrA was replaced with the constitutive promoter Ptac, resulting in CVD 909, which expresses Vi even under high-osmolarity conditions [22]. Our hypothesis was that this might result in serum IgG and mucosal IgA Vi antibody responses in orally vaccinated subjects, thereby enhancing the protection against typhoid fever. We recently conducted a vaccine trial in which healthy subjects were orally immunized with one dose of CVD 909. This vaccine was very well tolerated and 16 of the 20 volunteers (80%) who received 108–9 cfu exhibited anti-Vi ASC responses following immunization, while only 2 of the 20 volunteers who received 108–9 cfu generated anti-Vi IgG or IgA antibody in serum [21]. This is the first live attenuated S. Typhi vaccine candidate shown to elicit anti-Vi responses.
To explore the possibility that stronger immunity could be elicited by a second dose, a separate phase 2 vaccine trial was conducted in which volunteers were immunized with 2 oral doses of CVD 909, 1 dose on day 0 and a subsequent dose on day 14. In the present study we investigated whether the genetic manipulations involved in modifying CVD 908-htrA to constitutively express Vi altered its ability to induce potent CMI after a single dose and whether a second dose further enhanced the immunogenicity of oral live attenuated typhoid vaccine CVD 909.
2. Materials and Methods
2.1.Subjects, vaccinations and isolation of peripheral blood mononuclear cells (PBMC)
PBMC from subjects participating in 2 separate vaccine trials directed to evaluate the safety and immunogenicity of CVD 909 were used in these studies. One study (hereafter Ty32004) consisted of six subjects (median age 28.3 yrs, range 20 yrs to 39 yrs) who received a single dose of CVD 909 (2.5 X 109 cfu vaccine strain with sodium bicarbonate buffer) and blood samples were collected on days 0, 28 and 91 post immunization. The second study (hereafter Ty35000) included eight healthy subjects (median age 24 years, range 19 to 39 yrs) who were immunized with 2 oral doses of CVD 909 (6.2 X 109 cfu vaccine per dose with sodium bicarbonate buffer), 1 dose on day 0 and 1 dose on day 14. Blood samples from these subjects were obtained on days 0, 28 and 60 post vaccination [21]. In the latter study, from a total of 8 volunteers who were immunized, PBMC were not available from volunteer 35000–5 at day 60 and from volunteer 35000–8 at day 28. PBMC were isolated immediately after the blood draws by density gradient centrifugation and cryopreserved in liquid N2 following standard techniques until used in the assays [20,23].
All subjects in both trials were from the Baltimore-Washington, DC area and University of Maryland Baltimore community. A thorough screening was done to ensure that they were in good physical and mental health by medical history, physical examination, and laboratory tests. Before the blood collection, volunteers were explained the purpose of this study and signed informed consents. To document comprehension of the study, all volunteers passed a written examination. The human experimentation guidelines of the US Department of Health and Human Services and those of the University of Maryland, Baltimore, were followed in the conduct of the present clinical research.
2.2. Antigens
S. Typhi flagella H-antigen
Flagellar antigen was purified from the rough S. Typhi strain Ty2R following standard procedures and used at a concentration of 2 or 10 μg/ml as indicated in the text [8,23].
Heat-phenolyzed whole-cell S. Typhi (TypVac)
The particulate S. Typhi antigen consisted of Ty2 heat-killed, phenol-preserved whole cell bacteria (typhoid vaccine, Wyeth Laboratories, Marietta, PA, lot#4978038, ~2.5x108 organism/ml) washed several times in serum-free media (AIM-V, Gibco, Grand Island, NY) to remove residual phenol used as preservative in this vaccine. The bacterial pellet was resuspended to a concentration of 1X107 organism/ml in serum free media and kept at 4°C until used [23].
2.3. Proliferation assays
A standard 3H-thymidine incorporation assay was used to measure the proliferative responses to particulate S. Typhi and S. Typhi flagella [24]. PBMC were resuspended in Aim-V serum free media supplemented with 2mM L-glutamine (Gibco) and incubated at 37°C, 5% CO2, in 96 well round bottom plates (1.5 X 105 PBMC/well, triplicate wells) in the presence or absence of the antigens or mitogens at the following concentrations; S. Typhi flagella (2 μg/ml), TypVac particles (2 X 105 organisms/well), anti-CD3/CD28 beads (Dynabeads, Dynal, Oslo, Norway; 0.6 μl/ml); PHA-L (Sigma, St. Louis, MO, 1 μg/ml) or Bovine Serum Albumin (BSA, Fraction V, Sigma, 2 μg/ml). Cultures without stimulants (media only) were used as negative controls. Six days after the initiation of the assays, cultures were pulsed with 1 μCi of 3H-thymidine (Perkin Elmer, Boston, MA) and incubated for an additional 18 hrs. Cultures were terminated by automated harvesting (Harvester 96, Tomtec, Orange CT, USA), and thymidine incorporation was measured in an automated Wallac Trilux Microbeta liquid scintillation counter (Wallac, Finland). PHA-L stimulated wells were pulsed with 3H-thymidine 2 days after the initiation and harvested 18 hrs later. Data are presented as mean ± SE of fold increases (stimulation indices) for each antigen calculated according to the formula: net cpm with antigen /net cpm without antigen (media alone) for each individual volunteer on each day (i.e., pre-inoculation or post-inoculation days).
2.4. Cytokine measurements in culture supernatants
Cytokine production was measured using the flow cytometry-based BD Cytometry Bead Array (CBA) human TH1/Th2 kit assay (BD biosciences Pharmingen, San Diego, CA, USA) according to the manufacturer’s recommendations with minor modifications (i.e., supernantants were incubated with the beads for 3 days). Briefly, cells were plated in 96-well plates as described in proliferation assays and supernatants were collected from PHA-L stimulated cultures after 2 days. From the cultures with other antigens, 60 μl of each supernatant was collected on day 3 and an equal volume of fresh serum free media added to the wells. After 3 additional days of culture (Day 6), a second set of supernatants was harvested. Supernatants were kept at –70°C until tested. To ameliorate the loss of cytokines during freezing/thawing in serum free lymphocyte medium (AIM-V Media), as well as to prevent non-specific binding to the plates, 5 μl of 20% BSA in PBS was added to each well of the collection plates before harvesting the supernatants. The limits of sensitivity of the CBA assays for IFN-γ, IL-2, IL-4, IL-5, IL-10 and TNF-α were 2.5 to 10 pg/ml depending on the cytokine and the individual assays. Data are presented as net cytokine production ± SE. Net cytokine production was calculated by subtracting the cytokine levels (in pg/ml) of the “media alone” control wells from cytokine levels of antigen-stimulated wells for each individual volunteer on each day.
2.5. Preparation of target/stimulator cells for use in IFN-γ Elispot and cytotoxic T cell (CTL) assays
Autologous PHA-stimulated PBMC (henceforth called “blasts”) were used in this study as target/stimulator cells. Blasts were obtained by incubating 5–10 x 106 PBMC with 1 μg/ml PHA-L for 24 hours in RPMI 1640 (Gibco) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 2 mM L-glutamine, 2.5 mM sodium pyruvate, 10 mM HEPES buffer and 10% heat-inactivated fetal bovine serum (complete RPMI). PHA-activated PBMC were then washed three times with RPMI 1640, and cultured in complete RPMI supplemented with 20 IU/ml human recombinant interleukin-2 (rhIL2) (Boehringer Gmbh, Mannheim, Germany) for 5 to 6 days.
2.6. Infection of target/stimulator cells with S. Typhi for use in IFN-γ Elispot and CTL assays
Blasts were incubated in RPMI 1640 (without antibiotics) for 3 hours at 37 °C in the absence or presence of wild-type S. Typhi strain ISP1820 (wt S. Typhi) (obtained from Dr. J. Nataro, Center for Vaccine Development) at different multiplicity of infection (MOI) as previously described [20,25]. After exposure to S. Typhi, cells were washed and incubated overnight at 37 °C in complete RPMI containing 20 IU/ml rhIL2. The following day cells were γ-irradiated (4,000 rads) and used as stimulators to expand effector cells for CTL assays or in Elispot assays. Alternatively, infected and uninfected blasts were labeled with 200 μCi of sodium chromate (51Cr) (Amershan Pharmacia Biotech, Piscataway, NJ), for 1 h at 37 °C, washed three times and used as targets in CTL assays. To confirm that targets were infected with S. Typhi, cells were stained with FITC-labeled polyclonal antibody recognizing Salmonella Common Structural Antigens (CSA-1)(KPL, Gaithersburg, MD) and expression of bacterial antigens on the cell surface was measured by flow cytometry [25].
2.7. Preparation of effector cells for use in IFN-γ Elispot and CTL assays
For CTL assays, effector cells were obtained using a modification of previously described techniques [5–7,20,25]. Briefly, PBMC were co-cultured with stimulator cells at an effector to stimulator cell ratio of 7:1 in complete RPMI supplemented with 20 IU/ml of rhIL2 for 7–8 days. Stimulator cells consisted of autologous blasts infected with S. Typhi and γ irradiated (4,000 rads) as described above. For IFN-γ Elispot assays, PBMC from immunized volunteers were used ex vivo as effector cells after an overnight incubation in complete RPMI.
2.8. Cytotoxicity (CTL) assays (Chromium Release Test)
Cytotoxicity was determined in a 4-hour 51Cr-release assay against autologous blasts infected with S. Typhi as previously described [5–7,20,25]. Uninfected blasts were used as controls. Consistent with our previous observations, the cut-off for positive responses in CTL assays was defined as >10% specific cytotoxicity observed in at least 2 effector:target ratios [5–7,20,25].
2.9. Granzyme B and IFN-γ ELISPOT assays
The human Granzyme-B ELISPOT assay was performed using a commercial kit (BD Immunocytometry systems) following the manufacturer’s recommendations. IFN-□ ELISPOT assays were performed as previously described [5–7,20,25]. S. Typhi-infected cells were used as stimulator cells. Effector cells cultured without stimulator cells or with CD3/CD28 beads (0.6 μl/ml, Dynal), were used as negative and positive controls, respectively. Target cells uninfected or infected with S. Typhi without effector cells were also used as controls. Each sample was tested in triplicate. The data were read with an automated ELISPOT reader (Bioreader 3000 PRO, Bio-Sys, Karben, Germany). As previously described [5–7,20,25], net frequencies of spot forming cells (SFC) were calculated by using the following formula: [(number of SFC in effector cell populations incubated with S. Typhi-infected targets) – (number of SFC in effector cell populations incubated with uninfected targets + number of SFC in cultures containing S. Typhi-infected target cell populations alone)]. The cut-off for assays was established as the frequency of Granzyme-B or IFN-□ SFC/106 PBMC in co-culture of effectors with not-infected targets + 2 SE.
2.10. Statistical analysis
All tests were performed using SigmaStat software (version 3.1, Systat Software, Inc., Point Richmond, CA). P values <0.05 were considered significant.
3. Results
3.1. Lymphoproliferative responses to S. Typhi flagella and whole-cell S. Typhi particulate (TypVac)
We have previously reported that significant increases in proliferative responses to S. Typhi antigens were observed in 20–67 % of subjects who received CVD 909 depending on the dosage of the inoculum and S. Typhi antigen evaluated [21]. To investigate whether a second dose vaccine dose resulted in increased levels of CMI, we determined whether immunization with a second oral dose of CVD 909 14 days after the primary immunization elicited stronger proliferative responses than those observed previously after a single dose of CVD 909 [21]. To this end, PBMC from the eight subjects who received two doses of CVD 909 were cultured in presence or absence of S. Typhi flagella or TypVac and their lymphoproliferative responses analyzed by 3H-thymidine incorporation (Fig. 1, panel A). Compared to the corresponding pre-immunization (day 0) levels, significant increases in response to S. Typhi flagella at either days 28 or 60 were observed in 4 of the 8 volunteers (Fig. 1, panel A). Significant increases to TypVac were observed in 7 of the 8 volunteers at days 28 and/or 60 (Fig. 1, panel B). No significant differences in proliferative responses to BSA (negative control) or PHA-L or CD3/CD28 beads (positive controls) were observed among PBMC obtained before and after immunization (data not shown). These results indicate that immunization with 2 doses of CVD 909 elicited high lymphoproliferative responses to S. Typhi antigens in the majority of volunteers (Table 1). However, when comparing these responses with those observed after a single dose of CVD 909 [21] the levels were similar. Taken together, these results demonstrated that immunization with 2 doses of CVD909 elicited lymphoproliferative responses but no booster effect was observed.
Figure 1.
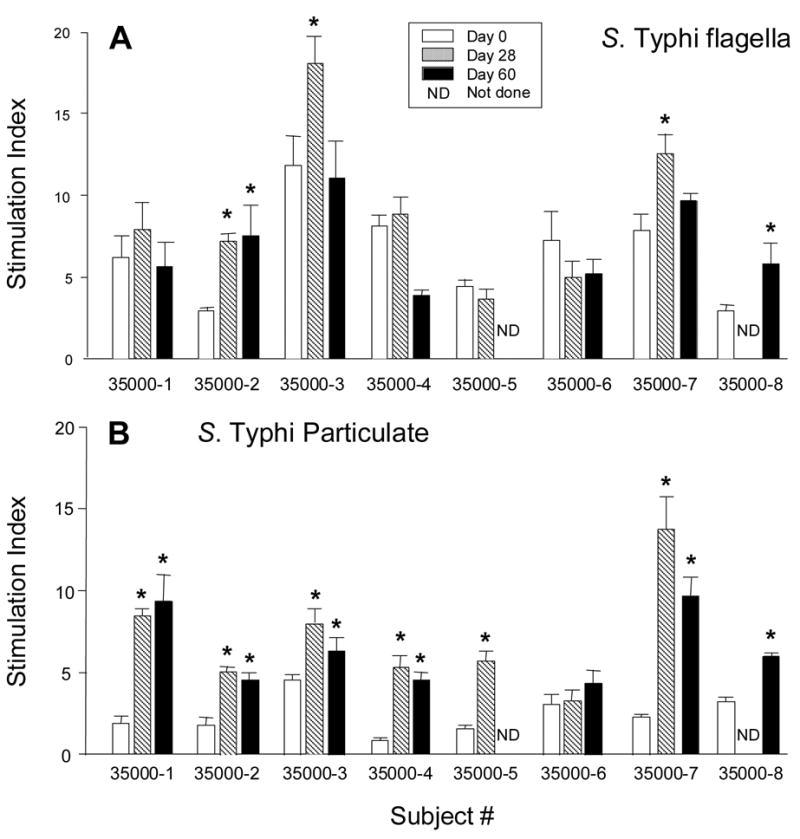
Proliferative responses to antigenic stimulation with (A) S. Typhi flagella and (B) Whole-cell S. Typhi particulate by PBMC obtained from subjects immunized with 2 doses of CVD 909. PBMC were obtained before immunization (Day 0), and at days 28 and 60 after the first immunization as indicated. Bars represent the mean ± SE of the fold increases (stimulation index) calculated as described in Materials and Methods. *p<0.05 compared to pre-immunization levels.
Table 1.
Summary of cell-mediated immune responses in volunteers immunized with CVD 909
|
S .Typhi Infected Targets
|
S .Typhi Antigens (Day 3 Supernatants)
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S.Typhi Flagella (Hdf)
|
S .Typhi Particulate (TypVac)
|
|||||||||||||||||
| Subject # | IFN-γ 1 | CTL2 | Granzyme 3 | Prolif 4 | IFN-γ 5 | TNF-α | IL-10 | IL-5 | IL-4 | IL-2 | Prolif. | IFN-γ 5 | TNF-α | IL-10 | IL-5 | IL-4 | IL-2 | |
| Ty32004 (One dose) | 32004-1 | - 6 | - | + 6 | + | + | + | + | - | - | - | + | + | - | - | - | - | - |
| 32004-2 | - | NE7 | - | - | + | + | + | - | - | - | - | + | + | + | - | - | - | |
| 32004-3 | + | - | - | - | - | + | - | - | - | - | - | - | + | + | - | - | - | |
| 32004-4 | NA8 | NA | NA | - | + | + | + | - | - | - | - | + | + | + | - | - | - | |
| 32004-5 | + | + | - | - | + | + | + | - | - | - | - | + | + | + | - | - | - | |
| Ty35000 (Two doses) | 35000-1 | + | - | + | - | + | + | + | - | - | - | + | + | + | + | - | - | - |
| 35000-2 | - | - | + | + | + | - | + | + | - | - | + | + | + | + | + | - | - | |
| 35000-3 | + | + | - | + | + | + | + | - | - | - | + | + | + | + | - | - | - | |
| 35000-4 | - | - | - | - | + | + | + | - | - | - | + | + | + | + | - | - | - | |
| 35000-5 | NA | NA | NA | - | + | - | - | + | - | - | + | + | - | - | + | - | - | |
| 35000-6 | + | NE | - | - | + | - | + | - | - | - | - | + | - | + | - | - | - | |
| 35000-7 | + | - | - | + | - | + | + | - | - | - | + | + | + | + | - | - | - | |
| 35000-8 | NA | NA | NA | + | - | - | - | - | - | - | + | + | - | - | - | - | - | |
IFN-γ detected by ELISPOT. Responder: a net increase of >99 SFC/106 PBMC in the presence of S. Typhi-infected targets over those observed with uninfected targets
Cytotoxic T cells (CTL). Responder: a specific lysis of >10% at two or more Effector:Target ratios
Granzyme B detected by ELISPOT (Granzyme): Responder: a net increase of >59 SFC/106 PBMC in the presence of S. Typhi-infected targets over those observed with uninfected targets
Proliferation (Prolif): Responder: significant (p<0.05) increases in stimulation index of proliferative responses at either days 28 or 60/91compared to day 0
IFN-γ, TNF-α, IL-10, IL-5, IL-4 and IL-2 in day 3 supernatants detected by cytokine bead arrays. Responder: significant (p<0.05) increases in net cytokine production at either days 28 or 60/91compared to day 0
Responder: (+); Non-responder: (-)
NE: Not evaluable (Responder at day 0)
NA: PBMC not available
3.2. Cytokine production in response to S. Typhi flagella and whole-cell S. Typhi particulate
To further evaluate the CMI responses elicited by one or two doses of CVD 909, we evaluated the patterns of cytokine production of PBMC from immunized subjects following S. Typhi flagella or TypVac stimulation. To this end, PBMC were cultured in the presence of S. Typhi flagella (2 μg/ml), TypVac particles (2 X 105 organisms/well) for 6 days. At days 3 and 6 after culture initiation, supernatants were collected and the levels of six key cytokines, IFN-γ, TNF-α, IL-10, IL-5, IL-4 and IL-2, measured by CBA. Marked increases in IFN-γ production were observed in post-immunization PBMC from both the Ty32004 and Ty35000 trials after 3 days of in vitro culture (Fig. 2). Significant increases (at either days 28 or 60/91 post-immunization) in response to S. Typhi flagella were observed in 4 of the 5 volunteers in the single-dose study (Ty32004; Fig. 2, panel A) and in 6 of the 8 volunteers in the two-dose study (Ty35000; Fig. 2, panel B). Similar levels of IFN-γ production in response to the S. Typhi particulate were also observed in 4 of the 5 and all 8 subjects in the Ty32004 and Ty35000 studies, respectively (Table 1).
Figure 2.
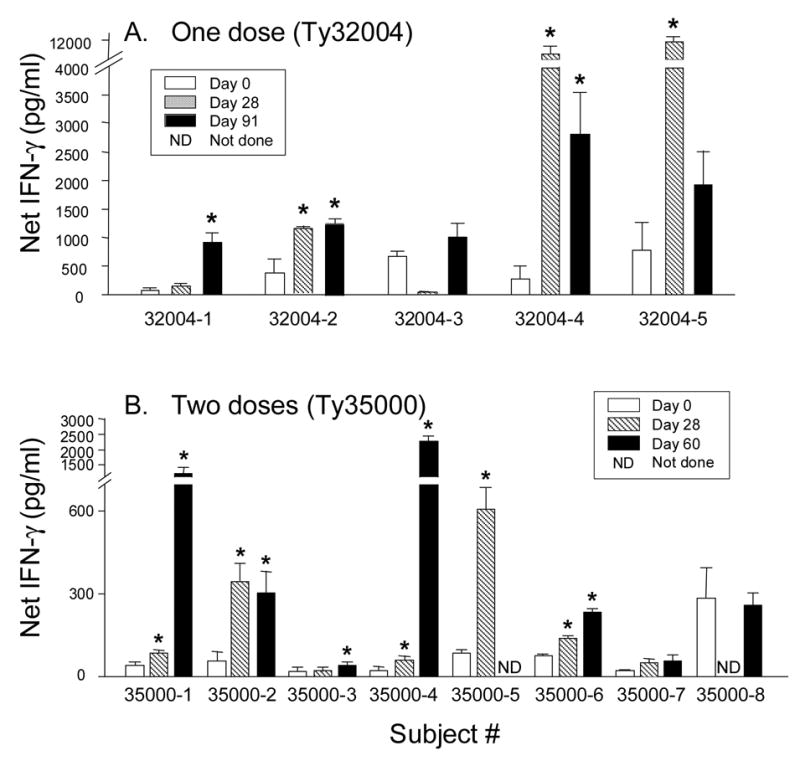
IFN-γ production in response to antigenic stimulation with purified S. Typhi flagella by PBMC obtained from subjects immunized with one (A) or two (B) doses (days 0 and 14) of CVD 909. PBMC were obtained before immunization (Day 0), and at days 28 and 60 or 91 after the first immunization as indicated. Bars represent the mean ± SE of the net IFN-γ levels in 3-day culture supernatants calculated as described in Materials and Methods. *p<0.05 compared to pre-immunization levels.
Immunization also resulted in significant increases in TNF-α and IL-10 production in 3-day culture supernatants in response to S. Typhi flagella (Fig. 3). With regard to TNF-α production, all 5 subjects in the Ty32004 study and 4 of the 8 subjects in the Ty35000 study showed significant increases at either days 28 and/or 60/91 (Fig. 3, panels A and B, respectively). Similarly, increases in IL-10 production were observed in 4 of the 5 and in 6 of the 8 subjects in the Ty32004 and Ty35000 studies, respectively (Fig. 3, panels C and D, respectively). TNF-α and IL-10 production increases in response to the whole cell particulate TypVac were similar to those observed with S. Typhi flagella (Table 1). Interestingly, subjects 35000-5 and 35000-8 showed no increases in TNF-α secretion to either S. Typhi flagella or TypVac (Table 1). Except for 2 of the 8 subjects in the Ty35000 study who showed meager, and probably biologically inconsequential, increases in IL-5 production in response to TypVac in post-immunization samples (i.e., in Vol#35000–5 day 28 post-vaccination levels were 5.7 pg/ml compared to a pre-vaccination level of 2.5 pg/ml, while Vol# 35000-2 showed 12.9 pg/ml at day 60 post-vaccination compared to a pre-vaccination level of 3.2 pg/ml), there were no other significant increases in IL-5, IL-4 and IL-2 production after immunization with CVD 909 (Table 1). These results are consistent with previous findings using other attenuated S. Typhi strains [8,19,20,23,26] that also showed a dominance of Th1 responses (e.g., IFN-γ and TNF-α production), suggesting that these cytokines are likely to be important in S. Typhi immunity. Similar to the results with proliferative responses, no differences were observed between volunteers immunized with one or two doses of CVD 909.
Figure 3.
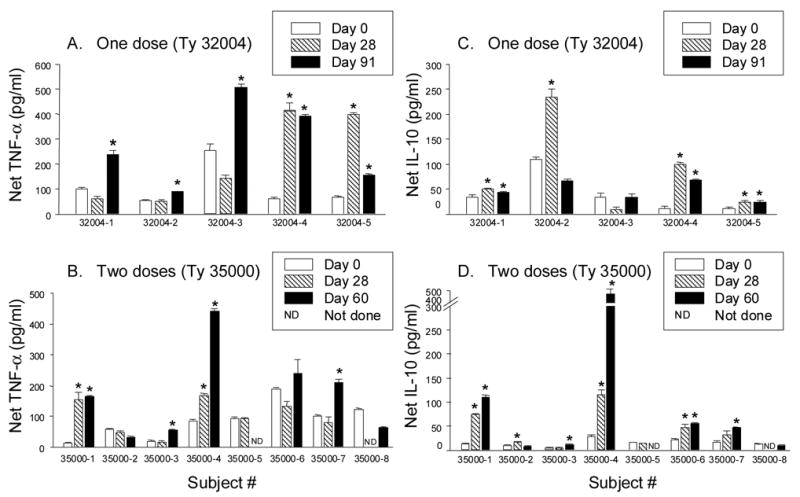
TNF-α and IL-10 production in response to antigenic stimulation with purified S. Typhi flagella by PBMC obtained from subjects immunized with one (A, C) or two (B, D) doses of CVD 909. PBMC were obtained before immunization (Day 0), and at days 28 and 60 or 91 after the first immunization as indicated. Bars represent the mean ± SE of the net TNF-α (A, B) and net IL-10 (C, D) levels in 3-day culture supernatants calculated as described in Materials and Methods. *p<0.05 compared to pre-immunization levels.
To investigate the kinetics of in vitro production, we also assayed cytokine levels 6 days after initiation of the cultures and compared these results with those observed in 3-day supernatants. We observed that while IFN-γ levels were considerably higher at day 6 than at day 3, levels of TNF-α and IL-10 peaked at day 3, suggesting that these cytokines were secreted, at least in part, by cells other than classical effector-memory specific T cells (data not shown).
3.3. Frequency of IFN-γ secreting cells in response to S. Typhi-infected stimulators
To provide a quantitative measurement of the specific T cells elicited by immunization, as well as to compare the magnitude of the responses induced by CVD 909 with that induced by Ty21a [7] and CVD 908htrA [20], we next determined the frequency of IFN-γ secreting effector cells elicited by immunization in response to ex vivo stimulation with S. Typhi-infected blasts. To this end, PBMC from immunized volunteers were used as effectors and autologous blasts infected with wt S. Typhi were used as stimulators. After overnight re-stimulation of effectors cells by S. Typhi-infected stimulators, the frequency of IFN-γ-SFC was quantified by using an IFN-γ Elispot assay. As we observed in previous studies, the cutoff for positive responses was determined to be 99 (mean + 2 SE of SFC observed in co-cultures with uninfected stimulators) [20].
When compared with pre-immunization levels, significant increases in the net frequency of IFN-γ-SFC were observed in six of ten volunteers following immunization (Table 2). These included 2 of 4 subjects who received one dose (#s Ty32004-3 and Ty32004-5) and 4 of 6 volunteers that received 2 doses (#s Ty35000-1, Ty35000-3, Ty35000-6 and Ty35000-7). Mean increases in the net frequency of IFN-γ-SFC following immunization ± 2 SE were 147 ± 128 SFC/106 PBMC and 239 ± 115 SFC/106 PBMC, in Ty32004 and Ty35000 subjects, respectively, showing no significant differences between subjects that received one or two doses of CVD 909.
Table 2.
Frequency of spot forming cells in the presence of S . Typhi-infected stimulator cells detected by a ex vivo IFN-γ ELISPOT in volunteers immunized with with one dose (Ty32004) or two doses (Ty35000) of CVD 909.
| Study | Subject # | IFN-γ ELISPOT 1 | ||
|---|---|---|---|---|
| Day 0 | Day 28 | Day 60/91 3 | ||
| Ty32004 (one dose) | 32004-1 | 19 | 0 | 0 |
| 32004-2 | 93 | 113 | 3 | |
| 32004-3 | 95 | 279 2 | 319 | |
| 32004-5 | 96 | 196 | 293 | |
| Ty35000 (two doses) | 35000-1 | 0 | 130 | 372 |
| 35000-2 | 0 | 0 | 0 | |
| 35000-3 | 0 | 122 | 106 | |
| 35000-4 | 256 | 0 | 0 | |
| 35000-6 | 243 | 538 | 160 | |
| 35000-7 | 277 | 439 | 444 | |
IFN-γ-spot forming cells (SFC) /106 PBMC.
Responders: >100 SFC/106 above day 0 levels are shown in bold type.
PBMC were obtained at days 0, 28 and 91, or at days 0, 28 and 60 for Ty32004 and Ty35000 vaccinees, respectively.
Of note, these responses are similar in frequency and magnitude to those observed in previous studies in which subjects were immunized with a single dose of 4.5 X 108 cfu of CVD 908htrA [20] or 4 doses of 2–6 x 109 cfu of Ty21a [7].
3.4. CTL responses measured by 51Cr release assays
We have previously shown in volunteers immunized orally with attenuated strains of S. Typhi, including Ty21a, as well as with the novel attenuated typhoid vaccine candidate strains CVD 908 or CVD 908htrA, that CD8+ T cells might be important in host defense against S. Typhi by lysing S. Typhi-infected cells [5–7,20,25]. Thus, it was important to evaluate whether CVD 909 retained the ability of its parent strain to elicit specific CTL activity in humans and whether this activity can be enhanced by a second dose immunization. To this end, PBMC from 6 Ty35000 and 4 Ty32004 typhoid vaccinees were expanded in vitro for 8-days in the presence of S. Typhi-infected blasts and used as effectors in 51Cr-release cytotoxicity assays. Significant increases in CTL activity following immunization were observed in 1 of 3 subjects receiving a single dose (subject Ty32004-5) and in 2 of 5 subjects receiving two doses (subjects Ty35000-3 and Ty35000-7) who were negative before immunization (Table 1). Of note, two subjects (32004-2 and Ty35000-6) were positive before immunization and could therefore not be evaluated for increased CTL activity since this is at best a semi-quantitiative assay. Specific cytotoxic activity was not observed when uninfected autologous blasts were used as targets in any of these assays, indicating that S. Typhi infection is necessary to enable killing of targets by effector CTL. In conclusion, as seen for other CMI measurements, comparable CTL activity was observed between the subjects who received one or two doses of CVD 909. Moreover, these responses were similar to those observed with other attenuated S. Typhi strains [5–7,20,25].
3.5. Granzyme-B production
Because the granule-dependent pathway is largely responsible for the killing of S. Typhi-infected targets by specific CTLs, we determine the magnitude of the specific cytotoxic response induced by CVD 909 by measuring the ex vivo frequency of CTL effectors by using granzyme-B Elispot assay [27]. Following infection with S. Typhi, autologous blasts were cultured with ex vivo PBMC and the frequency of S. Typhi specific T cells measured by their release of Granzyme-B using a commercially available Elispot assay. The cut-off for positive responses was determined to be 59 SFC/106. Immunization with CVD 909 resulted in increases in the net frequencies of granzyme-B SFC in 1 of 4 volunteers receiving a single dose (Ty32004-1) and 2 of 6 volunteers receiving two doses (vol#s Ty35000-1 and Ty35000-2). Surprisingly, no concordance was observed between responders by standard 51Cr-release CTL assays and granzyme B Elispot. Mean increases in the net frequency of Granzyme B following immunization were 145 ± 56 SFC/106 PBMC (range: 83-256 SFC/106 PBMC) (data not shown). A summary of the S. Typhi specific effector T cell responses observed in subjects orally immunized with CVD 909 is presented in Table 1.
4. Discussion
Over the past decade we showed that immunization with several strains, including Ty21a (the licensed vaccine), CVD 906 (an ΔaroC ΔaroD mutant), CVD 908 (an ΔaroC ΔaroD mutant) and CVD 908htrA (an ΔaroC ΔaroD ΔhtrA mutant) resulted in the appearance in peripheral blood of sensitized lymphocytes that exhibit significantly increased proliferative responses and cytokine production, as well as classical and non-classical classI restricted CTL activity to purified S. Typhi antigens and S. Typhi-infected targets [5–7,17–20,23,25]. Moreover, we have shown that a single dose of CVD 909 also induced lymphoproliferative responses [21]. In the present study we addressed two key questions, (1) did the genetic manipulations involved in engineering CVD 908-htrA to constitutively express Vi affect its ability to elicit the full spectrum of S. Typhi specific CMI and (2) could these responses be enhanced by a second immunization. Moreover, we compared the CMI responses elicited by CVD 909 with those previously reported to be induced by other attenuated S. Typhi strains in humans [5–7,9–11,17–21,23,25].
Different cytokines might have an important role in the Salmonella infection. Here, we observed marked increases in IFN-γ, TNF-α and IL-10, but not IL-2, IL-4 or IL-5 in 3-day culture supernatants of PBMC stimulated with S. Typhi flagella and TypVac. To investigate the kinetics of the in vitro response, cytokine levels were also measured 6 days after stimulation. We observed markedly higher levels of IFN-γ after 6 days than after 3 days in culture. These results are consistent with our observations in previous studies using intracellular cytokine staining and flow cytometry indicating that this key cytokine is produced predominantly by CD4+ and CD8+ antigen-specific T cells, although a small proportion of the IFN-γ originates in NK cells [7,20]. In contrast to IFN-γ, we observed that IL 10 and TNF-α production was significantly decreased in day 6 culture supernatants (data not shown). Because cytokines secreted by cells of the innate immune response typically reach peak levels at earlier time points than those produced by antigen-specific specific T cells, it is reasonable to hypothesize that TNF-α and IL-10, were secreted, by cells of the innate immune response (e.g., monocytes/macrophages, NK cells). In fact, we have previously reported that these cytokines in response to S. Typhi are produced, at least in part, by monocytes/macrophages [8,26]. However, the fact that higher levels of IL-10 and TNF-α were consistently observed in supernatants from PBMC obtained following immunization than those recorded in pre-immune specimens (Fig. 3), indicates that a substantial proportion of IL-10 and TNF-α is also secreted by antigen-specific cells. Of note, IL-10 is a counter-regulatory cytokine secreted, among others, by CD4+ CD25+ regulatory T cells [28]. We are currently characterizing the IFN-γ secreting T cell subpopulations in CVD 909 vaccinees by intracellular cytokine staining and multichromatic flow cytometry. Preliminary data are supportive of the notion that T cells, rather than NK cells (i.e., CD3− CD56+ cells), are the predominant IFN-γ secreting cells also in CVD 909 vaccinees. Taken together, these results, resemble those observed with other attenuated S. Typhi strains, including the parent CVD 908-htrA strain [8,19,20,23,26]. These studies showed a dominance of Th1 responses (e.g., IFN-γ and TNF-α production), suggesting that these cytokines are likely to be involved in the elimination of this intracellular organism. These cytokines are likely to be important in resistance to S. Typhi infection by a variety of mechanisms, including augmentation of macrophage bactericidal activity and enhancement of antigen presentation [29–32].
In addition to cytokine levels in culture supernatants that measure the aggregate response of all cells in culture, another key measurement that enables comparisons among attenuated S. Typhi vaccine candidates is the determination of the ex vivo frequency of IFN-γ secreting cells, as well as that of CTL effectors able to kill S. Typhi-infected targets, both hallmarks of the host response elicited by oral immunization with attenuated S. Typhi strains [5–7,20,25]. Using a modified IFN-γ Elispot assay we have previously reported that the frequencies of S. Typhi-specific cells in responders ranged from 175–548 and from 117-752 SFC/106 PBMC in subjects vaccinated with Ty21a and CVD 908htrA, respectively [7,20]. These frequencies are similar to those observed in the present study (122–538 SFC/106 PBMC, Table 2) following immunization with CVD 909.
We have previously shown that CTL activity in subjects immunized with attenuated strains of S. Typhi is mediated by a granule-dependent, FAS-independent, mechanism [6]. In the present study we took advantage of this observation to determine the frequency of CTL effectors using a novel technique that measures the number of granzyme B secreting cells by Elispot [27]. We observed mean increases of 145 ± 56 SFC/106 PBMC (range: 83–256) in subjects immunized CVD 909. This is the first report of the frequency of CTL effectors in subjects immunized with attenuated strains of S. Typhi.
In spite that the present clinical trials involved a small number of volunteers, we were able to correlate the various CMI responses in a volunteer by volunteer basis (Table 1). As we reported previously in Ty21a vaccinees [7], robust correlations were observed between IFN-γ production and CTL activity by 51Cr-release assays (p<0.001, Pearson Product Moment Correlation Test). However, no correlations were observed either between CTL by 51Cr-relase assays and Granzyme-B production or between IFN-γ and Granzyme-B production. Among the factors that might be responsible for this lack of correlation are a lower level of sensitivity and a certain degree of non-specificity in Granzyme-B Elispot assays when compared to other techniques. However, because the Granzyme-B Elispot assay is a novel technique, and the number of volunteers evaluated to date is limited, it is not possible at present to draw firm conclusions from the results observed using this assay.
As discussed above, in the present study we addressed the important question of whether proliferation and other CMI responses could be enhanced by the administration of a second dose of CVD 909 14 days after the first dose. We observed that the administration of a second dose did not result in increases in the number of responders or the magnitude of the responses (Tables 1, 2; Figs. 2, 3; data not shown). Since the second dose was administrated 14 days after the first one, the host might have had sufficient time to mount specific immunity against the vaccine strain. In fact, it is generally accepted that CMI might develop as early as 7 to 10 days after exposure to the organism. Thus, it is reasonable to speculate that immunity against CVD 909 generated by the first dose interferes with a subsequent second dose by contributing to rapidly eliminate the organism, leading to decreased immunogenicity. Alternatively, it is possible that a single dose of attenuated S. Typhi CVD 909 induces a “near maximal” response in terms of numbers and effectiveness of memory T cells above which threshold increases in magnitude or function are difficult to achieve and do not result in further benefits for protection [33]. This might be the case with CVD 909 and other attenuated vaccines which are able to replicate for a short period of time in the host leading to effective immunity to the vaccine strain. Another possibility is that macrophages secreting large amounts of nitric oxide might still be present 14 days after the first dose lead to depressed adaptive immune responses, a phenomenon reported in mice using attenuated Salmonella typhimurium strain SL3235 [34,35]. Interestingly, no increases in CMI were observed with the 2 dose regimen despite the fact that the inocula with this regimen were somewhat higher than the CFU administered in the single dose study. Detailed longitudinal studies with larger numbers of volunteers will be required to confirm our results and to shed additional light on the mechanisms underlying this phenomenon. If confirmed, these observations might have broad implications for other attenuated vaccines.
Taken together, these observations, as well as those recently published [21], add further support to the contention that CVD 909 appears to be as immunogenic after one or two doses as the parent strain (CVD 908htrA) or the licensed Ty21a vaccine, which confers moderate protection in subjects following 3–4 doses of the lyophilized formulation in enteric-coated capsules [2,12,14,15,36–38]. These results support the notion that expression of the capsule did not interfere with the ability of CVD 909 to elicit a wide array of CMI responses, and provide additional impetus for the continuing evaluation of CVD 909 as a leading oral typhoid vaccine candidate.
Acknowledgments
We are indebted to the volunteers who allowed us to perform this study. We also thank Kathleen Palmer, Catherine Black, Ron Grochowski, Brenda Berger, Theresa Mowry, Elizabeth Peddicord, and Elisa Sindall from the Adult Clinical Studies Section at the Center for Vaccine Development for their help in collecting blood specimens and Ms. Regina Harley for excellent technical assistance.
Footnotes
This work was supported by grants R01-AI036525 (to M.B.S) and M01-6616500 (GCRC, to C.O.T.) and contracts N01-AI30028 (IRU-FWD-IRN, to M.B.S.) and N01-AI65299 (EPRU, to C.O.T.), all from the National Institute of Allergy and Infectious Diseases.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Levine MM, Galen JE, Tacket CO, Barry EM, Pasetti MF, Sztein MB. Attenuated strains of Salmonella enterica serovar Typhi as Live Oral Vaccines Against Typhoid Fever. In: Levine MM, Kaper JB, Rappuoli R, Liu M, Good M, editors. New Generation Vaccines. 3. New York, NY: Marcel Dekker, Inc.; 2004. pp. 479–86. [Google Scholar]
- 3.Rowe B, Ward LR, Threlfall EJ. Multidrug-resistant Salmonella typhi: a worldwide epidemic. Clin Infect Dis. 1997;24:S106–9. S106–S109. doi: 10.1093/clinids/24.supplement_1.s106. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 4.Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine. 1996;14:435–38. doi: 10.1016/0264-410x(95)00186-5. [DOI] [PubMed] [Google Scholar]
- 5.Salerno-Goncalves R, Wahid R, Sztein MB. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vβ repertoires. Infect Immun. 2005;73:3521–30. doi: 10.1128/IAI.73.6.3521-3530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a Human HLA-E-Restricted CD8+ T Cell Subset in Volunteers Immunized with Salmonella enterica Serovar Typhi Strain Ty21a Typhoid Vaccine. J Immunol. 2004;173:5852–62. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 7.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2002;169:2196–203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 8.Wyant TL, Tanner MK, Sztein MB. Potent Immunoregulatory Effects of Salmonella typhi Flagella on Antigenic Stimulation of Human Peripheral Blood Mononuclear Cells. Infect Immun. 1999;67:1338–46. doi: 10.1128/iai.67.3.1338-1346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilhamn J, Lundin SB, Brevinge H, Svennerholm AM, Jertborn M. T- and B-cell immune responses of patients who had undergone colectomies to oral administration of Salmonella enterica serovar Typhi Ty21a vaccine. Clin Diagn Lab Immunol. 2003;10:426–30. doi: 10.1128/CDLI.10.3.426-430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viret JF, Favre D, Wegmuller B, Herzog C, Que JU, Cryz SJ, Jr, et al. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect Immun. 1999;67:3680–85. doi: 10.1128/iai.67.7.3680-3685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundin BS, Johansson C, Svennerholm AM. Oral immunization with a Salmonella enterica serovar typhi vaccine induces specific circulating mucosa-homing CD4(+) and CD8(+) T cells in humans. Infect Immun. 2002;70:5622–27. doi: 10.1128/IAI.70.10.5622-5627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine MM. Typhoid Fever Vaccines. In: Plotkin SA, Mortimer EA, editors. Vaccines. 2. Philadelphia: W. B. Saunders, Co.; 1994. pp. 597–633. [Google Scholar]
- 13.Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-Old children in vietnam. Infect Immun. 1999;67:5806–10. doi: 10.1128/iai.67.11.5806-5810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17 :S22–S27. doi: 10.1016/s0264-410x(99)00231-5. Suppl 2. [DOI] [PubMed] [Google Scholar]
- 15.Engels EA, Falagas ME, Lau J, Bennish ML. Typhoid fever vaccines: a meta-analysis of studies on efficacy and toxicity. BMJ. 1998;316:110–16. doi: 10.1136/bmj.316.7125.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine MM, Galen J, Barry E, Noriega F, Chatfield S, Sztein M, et al. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J Biotechnol. 1996;44:193–96. doi: 10.1016/0168-1656(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 17.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Nataro JP, Edelman R, et al. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–56. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tacket CO, Galen J, Sztein MB, Losonsky G, Wyant TL, Nataro J, et al. Safety and Immune Responses to Attenuated Salmonella enterica Serovar Typhi Oral Live Vector Vaccines Expressing Tetanus Toxin Fragment C. Clin Immunol. 2000;97:146–53. doi: 10.1006/clim.2000.4924. [DOI] [PubMed] [Google Scholar]
- 19.Tacket CO, Sztein MB, Wasserman SS, Losonsky G, Kotloff KL, Wyant TL, et al. Phase 2 clinical trial of attenuated Salmonella enterica serovar typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers . Infect Immun. 2000;68:1196–201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, Levine MM, et al. Concomitant Induction of CD4(+) and CD8(+) T Cell Responses in Volunteers Immunized with Salmonella enterica Serovar Typhi Strain CVD 908-htrA. J Immunol. 2003;170:2734–41. doi: 10.4049/jimmunol.170.5.2734. [DOI] [PubMed] [Google Scholar]
- 21.Tacket CO, Pasetti MF, Sztein MB, Livio S, Levine MM. Immune responses to an oral typhoid vaccine strain that is modified to constitutively express Vi capsular polysaccharide. J Infect Dis. 2004;190:565–70. doi: 10.1086/421469. [DOI] [PubMed] [Google Scholar]
- 22.Wang JY, Noriega FR, Galen JE, Barry E, Levine MM. Constitutive expression of the Vi polysaccharide capsular antigen in attenuated Salmonella enterica serovar typhi oral vaccine strain CVD 909. Infect Immun. 2000;68:4647–52. doi: 10.1128/iai.68.8.4647-4652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sztein MB, Wasserman SS, Tacket CO, Edelman R, Hone D, Lindberg AA, et al. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–17. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 24.Samandari T, Kotloff KL, Losonsky GA, Picking WD, Sansonetti PJ, Levine MM, et al. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164:2221–32. doi: 10.4049/jimmunol.164.4.2221. [DOI] [PubMed] [Google Scholar]
- 25.Sztein MB, Tanner M, Polotsky Y, Orenstein JM, Levine MM. Cytotoxic T Lymphocytes After Oral Immunization with Attenuated Vaccine Strains of Salmonella typhi in Humans. J Immunol. 1995;155:3987–93. [PubMed] [Google Scholar]
- 26.Wyant TL, Tanner MK, Sztein MB. Salmonella typhi Flagella are Potent Inducers of Proinflammatory Cytokine Secretion by Human Monocytes. Infect Immun. 1999;67:3619–24. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rininsland FH, Helms T, Asaad RJ, Boehm BO, Tary-Lehmann M. Granzyme B ELISPOT assay for ex vivo measurements of T cell immunity. J Immunol Methods. 2000;240:143–55. doi: 10.1016/s0022-1759(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 28.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–71. 256–71. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 29.Mastroeni P, Villarreal Ramos B, Hormaeche CE. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro-Salmonella vaccines. Microb Pathog. 1992;13:477–91. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 30.Tite JP, Dougan G, Chatfield SN. The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol. 1991;147:3161–64. [PubMed] [Google Scholar]
- 31.Green SJ, Crawford RM, Hockmeyer JT, Meltzer MS, Nacy CA. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990;145:4290–97. [PubMed] [Google Scholar]
- 32.Mastroeni P, Harrison JA, Robinson JH, Clare S, Khan S, Maskell DJ, et al. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–76. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Lee JC, Gibson CW, Eisenstein TK. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985;91:75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- 35.al Ramadi BK, Brodkin MA, Mosser DM, Eisenstein TK. Immunosuppression induced by attenuated Salmonella Evidence for mediation by macrophage precursors. J Immunol. 1991;146:2737–46. [PubMed] [Google Scholar]
- 36.Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet. 1990;336:891–94. doi: 10.1016/0140-6736(90)92266-k. [DOI] [PubMed] [Google Scholar]
- 37.Ferreccio C, Levine MM, Rodriguez H, Contreras R. Comparative efficacy of two, three, or four doses of Ty21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. JID. 1989;159:766–69. doi: 10.1093/infdis/159.4.766. [DOI] [PubMed] [Google Scholar]
- 38.Black RE, Levine MM, Ferreccio C, Clements ML, Lanata C, Rooney J, et al. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Vaccine. 1990;8:81–84. doi: 10.1016/0264-410x(90)90183-m. [DOI] [PubMed] [Google Scholar]


