Abstract
When Drosophila adults are placed into an open field arena, they initially exhibit an elevated level of activity followed by a reduced stable level of spontaneous activity. We have found that the initial elevated component arises from the fly's interaction with the novel arena since: (1) the increased activity is independent of handling prior to placement within the arena, (2) the fly's elevated activity is proportional to the size of the arena, and (3) the decay in activity to spontaneous levels requires both visual and olfactory input. These data indicate that active exploration is the major component of elevated initial activity. There is a specific requirement for the kurtz nonvisual arrestin in the nervous system for both the exploration stimulated by the novel arena and the mechanically stimulated activity. kurtz is not required for spontaneous activity; kurtz mutants display normal levels of spontaneous activity and average the same velocities as wild-type controls. Inhibition of dopamine signaling has no effect on the elevated initial activity phase in either wild-type or krz1 mutants. Therefore, the exploratory phase of open field activity requires kurtz in the nervous system, but is independent of dopamine's stimulation of activity.
LOCOMOTION is a fundamental and vital behavior. Through movement, animals can exert control over their surroundings to locate essential resources and avoid hazards. Without efficient and productive movement, animals can be easily preyed upon. Experimentally, we rely on an animal's movement to instruct us on the acuity of sensory systems, the vigor of courtship, and even the strength of learned associations. Locomotion, however, is a complex biological response encompassing sensory processing, integration of stimuli, executive functions, and motor response pathways. By understanding the genetic determinants underlying an animal's decision to move, we may gain significant insights into the molecular mechanisms involved in all of these levels of processing.
Activity in an open field arena is one of the oldest and most widespread experimental behavioral tasks (Hall 1936; Walsh and Cummins 1976). This task is typically used as a simple measure of general activity. Many species, however, display nonlinear activity profiles when first placed into an open field, indicating a complexity in this behavior. Drosophila melanogaster, several species of rodents, chickens, domestic cats, and dogs all demonstrate an elevated level of initial activity, followed by a rapid decline to a lower steady-state level of ambulation when assayed in an open field arena (Glickman and Hartz 1964; Connolly 1967; Candland and Nagy 1969; Lát and Gollová-Hemon 1969). Some insight into the initial elevated activity component came when Ewing (1963) and Connolly (1967) selectively bred Drosophila for differences in locomotor activity. The selected genotypes were actually found to have differences in how they react to stimulation, including the presence of other flies. The highly reactive flies had more activity because they were more vigorously running away from the other flies present in the apparatus (Ewing 1963). These flies also displayed differences in initial activity when individually placed into an open field arena, but not in the steady-state level of spontaneous activity (Ewing 1963; Connolly 1967). This early, elevated component of activity has been consequently termed reactivity or stimulated activity (Meehan and Wilson 1987). Surprisingly, very little is known about why animals increase their activity when placed into the open field, what the proximal causes for this stimulated activity are, or what the genetic determinants are that govern this response.
Herein we demonstrate that the kurtz (krz) gene of D. melanogaster is required within the nervous system for the elevated initial activity phase in an open field arena, but is not required for spontaneous activity. The krz gene encodes the only nonvisual arrestin in Drosophila (Roman et al. 2000). Nonvisual arrestins are important scaffolding proteins that regulate the activity of several families of cell-surface receptors, including G-protein-coupled receptors (GPCRs) (Lefkowitz and Whalen 2004). The arrestins are indispensable players in the agonist-dependent desensitization of GPCRs and are required to keep low levels of ligand from saturating cellular responses.
Since the nature of the elevated initial activity phase in Drosophila was unknown, we further explored this behavior to better understand the krz phenotype. We show here that the elevated initial activity phase is an evolutionarily conserved response to the arena and is principally composed of exploration, defined as behavioral acts that are evoked by novelty, and we provide information about the surrounding environment (Crusio and van Abeelen 1986). A role for dopamine in initial activity had been previously suggested (Meehan and Wilson 1987). We show that the requirement for krz in elevated initial activity is independent of dopamine signaling and that dopamine is most likely dispensable for the response evoked by the novel arena.
MATERIALS AND METHODS
Fly stocks and genetics:
All stocks were raised and maintained on standard yeast–cornmeal agar food at room temperature. Flies that were used in behavioral assays, unless otherwise noted, were raised on standard food at 25°, 60% humidity, with 12 hr of light/day. The krz1 allele, the b5.8T4 genomic krz transgene, and the UASkrzT5 and UASkrzT12 cDNA transgenes were originally described in Roman et al. (2000). The following fly stocks were all obtained from the Bloomington Stock Center: P{hspGal489-2-1}, c155elavGal4, P{UASLacZ}Bg4-1-2, norpA7, gl2, AntpNS/+, and Dr1/TM3, P{hs-hid}14, Sb1. The Drosophila virilis and Drosophila simulans stocks were obtained from the Tucson Stock Center. The TH-Gal4 driver was as described in Friggi-Grelin et al. (2003). The or83b2-bearing line was the generous gift of Leslie Vosshall (Rockefeller University). The AntpNS, Dr1, and or83b2 mutations were all outcrossed into Canton-S for a minimum of six generations before behavioral testing. The gl2 mutation was outcrossed to Canton-S for three generations prior to behavioral testing. Additionally, all transgenes were crossed into w1118[CS10] for a minimum of six generations; the Canton-S X chromosome was subsequently used to replace the w1118[CS10] X chromosome where noted. To generate inducible tetanus toxin light-chain expression, we recombined onto the same second chromosome the Gal80ts20 transgene (McGuire et al. 2003) and the UASTNT-H transgene (P{UAS-TeTxLC.tnt}H2) (Sweeney et al. 1995). The cross of TH-Gal4 × Gal80ts20, UASTNT-H was incubated at 18° for 1 week before parents were cleared. The progeny were raised at 18°; under these conditions, Gal80ts20 effectively represses Gal4 (McGuire et al. 2003). The day before the experiment, males were selected randomly and placed in normal food vials either in the Gal80ts nonpermissive 32° for 14 hr or back into the permissive 18°. The flies were then removed to room temperature and used for open field experiments. A 14-hr induction at 32° was chosen for the Gal80ts20-containing genotypes since it is sufficient to induce lethality with several different Gal4 drivers and the UASTNT-H transgene and to induce UAS reporter expression to levels approximating the Gal4 expression in the absence of the Gal80ts20 repressor (data not shown).
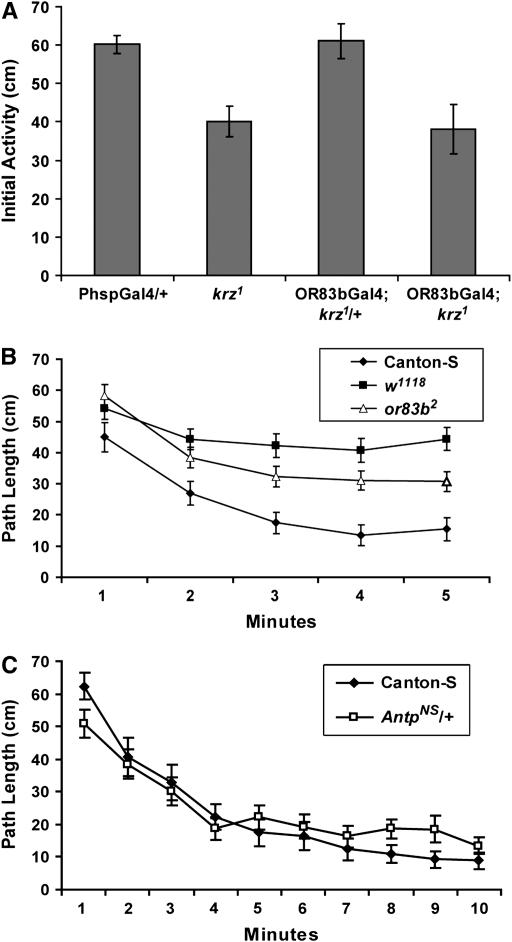
To generate adult flies that are deficient in krz expression, we used heat-shock Gal4-induced krz activity to rescue the developmental lethality of the krz1 allele (Roman et al. 2000). The following cross was used to generate the krz1 homozygotes: krz1, P{hspGal489-2-1}/TM3, P{hs-hid}14 × P{UASkrzT5}; krz1/TM3, P{hs-hid}14. The embryo and first instar larva progeny from this cross were placed in a cycling incubator for 10 days. This incubator provided 1.5 hr of 37° heat shocks every 12 hr. After the 10th day in the cycling chamber, pupa and late third instar larvae were removed to room temperature until eclosion. The homozygous adults were selected and then placed at 18° for 4 days immediately after eclosion. These same conditions were used to raise all control flies for experiments that included the krz1 homozygotes. The OR83bGal4; krz1 rescued flies were generated as stated in Ge et al. (2006), using the second chromosome OR83bGal4 driver (Kalidas and Smith 2002).
The pharmacological manipulation of dopamine synthesis was accomplished by feeding the flies 3-iodatyrosine (3-IY) or l-dopa (Sigma, St. Louis) according to the methods of Bainton et al. (2000).
Immunohistochemistry and in situ hybridization:
The anti-krz polyclonal antibodies were prepared as described in Ge et al. (2006). The immunohistochemistry on paraffin sections and in situ hybridization on cryosection experiments were performed largely as previously described with a few modifications (Roman et al. 1998). A sense digoxigenin–UTP-labeled riboprobe for in situ hybridization was made by digesting the p478d cDNA (pSK vector) with XhoI and utilizing T3 RNA polymerase according to the manufacturer's protocols (Boehringer Mannheim, Indianapolis). The antisense riboprobe was made using the p478d cDNA, digesting with XbaI, and the transcription with T7 RNA polymerase. For immunohistochemistry, paraffin was removed from the sections with 2-min incubations in xylenes (Fisher Scientific, Hampton, NH). The sections were next hydrated by successive 5-min incubations in a series of decreasing ethanol concentrations (100, 80, 60, 40, 20, and 0% ethanol). It was also necessary to perform antigen retrieval to detect KRZ. To uncover the KRZ antigen, the hydrated slides were placed in 1× PBS heated to 95° for 5 min. The slides were then removed and placed into 1× PBS at room temperature for 10 min prior to immunoblocking. Nonspecific antibody binding was blocked with a 4-hr incubation at room temperature in 5% normal goat sera (Sigma Chemicals, St. Louis) in 1× PBS. For these experiments, affinity-purified antibody was used at a concentration of 1:100. Antibody was detected using the Vectastain ABC kit (Vector Labs, Burlingame, CA).
Behavioral analysis:
The base of the open field arena was the bottom a 9.1-cm polystyrene petri plate and the top of the arena was made from the lid of a 15-cm petri plate (Fisher Scientific). A 2-mm hole was drilled in the top of the arena, near the side to allow for the aspiration of a fly into the arena. Since the top of the arena was larger than the bottom, the hole could be shifted out of the active arena area after the fly was added. The arena was illuminated by two 120-W flood lights. The output of these flood lights was controlled by rheostats and set to 4000 lx on the arena unless otherwise noted. The movement of the fly within the arena was tracked with either the HVS Image automated tracker or the Ethovision Tracking system (Noldus Information Technology, Leesburg VA). The trackers record the position of the fly in the X–Y plane every 100 msec. The collected data were then analyzed with Water2020 software (HVS Image), Ethovision Pro (Information Technology, Leesburg VA) or with the Wintrack software (Wolfer et al. 2001). Before beginning the experiments, it was determined that Canton-S had no preferences for individual arena quadrants. The measured variables included total path length, distance from center, the percentage of time spent in outermost one-third of arena, the percentage of time active, the number of stops (a stop is a pause lasting <5 sec), the number of rests (a rest is defined as a pause lasting >5 sec), and the average duration of a rest. The average velocity when moving was computed as path length/(percentage of time active × 60 sec). Each measure was determined for each successive minute in the apparatus. The percentage of time spent in each section of the 5.5-cm2 arena was determined using the HVS field data acquisition and analysis software (HVS Image). The automated video trackers were able to follow the flies for a minimum of 97.1% of the time. The analyzed data were imported into StatView v5.0.1 (SAS Institute, Cary NC) for statistical analysis. The activity in the Trikinetics (Waltham, MA) activity monitor was determined according to the manufacturer's recommendations. Flies that died during the experiment were removed from the subsequent analyses.
To measure activity after mechanical stimulation, the 9.1-cm circular arena was mounted on a Vortex Genie II (VWR Scientific). The arena's distance from the lighting and camera were kept the same as in previous experiments. Immediately after the end of the 5 min within the arena, the vortex (set at level 2.5) was pulsed for 1 sec. This pulse generated a moderate shaking of the arena, without dislodging the fly from its position. After the pulse, tracking was again initiated for another 5 min.
RESULTS
Rescued krz1 homozygotes have undetectable levels of krz immunoreactivity within the central nervous system:
We examined the sites of krz expression using both immunohistochemistry and in situ hybridization (Figure 1). In paraffin sections, KRZ was detected in almost all tissues of the adult, in both males and females. However, the highest level of KRZ expression was within the neuropil of the central nervous system (CNS) (Figure 1A). Previously, KRZ was also detected at high levels in the sensory systems of the second and third antennal segments (Ge et al. 2006). The second antennal segment contains Johnston's organ, the primary auditory organ for adult Drosophila, while the third antennal segment is the primary olfactory organ of the fly (Miller 1950). Within the CNS, high levels of expression were found in segments of the olfactory system, including the antennal lobe, and the calyces of the mushroom bodies (Figure 1A). Relatively lower levels of KRZ were found in the lobes of the mushroom bodies and in the ellipsoid body. KRZ levels were essentially undetectable within the cell body layer of the central cortex and the photoreceptor neurons (Figure 1A; data not shown). The krz mRNA was also more highly expressed within the nervous system than the surrounding tissues (Figure 1B). The expression of krz message within the CNS was both widespread and uniform. Hybridization was not detected in the sense probe control (data not shown). The expression of the krz mRNA appears to be ubiquitous within the CNS, consistent with the expression of KRZ protein. This general expression of krz, a modulator of G-protein signaling, within the nervous system suggests a broad role for this gene in behavioral plasticity.
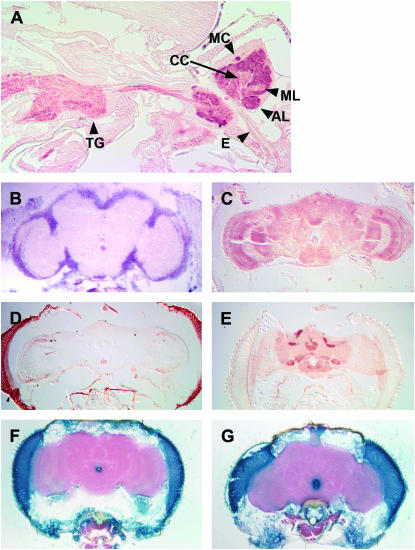
Figure 1.—
kurtz expression in the CNS of wild-type and mutant animals. KURTZ was detected in paraffin sections using immunohistochemistry (A, C, D, and E). The frontal sections were performed and developed in parallel; sections shown in C and D are from the same slide. The krz message was detected by in situ hybridization on cryosections (B). (A) A sagittal section of a w1118 adult female. The head is slightly tilted in this section. KURTZ is seen as reddish-brown staining throughout the neuropil of the CNS, including in the thoracic ganglia (TG). The esophagus (E) divides the brain in this section. The edge of the mushroom body's calyx (MC) is seen as the dark reddish-brown spot on the posterior slope of the brain. The antennal lobe (AL) is also heavily stained. The mushroom body horizontal lobes (ML) and the central complex (CC) are more lightly stained. (B) krz message is detected by in situ hybridization on a w1118 female head. Abundant and widespread expression is detected throughout the CNS cell-body layer. (C) KURTZ expression in a wild-type female head, frontal section. (D) KURTZ expression in the CNS of a female P{UASkrzT5}/+; krz1/krz1, PhspGal4 fly, frontal section. (E) KURTZ expression in the CNS of a female c155/+; P{UASkrzT5}/+; krz1 fly. (F) P{UASLacZ}/+; PhspGal4/+, raised at 18°. β-Galactosidase activity is shown in blue. (G) P{UASLacZ}/+; PhspGal4/+, 3 hr after a 30-min, 37° heat shock. β-Galactosidase expression is shown in blue.
To investigate a role for krz in regulating behavior, it was necessary to rescue the krz1 loss-of-function allele through development. The krz1 allele has a lethal phenotype that results from a P-element insertion within the only intron of this gene. In krz1 homozygous larvae, krz mRNA is undetectable by RT–PCR, indicating a loss of function (Roman et al. 2000). The krz1 lethal phase is protracted, occurring throughout embryonic and larval development. To surmount this problem, krz1 homozygotes were rescued to adulthood by using heat-induced Gal4 to drive the expression of a krz cDNA ubiquitously during development (Ge et al. 2006). The genotypes of these rescued homozygotes are listed in Table 1. The expression of KRZ in wild-type control and krz1 rescued homozygotes were compared in parallel by immunohistochemistry (Figure 1, C and D). Under these conditions, KRZ immunoreactivity is prominent in the CNS of wild-type flies (Figure 1C). In the developmentally rescued krz1 homozygotes, we detected strong immunoreactivity in eyes and slight immunoreactivity in the optic lobes (Figure 1D). No immunoreactivity was ever detected in the central brain neuropil of the krz1 developmentally rescued homozygotes during seven independent experiments. In contrast to these krz1 rescued homozygotes, the c155 pan-neuronal rescued krz1 homozygotes expressed krz in all areas of the nervous system (Figure 1E). In these animals, however, krz expression is elevated in the central complex, antennal lobes, and mushroom bodies as compared to wild type (Figure 1E). The absence of krz expression in the CNS of adult krz1 rescued homozygotes is facilitated by a property of the PhspGal4 driver. This driver is very poor at inducing the LacZ reporter gene expression in the adult CNS (Figure 1, F and G). In the uninduced state, β-galactosidase activity was detected in a manner very similar to that of krz in the krz1 developmentally rescued homozygotes (Figure 1, D and F). This driver therefore provides krz expression during development to rescue lethality, but then turns off in the adult nervous system. The KRZ protein found in the optic lobe neuropil may be located in the axons of the photoreceptor neurons that innervate both the lamina (R1-6) and the medulla (R7 and R8), and not within the neurons of the CNS. The P{UASLacZ}/+; PhspGal4/+ flies have abundant β-galactosidase activity within these pho-toreceptor cells, but not within the neurons of the optic lobes (Figure 1, F and G). KRZ protein was detected in krz1/+ flies at levels similar to the those of control wild-type flies (data not shown). These data indicate that KRZ levels are very low in the central brain of the developmentally rescued krz1 homozygotes. The absence of krz expression within the CNS and antenna indicates that these krz1 rescued homozygous adults, which have been rescued through development by the induced expression of a krz cDNA, can be used as strong reduction-of-function mutants for behavioral analysis.
TABLE 1.
Genotypes of the flies examined in the open field assays
| Flies | Expanded genotype | Description | References |
|---|---|---|---|
| Canton-S | +; +; + | Wild-type D. melanogaster | |
| w1118 | w1118; +; + | Background white allele outcrossed to the Canton-S for 10 generations | |
| krz1 | w1118; P{UASkrzT5}/+; krz1/krz1, P{hspGal489-2-1}a | krz1 heat-shocked rescued genotype; missing krz in the adult CNS | Roman et al. (2000); this study |
| krz1/+ | w1118; P{UASkrzT5}/+; krz1/ P{hspGal489-2-1} | Heterozygous control for krz1 | Roman et al. (2000) |
| PhspGal4/+ | w1118; +; P{hspGal489-2-1}/+ | Heat-inducible Gal4 driver | Brand and Perrimon (1993) |
| c155; krz1 | c155, w1118; P{UASkrzT5}/+; krz1 | elavGal4; krz1 rescued genotype, expressing a krz cDNA throughout the nervous system | Roman et al. (2000) |
| c155; krz1/ + | c155, w1118; P{UASkrzT5}/+; krz1/+ | Heterozygous control for c155; krz1 | Roman et al. (2000) |
| b5.8T4; krz1 | w1118; P{krzb5.8T4}; krz1 | krz1 genomic transgene rescued genotype | Roman et al. (2000) |
| w+; krz1 | +; P{UASkrzT12}/+; krz1/krz1, P{hspGal489-2-1} | krz1 heat-shocked rescued genotype containing wild-type first chromosome | This study |
| norpA7 | +; +; norpA7 | Phospoholipase C mutation; blind | Harris and Stark (1977) |
| gl2 | +; +; gl2 | Missing photoreceptor cells; blind | Moses et al. (1989) |
| Dr1/+ | +; +; Dr1/+ | Severely reduced number of ommatidia | Krivshenko (1954) |
| THGal4/TNT | +; THGal4/P{UASTNT-H}, Gal80ts20; + | Inducible tetanus toxin light-chain expression in most dopaminergic neurons | Friggi-Grelin et al. (2003) |
| or83b2 | +; +; or83b2 | Or83b mutation; anosmic | Larsson et al. (2004) |
| OR83bGal4; krz1 | w1118; P{UASkrzT12}/OR83bGal4; krz1/krz1, P{hspGal489-2-1} | krz1 heat-shocked rescued genotype with krz expression in olfactory receptor neurons | Ge et al. (2006) |
| OR83bGal4; krz1/+ | w1118; P{UASkrzT12}/OR83Gal4; krz1, P{hspGal489-2-1}/+ | Heterozygous control for ORGal4; krz1 | Ge et al. (2006) |
| AntpNS/+ | +; +; AntpNS/+ | Antenna is converted to mesothoracic leg, missing arista | Scott et al. (1983) |
In Figure 2D, krz1 homozygotes were generated with the P{UASkrzT12} transgene in place of P{UASkrzT5}.
krz is specifically required for the elevated initial activity phase in Drosophila:
As a first step in defining the potential functions for krz in behavioral plasticity, we examined the role for this gene in regulating locomotor activity in adults. Locomotor activity of the developmentally rescued krz1 homozygous adults was measured in an open field circular arena, 0.7 cm high and 9.1 cm in diameter and constructed of clear polystyrene. The limited height restricted most movement to the X–Y plane. The data were collected with an automated video tracking system, and activity was measured as the path length per minute. The krz1 developmentally rescued adult males were examined in this open field assay for 10 min and compared to both wild-type Canton-S and a w1118 genetic background control group (Figure 2A). The w1118 allele used in these experiments had previously been outcrossed into Canton-S for 10 generations [w(CS10)]. Furthermore, we tested the rescued krz1 homozygotes with a wild-type X chromosome, providing the mutants with wild-type levels of eye pigments and a Canton-S genetic background.
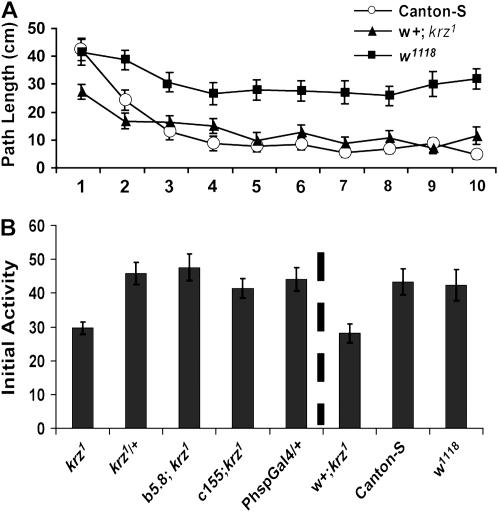
Figure 2.—
krz is required for the initial activity phase in an open field arena. (A) Activity was measured in the circular open field arena. n = 24 for each genotype. There is a significant effect of both time (F = 9.02; P < 0.001) and genotype (F = 72.44; P < 0.001) on activity. The w+; krz1 homozygotes were significantly different from Canton-S control flies only during the first minute (Bonferroni–Dunn, P = 0.005). (B) The path length during the first minute in the open field arena (“Initial Activity”) for male krz1 and control genotypes (Table 1) is shown. n = 24 for each genotype. The initial activity from the first experiment is also shown for comparison, separated by a vertical dashed line. The krz1 homozygotes are significantly less active than PhspGal4/+ control flies (Bonferroni–Dunn, P < 0.002). There were no significant differences between the krz1 heterozygotes, c155; krz1 and the b5.8; krz1, and the PhspGal4/+ controls.
The initial activities of the w1118 and Canton-S flies were virtually the same (Figure 2A; P = 0.866). Canton-S flies then demonstrated a decline in activity during the next few minutes. This activity profile of Canton-S is qualitatively equivalent to the previously reported stimulated activity and the decay in stimulated activity to a steady-state level of spontaneous activity seen in D. melanogaster (Connolly 1967; Meehan and Wilson 1987; Martin et al. 1999). Yet, the w1118 flies did not reduce their activity to the same extent as Canton-S males did during the first 10 min, suggesting a role for visual acuity in the activity decay or in establishing the baseline of spontaneous activity (Kalmus 1943). The w1118 flies containing mini-white transgenes, such as c155, show partial-to-full rescue of the decay from elevated initial activity (data not shown).
During the first minute in the arena, the developmentally rescued w+; krz1 homozygotes had significantly lower levels of activity than the control genotypes. This initial difference in path length between Canton-S and w+; krz1 is primarily due to the percentage of time that the flies were moving (Canton-S, 82.0 ± 0.3%, w+; krz1, 67.0 ± 4.3%; P = 0.007) and not to the speed at which the flies were moving (Canton-S, 0.92 ± 0.09 cm/sec, w+; krz1, 0.81 ± 0.11 cm/sec; P = 0.464). During the first minute, the w+; krz1 homozygotes had the same number of stops as Canton-S (1.54 ± 0.23 and 1.63 ± 0.23, respectively; P = 0.989). However, the w+; krz1 homozygotes had longer periods of immobility as seen by a significant increase in the time spent resting compared to Canton-S flies (Canton-S, 6.65 ± 0.1.9 sec, w+; krz1, 16.0 ± 2.7 sec; P = 0.007). Rest is defined as a pause in movement lasting at least 5 sec.
The spontaneous activity of the developmentally rescued w+; krz1 mutants was not significantly different from Canton-S starting at the second minute (P = 0.114, Bonferroni–Dunn) and continuing through the tenth minute (Figure 2A; P = 0.249, Bonferroni–Dunn). By the second minute in the open field arena, the difference in the percentage of time spent in movement between these two genotypes had decreased (Canton-S, 65.2 ± 5.4%, w+; krz1, 50.7 ± 5.4%; P = 0.064), and the average speed stayed about the same (Canton-S, 0.81 ± 0.20 cm/sec, w+; krz1, 0.73 ± 0.22 cm/sec; P = 0.795). During the third minute, the differences between these genotypes in the percentage of time spent in movement had disappeared (Canton-S, 50.7 ± 5.4%, w+; krz1, 51.0 ± 4.4%; P = 0.957) and the average speed of these genotypes remained the same (Canton-S, 0.88 ± 0.34 cm/sec, w+; krz1, 0.84 ± 0.24 cm/sec; P = 0.919). Furthermore, there were no significant differences in spontaneous activity between the developmentally rescued krz1 homozygotes and w1118 measured in the dark over a period of days using a Trikinetics activity monitor (data not shown). In summary, the developmentally rescued krz1 homozygous flies are capable of moving as fast as wild-type flies and do not differ from wild type in spontaneous activity levels, indicating that the these krz1 homozygotes do not have a problem with coordinated movement. The data therefore suggest that krz is specifically required for the elevated initial activity phase in an open field arena.
We examined several control genotypes to verify a role for krz1 in elevated initial activity (Figure 2B). Heterozygous flies (w1118; P{UASkrzT5}/+; krz1/P{hspGal489-2-1}; Table 1) were used as controls for possible dominant effects of any of the transgenes found within the developmentally rescued homozygous krz1 adults. Additionally, we examined krz1 homozygotes rescued by a genomic transgene and the c155 rescued flies, which have krz expression throughout the nervous system in a krz1 background (c155, w1118;P{UASkrzT5}/+; krz1; Table 1; Figure 1E). In this experiment, the krz1 homozygous flies demonstrated a severe reduction in path length traveled during the first minute compared to both wild-type and heterozygous controls (Figure 2B). The heterozygous controls in these experiments demonstrate that the krz1 activity deficit in the initial minute is recessive, which rules out the possibility that it is caused by the P{UASkrz}, PhspGal4, or a combination of both P-element insertions. Furthermore, this initial activity deficit was fully rescued both by a genomic krz transgene and by the directed expression of a full-length krz cDNA throughout the nervous system (Figure 2A). Hence, the activity during the first minute within the open field arena is dependent on krz activity within the nervous system. Since the developmentally rescued krz1 mutant flies display structural antenna defects with variable expressivity and penetrance (Ge et al. 2006), we also separately examined krz1 homozygotes with either severe antennal defects or normal antennal structures, but failed to find any significant differences between these phenotypic classes in open field arena activity (data not shown).
The high level of initial activity is a conserved response to the arena:
Since krz is the first gene identified with a defect in the elevated initial activity phase, and there is a paucity of data defining this particular behavior, we sought to learn more about this activity phase by investigating the proximal and ultimate causes for this behavior (Tinbergen 1963). If the elevation of initial activity has a general survival or reproductive value, then it should be conserved through evolution. When wild-type members of D. melanogaster, D. simulans, and D. virilis were placed into the open field arena, the stimulated activity, the decline in stimulated activity, and the spontaneous activity phases all were remarkably well conserved (Figure 3A). The maintenance of these components through evolution suggests that these behaviors confer a significant survival or reproductive advantage.
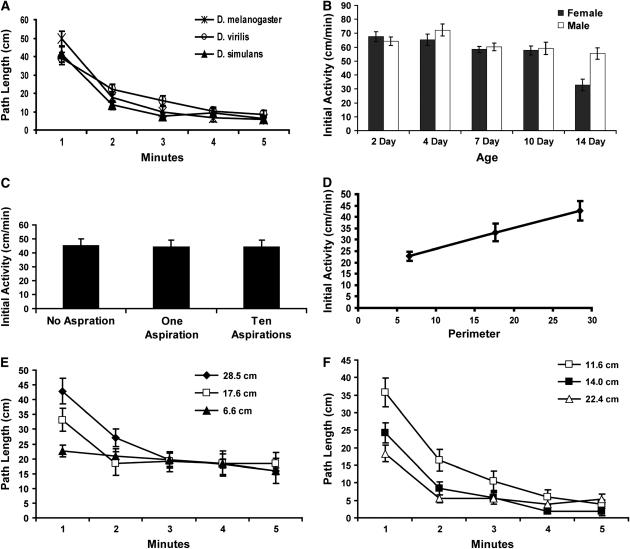
Figure 3.—
Elevated initial activity is a conserved response to the arena. (A) The open field activity is conserved in three species: D. melanogaster (Canton-S), D. simulans, and D. virilis. (B) The elevated initial activity phase is present in both male and female flies and in both young and older flies. Fourteen-day-old females displayed significantly reduced path lengths during the first minute in the open field arena (Bonferroni–Dunn, P < 0.001 for each comparison). There were no other significant differences in path length within the first minute. The extended data set can be found in supplemental Figure 1 at http://www.genetics.org/supplemental/. (C) The elevated initial activity phase is not affected by handling. The activity during the first minute within the circular arena is shown. n = 24 for each treatment. (D) The activity of Canton-S flies was measured in circular arenas of three sizes. n = 24 for each arena size. The elevated initial activity increased linearly with the perimeter size of these circular arenas (r2 = 0.999). (E) The path length of Canton-S was measured in circular arenas of three sizes. The circumferences of these arenas are shown. (F) The path length of Canton-S flies was measured in square arenas of three sizes. The perimeter lengths of these arenas are shown. Since in either square or circular arenas the flies spend the majority of time near the edge, the perimeter is the more relevant measure for arena size.
A distinctive ontogeny or sex specificity of elevated initial activity could suggest potential developmental or reproductive functions for the behavior. The elevated initial activity phase of both males and females is stable for the first 10 days posteclosion (Figure 3B; supplemental Figure 1 at http://www.genetics.org/supplemental/). However, at 14 days of age, females, but not males, show a significant decline in this stimulated activity phase (Bonferroni–Dunn, P < 0.001 for each comparison). The spontaneous activity phase also appeared to show distinct sexual dimorphism. Although path length was similar between males and females, males spent less time moving and had a longer duration of rests (supplemental Figure 1 at http://www.genetics.org/supplemental/). These data are consistent with males taking longer pauses, but compensating with faster movement, as was seen previously with a dissimilar apparatus (Martin et al. 1999). Thus, although spontaneous activity is modified by both age and sex, the elevated initial activity phase is present in both genders and in flies up to 10 days old, suggesting that this behavior has general utility for survival or reproduction.
We hypothesized that the elevated initial activity is likely a conserved response to novel stimuli. A source of these stimuli may be the experimenters handling of the fly—the fly may be agitated from being aspirated into the arena—or it may be due to a reaction to stimuli provided by the arena, such as novelty, open space, the absence of food, or bright lights. To address these hypotheses, we initially manipulated the amount of handling the flies received prior to being placed into the arena. In this experiment, posteclosion flies were housed individually for 3 days. They were then allowed to passively crawl from the food vial into the arena without aspiration, placed into the arena as before with a single aspiration, or aspirated into and out of a food vial 10 times within a span of 30 sec immediately before being placed into the arena (Figure 3C). Surprisingly, neither hyperagitation nor the lack of agitation had a measurable effect on the magnitude of the stimulated activity. The decline in initial activity and the amount of spontaneous activity also were not changed by these conditions (data not shown). We also found that posteclosion social isolation also did not affect open field behavior when compared to group-housed animals (data not shown).
If stimulated activity is not due to handling, then it may be a response to the arena itself. To test this hypothesis, we examined Canton-S flies for activity in arenas of different sizes and shapes (Figure 3, D–F). When the size of a circular arena is decreased, there is a coincident and linear decrease in the initial activity (Figure 3, D and F). In fact, in the smallest circular arena, the stimulated activity phase appeared to be completely absent. By the fifth minute, the activities in these circular arenas were essentially identical, suggesting that arena size does not alter spontaneous activity. In square arenas, there was a decrease in initial activity when the distance between corners was increased (Figure 3F). The stimulated activity changes in response to arena size and the interaction of this change with arena shape indicate that the flies respond to stimuli intrinsic to the different arenas.
Although the spontaneous activity in square arenas was not affected by arena size, it was significantly lower than the spontaneous activity in circular arenas (Figure 3, E and F). This result suggested that a component of the square arena, most probably the corners, may inhibit spontaneous activity relative to the circular arenas. To test this hypothesis, we reexamined wild type and the developmentally rescued w+; krz1 homozygote flies in a square arena (Figure 4). Both w+; krz1 and wild-type Canton-S males spend significantly more time in the corners than in other areas of the arena (Figure 4B). Loitering in the corners would explain the decreased spontaneous activity in square arenas as compared to the circular arenas. The sensory systems and executive functions that control the corner preference are not significantly affected in the rescued krz1 mutant flies, indicating that the activity of this gene is not required for these functions. Intriguingly, the two activity phases in the 22.4-cm2 arena of the developmentally rescued w+; krz1 homozygotes were also not significantly different from Canton-S (Figure 4C). There were, however, significant differences between the developmentally rescued w+; krz1 homozygotes and Canton-S in the smaller square arena (Figure 4D). Thus, the apparent inhibition of the elevated initial activity phase in the larger square arenas is capable of masking the krz1 phenotype.
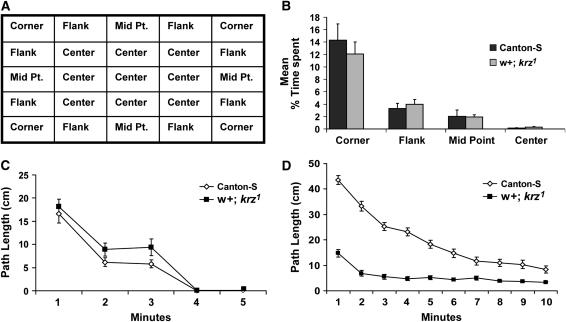
Figure 4.—
Wild-type and krz1 mutant flies prefer corners when in a square arena. Open field assays were performed with both male Canton-S and w+; krz1 homozygous flies within square arenas. (A) For this analysis, the 22.4-cm2 arena was divided into 25 sectors. These sectors were characterized as a corner, a flank located adjacent to a corner, a perimeter midpoint, and a center. The position of these sectors within the arena is shown. (B) The mean percentage of time that flies spent in each sector category ±SEM is shown. The time spent in a corner sector was significantly greater than the mean time spent in any of the other sector categories (Bonferroni–Dunn, P < 0.001). There were no significant differences in time spent in each sector between Canton-S and the w+; krz1 homozygous flies. The males spent a total of ∼54% of the time in the four corner sectors, 32% of the time in the eight flanking sectors, and 10% of the time in the midpoint sectors. These flies were also centrophobic, spending only 3% of the time in the nine central sectors. (C) The average path lengths ±SEM in w+; krz1 and Canton-S of the 22.4-cm2 arena are shown. There were no significant differences in activity in path length between these two genotypes in the square arena (F = 2.02, P = 0.156). n = 24 for each genotype. (D) The average path lengths ±SEM of w+; krz1 and Canton-S in the 11.6 cm2 arena are shown. There were significant differences in path length between the two genotypes (F = 617.6, P < 0.0001). These differences in path length were significant at each time point (Bonferroni–Dunn).
Dopamine is not required for exploratory activity:
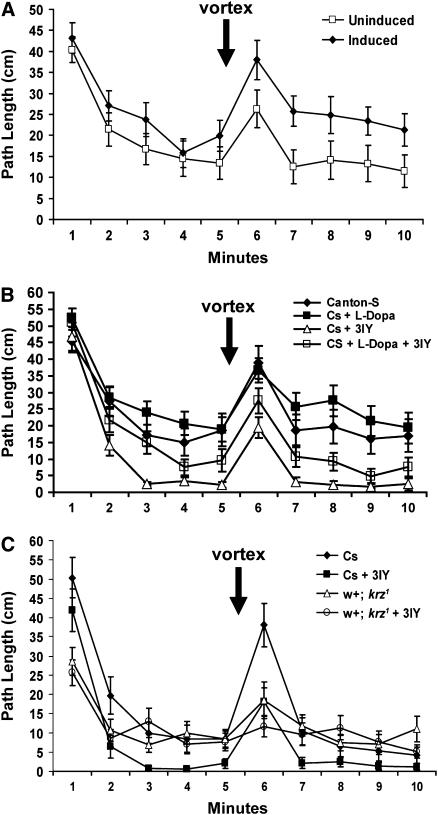
Previous experiments have suggested a role for dopamine in both ethanol-induced hyperactivity and mechanically stimulated activity (Bainton et al. 2000; Friggi-Grelin et al. 2003; Kume et al. 2005). It has been postulated that dopamine is a general regulator of arousal in Drosophila (Andretic et al. 2005; Kume et al. 2005). Since the elevated initial activity phase may represent stimulation by the novel arena, which should in principle be effected by arousal, we have examined the effects of dopamine on both initial activity and after mechanical stimulation using both the transgenic inhibition of dopaminergic neurotransmission and the reduction of dopamine levels pharmacologically (Figure 5). In our first experiment, we used a tyrosine hydroxylase-Gal4 line (TH-Gal4) to drive tetanus toxin light chain (TNT) in dopaminergic neurons (Friggi-Grelin et al. 2003). In this experiment we also utilized a temperature-sensitive Gal80 transgene to repress TNT expression during development [temporal and regional gene expression targeting (TARGET) system; McGuire et al. 2003]. In the TARGET system, Gal80ts is ubiquitously expressed and can conditionally repress Gal4. This Gal80ts transgene allowed us to examine uninduced flies, raised at 18°, as a within-genotype control. Interestingly, the induction of TNT in the dopaminergic neurons with a 14-hr incubation at the restrictive 32° had no apparent effect on either elevated initial activity phase or spontaneous activity phase (Figure 5A). However, after mechanical stimulation following the fifth minute, the induced flies exhibited significantly greater levels of activity, consistent with the results of Friggi-Grelin et al. (2003). These data suggest that neurotransmission from the neurons defined by the TH-Gal4 inhibits mechanically stimulated activity, but does not play a role either in the elevation of initial activity or in spontaneous activity within a circular arena.
Figure 5.—
Dopamine signaling is dispensable for the elevated initial activity within the open field arena. All flies were tested for 5 min in the circular open field arena. Immediately after the initial 5 min, the flies were mechanically agitated by moderate vortexing of the arena. The locomotion during the subsequent 5 min was then also recorded. Path lengths ±SEM are shown for each experiment. n = 24 for all groups. (A) Flies of the genotype UASTNT-H, Gal80ts20/+; TH-Gal4/+ were tested in the circular open field arena either after a 14-hr 32° induction of tetanus toxin light chain or without induction. The induction of tetanus toxin in the TH neurons had a significant effect on activity (F = 17.57, P < 0.0001). There were no significant differences in path length between the induced and uninduced flies during first 5 min (P = 0.078), but after mechanical stimulation the induced flies had significantly higher levels of activity (P < 0.0001). (B) Wild-type Canton-S males were tested in the open field after treatment with 3-IY, l-dopa, both, or neither. During the first 5 min, 3-IY significantly inhibited spontaneous activity as measured during the fifth minute (P <0.0001). This affect of 3-IY was partially reversed by l-dopa. (C) Wild-type Canton-S and w+; krz1 homozygous males were fed either 3-IY or a vehicle. These flies were then examined for activity in the open field arena. The 3-IY had a significant effect on the path length of Canton-S (P = 0.0029), but not of w+; krz1 (P = 0.800). The 3-IY did not have a significant effect on initial activity in either Canton-S (P = 0.194) or w+; krz1 (P = 0.651) males. However, during minutes 3, 4, and 5, 3-IY-treated Canton-S flies were significantly different from vehicle-treated Canton-S (P < 0.0001) and the 3-IY and vehicle-treated w+; krz1 (P < 0.0001 and P = 0.0002, respectively). Immediately after mechanical stimulation, the only significant comparisons were vehicle-fed Canton-S and either vehicle or 3-IY-treated w+; krz1 (P < 0.0011 and P = 0.0002, respectively).
In a second experiment examining the role of dopamine in open field behavior, the synthesis of this neurotransmitter was pharmacologically inhibited with 3-IY using the protocols of Bainton et al. (2000; Figure 5B). This treatment has been shown to reduce total dopamine content in the whole fly to <10% of wild-type levels (Bainton et al. 2000). In the circular open field arena, the depletion of dopamine failed to alter the level of initial activity, consistent with the previous transgenic approach (Figure 5A). However, a significant reduction in the level of spontaneous activity was found after dopamine depletion (Bonferroni–Dunn, P < 0.0001). This inhibition of spontaneous activity was partially rescued by feeding the flies l-dopa, the product of tyrosine hydroxylase, suggesting that it is the inhibition of this enzyme that causes the reduction in spontaneous activity. Mechanical stimulation increased the activity of each treatment by ∼15 cm during the first minute. The resulting activity after mechanical stimulation closely matched the spontaneous activity prior to mechanical stimulation (Figure 5B).
We next utilized 3-IY to inhibit tyrosine hydroxylase in Canton-S and the developmentally rescued w+; krz1 homozygous flies (Figure 5C). The pharmacological reduction in dopamine levels in either of these two genotypes did not have a significant effect on initial activity. Interestingly, the reduction of spontaneous activity in 3-IY-treated wild-type Canton-S flies was not found in the developmentally rescued w+; krz1 homozygotes, suggesting that krz is required for the inhibition of spontaneous activity by lowered dopamine levels (Figure 5C). After mechanical stimulation, both treated and untreated Canton-S flies increased their activity, an increase over spontaneous activity of ∼15 cm for the 3-IY-treated wild-type flies and of 25 cm for the untreated wild-type flies. It is not clear why the untreated Canton-S males were more responsive to mechanical stimulation in this experiment than in the previous experiment, but these activity levels were significantly higher than the 3-IY treated flies (Bonferroni–Dunn, P < 0.0001). Both the 3-IY-treated and -untreated developmentally rescued w+; krz1 homozygotes in this experiment failed to show a robust increase in activity after mechanical stimulation (Figure 5C). The results from this experiment are consistent with a positive role for dopamine in regulating spontaneous activity and mechanically stimulated activity, but not elevated initial activity. krz activity is required for both elevated initial activity and mechanically stimulated activity. Furthermore, krz activity is required for the lowered spontaneous activity brought about by 3-IY-mediated inhibition of dopamine synthesis. Finally, we have found that the two independent techniques for inhibiting dopamine signaling produced markedly different effects on activity within the circular arena.
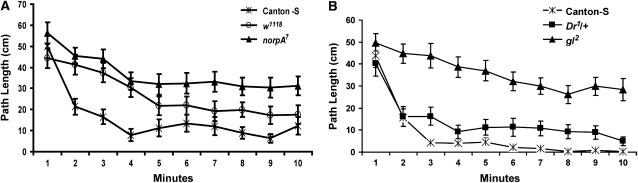
Visual acuity is required for the decay of initial activity:
The reduced decay of initial activity in the w1118 flies suggests that visual acuity is required for the decay from the elevated initial activity phase and is not required for the initiation of the elevated activity phase (Kalmus 1943; Figure 2A). The compound Drosophila eye is appositional, with each ommatidium being visually insulated by pigment cells. In the w1118 mutants, the screening pigments within these cells are not made and therefore neighboring ommatidia receive a superimposed image, resulting in poor visual acuity. These flies are positively phototactic, but perform some optimotor responses very poorly (Kalmus 1943). If poor visual acuity is responsible for the maintained high level of activity of w1118, then blind flies should also have similar defects in this assay. The norpA7 mutant flies lack phospholipase C activity, are physiologically blind, and maintain high levels of activity in the open field arena over the full 10-min assay (Table 1; Figure 6A). The gl2 mutant flies are also blind (they lack ommatidia) and they too maintain a high level of activity during the entire trial of 10 min (Table 1; Figure 6B). Moreover, we have found that the bw1; st1 double mutants, which also lack all screening pigments in the eye, phenocopy the w1118 activity phenotype (data not shown). These data show that flies with poor visual acuity maintain a high level of activity in the open field far longer than normally sighted flies. Remarkably, however, the Dr1/+ heterozygotes demonstrate a strong decline in activity after the first minute (Figure 6B). The Dr1 allele is a dominant gain-of-function mutation that results in a reduction from 700 to ∼10 ommatidia/eye. The activity profile of the Dr1/+ flies suggests that even a small number of functional ommatidia can lead to nearly normal activity decay, but the total lack of vision, or the poor acuity brought about by reduced screening pigments, leads to prolonged stimulated activity.
Figure 6.—
The decay of the elevated initial activity to spontaneous activity depends on visual acuity. (A) The average path length ±SEM in a circular arena for male Canton-S, w1118, and norpA7 flies. n = 24 for each genotype. There were no significant differences in path length between the three genotypes during the first minute. At 10 min, the path length of Canton-S was significantly less than norpA7 (Bonferroni–Dunn; P = 0.0065). This experiment was performed at 6000 lx. (B) Path length ±SEM in a circular arena for male Canton-S, gl2, and Dr1/+. There were no significant differences in path length between the genotypes during the first minute. Although Canton-S males were moving less than Dr1/+ during the third minute, the difference was not significant (P = 0.0446; Bonferroni–Dunn). The difference between gl2 males and either Canton-S or Dr1/+ flies during the third minute was significant (P < 0.0001 for both comparisons). During the tenth minute, the gl2 males were moving significantly more than either the Dr1/+ or the Canton-S males (P < 0.0001 for both comparisons), and the path lengths of the Dr1/+ and Canton-S males were not significantly different (P = 0.288). In both experiments, vision is required for the wild-type decay from stimulated activity. n = 24 for each genotype.
Olfaction is dispensable for elevated initial activity:
In addition to the elevated initial activity defect, the developmentally rescued krz1 homozygous adults also have blunted electrophysiological and behavioral responses to odorants (Ge et al. 2006). If the initial elevation in activity arises from a new odor in the open field, then the krz1 phenotype may be explained by the poor perception of this novel odor. We examined this possibility with three experiments. Both the physiological and behavioral olfactory defects of krz1 homozygotes can be rescued by the expression of krz in the olfactory receptor neurons (Ge et al. 2006). An OR83bGal4 driver that rescues the krz1 olfactory defect did not rescue the initial activity defect of developmentally rescued krz1 homozygotes, indicating that the olfactory phenotype of krz1 was not the source of the initial activity deficit (Figure 7A).
Figure 7.—
Olfaction is not required for elevated initial activity. (A) The rescue of the olfactory deficit in krz1 homozygotes does not rescue the elevated initial activity phenotype in the open field arena. The path length during the first minute in the circular open field arena is shown for the four genotypes. The full genotypes are as listed in Table 1. The OR83b; krz1 flies, which have normal levels of olfactory behavior and odorant receptor potentials (Ge et al. 2006), have significantly less initial activity than either the OR83bGal4; krz1/+ or the PhspGal4/+ control genotypes (P < 0.0015), but not significantly different from krz1 homozygotes (P = 0.754). (B) The anosmic or83b2 mutants display normal levels of initial activity in the open field arena. During the first minute, in the open field arena, there were no significant differences in path length between or83b2 and Canton-S (P = 0.023; significance with the Bonferroni–Dunn posthoc test requires a P-value of <0.0167) or w1118 (P = 0.47). However, during the fifth minute, the path length of the or83b2 mutants was greater than that of Canton-S (P = 0.003) and less than that of w1118 (P = 0.008). (C) The homeotic AntpNS/+ mutants do not show a significant difference from Canton-S in open field activity (F = 0.571, P = 0.45). n = 24 for each genotype.
We also examined flies homozygous for the broadly anosmic or83b2 mutation in the open field arena (Larsson et al. 2004; Figure 7B). Interestingly, the or83b2homozygotes did not move significantly more or less than the control genotypes during the first minute in the open field arena, but by the fifth minute their activity was intermediate between wild-type Canton-S and the visually defective w1118 (Figure 7B). This result suggested that olfactory input was an important component, albeit a lesser component than visual input, in the decay from initial activity to spontaneous activity.
In our third experiment to examine the role of the olfactory system in the elevated initial activity phase, we examined the homeotic AntennapediaNS mutation for activity defects (Figure 7C; Table 1). AntpNS is a dominant neomorphic allele that results in the transformation of the antenna into a mesothoracic leg with a high frequency of mutants lacking aristae (Jorgensen and Garber 1987; Kankel et al. 2004). The AntpNS/+ flies also demonstrate severe defects in the olfactory startle reflex, indicating a lack of olfactory acuity expected from the loss of the third antennal segment (McKenna et al. 1989). Prior to this experiment, the AntpNS mutation was outcrossed to Canton-S for 10 generations. The subsequently selected AntpNS/+ heterozygotes used for activity measures lacked arista, but appear to have normal maxillary palps (data not shown). Although the AntpNS/+ flies displayed lower levels of ambulation during the first minute, this difference was not significant (P = 0.0562). The overall level of activity between these flies without normal antenna sensory input and the control Canton-S genotype was also not significant (P = 0.45). The failure to see significant differences in activity suggests that the antenna plays a minor role, if any, in the expression of the elevated initial activity phase.
Thigmotaxis:
When placed into a well-lit open field arena, adult Drosophila avoid the center and mostly stay near the edge; a behavior most often referred to as centrophobicity (supplemental Table 2 at http://www.genetics.org/supplemental/; Götz and Biesinger 1985; Martin 2004). We quantified centrophobicity in the open field by measuring the fly's average distance from the center of the arena and the percentage of time that the fly spends in the outermost one-third of the arena. A fly moving about at random may be expected to spend ∼33% of the time within this outer region. Wild-type Canton-S flies spend ∼90% of the time within the outermost zone of the arena and average ∼4 cm from the center. By either measure, krz1 homozygotes displayed normal levels of centrophobicity (supplemental Table 2). Canton-S males were significantly less centrophobic than females. A similar sex difference in centrophobicity within square arenas has been previously reported (Martin 2004). The w1118 flies showed significantly less centrophobicity in two experiments, but the differences did not reach the level of significance in a third experiment (supplementary Table 2). The norpA7 flies also displayed less centrophobicity, although it was significant only with the percentage of time spent in the outermost one-third of the arena and not with the mean distance from the center. Nevertheless, both w1118 and norpA7 are quite centrophobic, spending more than twice as much time in the outer zone than the other two zones together. These data are consistent with a report in which a transgenic approach was used to inhibit visual perception in square arenas (Besson and Martin 2004). Our results strongly support a limited role for visual processing in the expression of centrophobicity. We have also found no role for olfaction in centrophobicity since the or83b2 mutant flies are as centrophobic as Canton-S (supplemental Table 2 at http://www.genetics.org/supplemental/). It is likely, therefore, that other factors, such as tactile stimulation or programmed radial search strategies, play a much larger role in the avoidance of the center of either square or circular arenas (supplemental Table 2 at http://www.genetics.org/supplemental/; Götz and Biesinger 1985; Besson and Martin 2004).
DISCUSSION
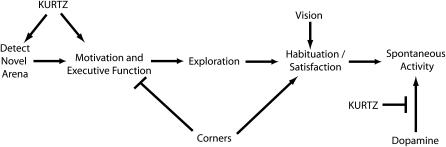
An understanding of the significance of the elevated initial activity brought about by the open field arena requires better insight into the relevance of this behavior for the fly. When trying to decipher the ethological basis of a specific behavior, one should consider the proximal causes of a behavior, including the inducing stimuli and the neurobiological requirements for the expression of that behavior, an evolutionary relationship for the behavior, and whether the behavior has any survival value (Tinbergen 1963). In dissecting the elevated initial activity, we have shown that the magnitude of the effect is dependent on the properties of the arena, independent of experimenter handling, and that the cessation of the elevated activity is dependent on vision and, to a lesser extent, on olfaction. We have also shown that elevated initial activity is present in diverged species, suggesting that it may underlie an important and general survival function. On the basis of these observations, we propose that the elevated initial activity constitutes exploration (Figure 8). Herein, we refer to the definition of exploration as stated by Crusio and van Abeelen (1986, p. 55): “exploration is evoked by novel stimuli and consists of behavioral acts and postures that permit the collection of information about new objects and unfamiliar parts of the environment.” A specific defect in exploration may arise either from a failure to detect or process the novel stimuli or from a failure in motivation and executive function.
Figure 8.—
A model for the exploration of an open field arena. In this model, a fly recognizes the novelty through multiple and redundant sensory inputs. The decision to explore the novel arena is then made through executive function centers and modified by motivational states. The KURTZ nonvisual arrestin is required in these early steps leading to exploration. The process of exploration can lead either to habituation of the novelty or to satisfaction if the fly has found what it sought. The result of either habituation or satisfaction is the much lower level of spontaneous activity. Poor visual and, to a lesser extent, olfactory acuity leads to prolonged exploration since the fly takes longer to habituate to the novelty. The presence of dispersed corners in a square arena leads to an overall reduced activity. The corners may provide a sense of shelter, which could satisfy the exploration drive. Alternatively, corners may generally affect activity through a different motivational or executive decision process. For example, a strong thigmotactic drive could supersede exploration and keep flies in corners.
Our hypothesis of Drosophila exploration occurring during the elevated initial activity phase in a circular open field arena is supported by the demonstration that the proximal causes of this stimulated activity come from properties of the arena and not from handling. The amount of activity during the first minute in a circular arena responded significantly to changes in arena size, indicating that some property of the arena itself is a proximal cause for this behavior: the greater the area to explore, the greater the amount of activity required to habituate the novelty stimulus. Sensory-deprived flies are deficient in the decay from elevated initial activity, which also supports the exploration hypothesis. The failure to visually observe the surroundings leads to an inability to recognize the surroundings and habituate to the stimulus, prolonging the exploration. The decline in exploration was also inhibited in the anosmic or83b2 mutants, suggesting that olfaction also has a role in the habituation to the novelty of the arena. The absence of an activity deficit in the antenna-transformed AntpNS/+ flies suggests that this sensory organ is largely dispensable for activation of exploration. The gross structural changes in AntpNS/+ flies lead to severe defects in olfactory jump responses (McKenna et al. 1989). Hence, it is apparent from AntpNS/+ that the elevated initial activity found in the open field arena and the olfactory jump responses have different sensory requirements.
Dopamine and stimulated activity:
Previous evidence has suggested that dopamine may generally regulate stimulated activity, including: initial activity within an open field, ethanol-vapor-stimulated activity, and mechanically stimulated activity (Meehan and Wilson 1987; Bainton et al. 2000; Friggi-Grelin et al. 2003). The only other mutation known in Drosophila to specifically affect initial activity in an open field arena activity is a spontaneous allele of the tyrosinase-1 gene; the tyr11 allele results in flies with significantly higher levels of initial activity than wild-type flies, but no differences are found in the level of spontaneous activity (Meehan and Wilson 1987). The tyr11 allele was identified as a spontaneous mutation having only 70% of the normal levels of dopamine, the molecular identity of this mutation (Lewis and Lewis 1963; Burnell and Daly 1982). In another study, Friggi-Grelin et al. (2003) selectively inhibited dopaminergic signaling with the targeted expression of tetanus toxin light chain in the majority of tyrosine-hydroxylase-expressing cells. The resulting flies were hyperactive after being banged to the bottom of a cylinder in a negative geotaxis assay but apparently have normal locomotion in the absence of this mechanical stimulation (Friggi-Grelin et al. 2003). In a third approach, Bainton et al. (2000) inhibited dopamine synthesis with 3-IY, an inhibitor of tyrosine hydroxylase that reduces total dopamine levels to ∼10% of the wild-type levels. The 3-IY-treated flies initially displayed normal activity in a 6-cm2 arena; however, after stimulation with ethanol vapor, the activity of the 3-IY-treated flies was reduced relative to the untreated flies (Bainton et al. 2000). These studies suggest that dopamine has a role in stimulated activity, although the direction of the response may differ, depending on either the treatment or the stimulus. There are, however, clear differences in how these approaches disrupt the dopamine pathway. In the transgenic approach, most but not all dopaminergic neurons express the toxin, leaving a small number of dopamine-signaling pathways intact (Friggi-Grelin et al. 2003). In both the tyr11 mutation and the pharmacologic inhibition of dopamine synthesis, it is not known if synthesis is globally inhibited (Burnell and Daly 1982; Bainton et al. 2000). It also remains possible that the residual dopamine levels in these flies may have enhanced effects due to a sensitization of their respective circuits.
More recently, several studies have highlighted a role for dopamine in regulating spontaneous activity and arousal in Drosophila. Lima and Miesenböck (2005) have found that activation of dopaminergic pathways results in state-dependent locomotor responses. In most flies, photostimulated dopamine release leads to an immediate increase in locomotor activity; however, in flies already expressing higher levels of ambulation, the forced release of dopamine leads to lower levels of activity (Lima and Miesenböck 2005). In two studies where synaptic levels of dopamine were likely to be increased, significant increases in locomotor activity were found (McClung and Hirsh 1998; Kume et al. 2005). The inhibition of dopamine reuptake with cocaine activates locomotor activity, whereas very high doses of this drug suppress ambulation (McClung and Hirsh 1998). Kume et al. (2005) identified a mutation in a dopamine transporter, fumin, which results in dramatically increased levels of spontaneous activity. The absence of fumin in neurons and glia may lead to higher synaptic levels of dopamine. These recent studies signify that dopamine probably has a dual role in regulating activity: an increase in locomotor activity occurs with lower levels of dopamine and higher levels suppress spontaneous activity.
It is possible that krz regulates elevated initial activity through an effect on dopamine signaling. If one presupposes that, in the developmentally rescued krz1 homozygotes, G-protein-coupled receptor signaling is generally extended and amplified due to the absence of agonist-dependent desensitization, then the reduction in the exploratory activity phase may result from an increased sensitivity to the effects of dopamine on activity. However, our data failed to show any effect of inhibition of dopamine signaling on the exploratory activity phase of wild-type flies or the developmentally rescued krz1 homozygotes, indicating that dopamine is not required in flies for the exploratory activity phase in an open field arena.
Spontaneous activity was dramatically reduced after feeding wild-type flies 3-IY, consistent with the increase in activity found with photostimulated dopamine release, with the increase in synaptic dopamine levels in the fumin mutant, or after cocaine treatment (McClung and Hirsh 1998; Kume et al. 2005; Lima and Miesenböck 2005). The affect of 3-IY on spontaneous activity was not found in the krz1 homozygous flies. Since the krz1 mutation suppresses the effect of 3-IY on spontaneous activity, krz most likely acts as a negative regulator of dopamine signaling during spontaneous activity. In the absence of krz, the dopamine receptors may perdure in an activated state, compensating for the shortfall of dopamine in the 3-IY-treated flies.
Conservation of exploration:
We have found a considerable conservation of the open field behavior in three species of drosophilids. D. simulans and D. melanogaster are closely related members of the melanogaster species group, having diverged ∼2.5–4 million years ago (MYA) (Ashburner et al. 1984; Cariou 1987). In contrast, the last shared ancestor for D. virilis and D. melanogaster is thought to have lived ∼65–70 MYA during the late Cretaceous period (Beverley and Wilson 1984; Cariou 1987). Remarkably, however, a potentially homologous behavior is also present in many species of vertebrates (Glickman and Hartz 1964; Connolly 1967; Candland and Nagy 1969; Lát and Gollová-Hemon 1969). The presence of a conserved behavioral response in such divergent species strongly suggests that it provides general advantages, for example, leading the animal to new food sources, mating partners, or protective shelter.
The responses to an open field arena have been most thoroughly studied in rodents where the behavior in an open field arena is thought to be shaped by at least two conflicting internal drives: emotionality and curiosity (Whimbey and Denenberg 1967; Walsh and Cummins 1976; Crusio 2001). The emotionality factor is thought to represent anxiety or fear and is typically measured by an inhibition of ambulation, an increased number of defecations, and decreased entries into the center of the open field (Hall 1936). These three ethological parameters in the open field arena are frequently reversible with anxiolytic drugs and enhanced by anxiogenic drugs, strengthening the association of these behaviors with anxiety (Prut and Belzung 2003). However, factor analysis has shown that anxiety in rodents is multidimensional, with decreases in locomotion and defecation influenced by different factors (Lister 1990; Ramos and Mormede 1998; Crusio 2001). In rodents, the inhibition of ambulation in the open field arena by an anxiety-like factor appears to be initially countered by a curiosity drive that leads to increased exploration (Walsh and Cummins 1976).
Outwardly, the behavior of the drosophilids in the open field resembles that of rats and mice: they all have an initially elevated period of activity, avoid the center of the arena, and prefer corners. It is not clear whether these behaviors of the drosophilids are functional analogs to the rodent behaviors since there is as yet much less known about the motivational factors that drive Drosophila behavior. The stressors of hyperagitation prior to placement in the arena or of social isolation do not affect the Drosophila exploratory activity phase, indicating that, if an emotionality factor exists in Drosophila, these potential stressors are insufficient to affect exploration (Figure 3C; G. Roman, unpublished result). We expect, however, that an exploratory drive similar to the one in rodents may exist in flies since it would serve the important survival functions of finding food, mating partners, or shelter. In fact, one explanation of the strong preference for corners and the striking thigmotactic responses in Drosophila is that the flies are seeking shelter. An increase in the size of a square arena resulted in a decrease in the amount of activity during the first minute—the opposite of the result in circular arenas. Although the corners inhibited the spontaneous activity phase independently of arena size, the exploration phase was most inhibited when the corners were farther apart: as the distance between shelters increases, the propensity to explore decreases. The phenomenon of staying in sheltered areas may also be associated with the avoidance of the arena's center and may represent the expression of fear or anxiety in Drosophila.
Alternatively, the centrophobicity and corner preference may be independent of any anxiety-like construct. Drosophila may have a powerful innate thigmotactic response that is independent of shelter seeking. In this case, the corners would offer more surface to rub against and would therefore become the preferred location. We do not favor this later explanation since the flies are, in general, not continuously rubbing against the arena's edge as much as maintaining proximity to it (L. Liu and G. Roman, unpublished observation). Drosophila may also have innate search strategies that drive them to the arena's edge through biased orientation and a persistence of direction during walking (Götz and Biesinger 1985).
The role of krz in exploration:
The rescue of the krz1 elevated initial activity deficit with both a genomic transgene and the pan-neural expression of a krz cDNA demonstrates a requirement for this gene in the nervous system for the expression of this activity phase. Since the krz1 homozygotes can ambulate at speeds identical to those of wild-type flies during the first minute within the arena and have wild-type levels of spontaneous activity in either the open field or the Trikinetics activity monitor, the krz1 phenotype in exploration is not due to a defect in motor function. Consequently, the requirement for krz in exploration more likely lies in either sensory- or executive-function-level processing. The developmentally rescued krz1 homozygotes have reduced olfactory sensitivity and antennal structural defects, which exhibit variable penetrance and expressivity (Ge et al. 2006). However, the olfaction defect appears not to be the proximal cause of the activity deficit, since we can rescue the olfaction phenotype without altering the exploration phenotype, and olfaction is dispensable for the exploration phase. The selection of krz1 homozygotes with or without antenna defects failed to affect the exploratory activity phase, suggesting that this exploration phenotype is independent of the antennal defect. Moreover, the more severe structure defects in AntpNS/+ flies failed to produce an elevated initial activity phenotype, further suggesting that the antennal structural defect is not responsible for the exploration phenotype seen in the developmentally rescued krz1 homozygotes. As the rescued krz1 homozygotes are unresponsive to novelty and mechanical agitation, this gene may have a more central role in gating the responses to certain forms of stimulation.
In the large square arena, the krz1 homozygotes behave indistinguishably from wild type: the same preference for corners, the same avoidance of the center of the arena, and the same activity profiles. In contrast, the rescued krz1 homozygotes show an exploration deficit in the small square arena, where the corners do not seem to inhibit exploration to nearly the same extent as in the larger arena. The presence of an initial activity deficit in both the circular arena and the smaller square arena and the absence of the initial activity deficit in large square arenas would occur if either (1) the repression of activity in large square arenas indirectly masks the krz1 deficit or (2) it more directly compensates for the absence of krz. An example of the former would be if the preference for corners is a general inhibitor of all locomotion, reducing the exploratory activity phase to a floor level commensurate with that of the developmentally rescued krz1 homozygotes. In an example of the later case, the effect of the corners may specifically reduce exploration by satiating a portion of the drive, which is reduced in krz1 homozygotes. In either case, the results do not argue against krz activity being required for exploration. The absence of this krz exploration phenotype also illustrates that tests of activity in some square arenas are restrictive—not all parameters of activity are identifiable in the 22.4-cm2 arena.
Mice have two nonvisual arrestins, βarr1 and βarr2 (Conner et al. 1997; Bohn et al. 1999). These proteins are required for the agonist-dependent desensitization of GPCRs and for the regulation of a number of other cell-surface molecules (Lefkowitz and Whalen 2004). The mouse βarr2−/− mutation also results in reduced locomotor activity, although it appears to affect both the elevated initial activity and the plateau phases of activity (Bohn et al. 2003). The causes of the innate βarr2−/− defect in activity are not currently known; these mice may have problems with emotionality or perhaps they have minor motor defects. The murine βarr2 may also have different roles from those of krz in Drosophila in regulating locomotion; however, there are also significant experimental differences in how these experiments were performed. The βarr2 mutants developed without this gene's activity, whereas the krz mutant flies were periodically supplied with krz activity throughout development (Bohn et al. 2003). The two nonvisual arrestins in mice are also at least partially redundant (Kohout et al. 2001; Bohn et al. 2003), whereas krz is the only nonvisual arrestin in Drosophila (Adams et al. 2000; Roman et al. 2000).
In humans, subtle defects in the agonist-dependent desensitization of G-protein-coupled receptors may be a considerable contributing factor to the severity of affective disorders. A variant of the human G-protein-coupled receptor kinase 3 gene has been identified as a candidate locus for bipolar disorder (Barrett et al. 2003). The levels of GRK3 are lower in the leukocytes of patients with severe bipolar symptoms (Niculescu et al. 2000). In the postmortem brains of depressed patients, there is a significant increase in the level of membrane-bound GRK2, which is not found in the brains of antidepressant-treated patients; βARR2 may be coordinately regulated with GRK2 in the brains of these depressed patients (Grange-Midroit et al. 2003). Avissar et al. (2004) found a strong correlation between lower βARR1 levels in leukocytes and the severity of patients with major depression. A greater understanding of arrestin function within the nervous system is required to understand how agonist-dependent desensitization of protein-coupled receptors may lead to pathological emotional states. Although these states are probably controlled by different neurotransmitter systems in Drosophila, they still most likely involve G protein signaling. Drosophila, with a single nonvisual arrestin and a simpler behavioral repertoire, provides an excellent opportunity for examining the role of agonist-dependent desensitization in behavior. Understanding how krz is involved in the responses to novelty and mechanical stimulation will likely provide us with important insights into how these molecules regulate emotional responses in vertebrates.
Acknowledgments
We are indebted to David Wolfer for his generous help with the Wintrack program. We thank Brigitte Dauwalder, Tammy Gutman, and Jin He for expert technical assistance. We also thank Chi-Shing Chan for helping us with the HVS automated tracking system and for a critical reading of the manuscript. Fly stocks were kindly provided by Leslie Vosshall and the Bloomington and Tucson stock centers. This work was funded by National Institutes of Health grant NS42185 (G.R.).
References
- Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne et al., 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Andretic, R., B. van Swinderen and R. J. Greenspan, 2005. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15(13): 1165–1175. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., M. Bodmer and F. Lemeunier, 1984. On the evolutionary relationships of Drosophila melanogaster. Dev. Genet. 4: 295–312. [Google Scholar]
- Avissar, S., A. Matuzany-Ruban, K. Tzukert and G. Schreiber, 2004. Beta-arrestin-1 levels: reduced in leukocytes of patients with depression and elevated by antidepressants in rat brain. Am. J. Psychiatry 161(11): 2066–2072. [DOI] [PubMed] [Google Scholar]
- Bainton, R. J., L. T. Tsai, C. M. Singh, M. S. Moore, W. S. Neckameyer et al., 2000. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 10(4): 187–194. [DOI] [PubMed] [Google Scholar]
- Barrett, T. B., R. L. Hauger, J. L. Kennedy, A. D. Sadovnick, R. A. Remick et al., 2003. Evidence that a single nucleotide polymorphism in the promoter of the G protein receptor kinase 3 gene is associated with bipolar disorder. Mol. Psychiatry 8(5): 546–557. [DOI] [PubMed] [Google Scholar]
- Besson, M., and J.-R. Martin, 2004. Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J. Neurobiol. 62: 386–396. [DOI] [PubMed] [Google Scholar]
- Beverley, S. M., and A. C. Wilson, 1984. Molecular evolution in Drosophila and the higher Diptera. J. Mol. Evol. 21: 1–13. [DOI] [PubMed] [Google Scholar]
- Bohn, L. M., R. J. Lefkowitz, R. R. Gainetdinov, K. Peppel, M. G. Caron et al., 1999. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286(5449): 2495–2498. [DOI] [PubMed] [Google Scholar]
- Bohn, L. M., R. R. Gainetdinov, T. D. Sotnikova, I. O. Medvedev, R. J. Lefkowitz et al., 2003. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J. Neurosci. 23(32): 10265–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118(2): 401–415. [DOI] [PubMed] [Google Scholar]
- Burnell, A. M., and B. A. Daly, 1982. Spontaneous locomotor activity and dopamine levels in tyr-1 mutants of Drosophila melanogaster, pp. 361–370 in Advances in Genetics, Development, and Evolution of Drosophila. Proceedings of the Seventh European Drosophila Research Conference, June 30–July 3, 1981, University of Oulu, Oulu, Finland.
- Candland, D. K., and Z. M. Nagy, 1969. The open field: some comparative data. Ann. NY Acad. Sci. 159(3): 831–851. [DOI] [PubMed] [Google Scholar]
- Cariou, M. L., 1987. Biochemical phylogeny of the eight species in the Drosophila melanogaster subgroup, including Drosophila sechellia and Drosophila orena. Genet. Res. 50: 181–185. [DOI] [PubMed] [Google Scholar]
- Conner, D. A., M. A. Mathier, R. M. Mortensen, M. Christe, S. F. Vatner et al., 1997. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ. Res. 81(6): 1021–1026. [DOI] [PubMed] [Google Scholar]
- Connolly, K., 1967. Locomotor activity in Drosophila III. A distinction between activity and reactivity. Anim. Behav. 15: 149–152. [DOI] [PubMed] [Google Scholar]
- Crusio, W. E., 2001. Genetic dissection of mouse exploratory behaviour. Behav. Brain Res. 125(1–2): 127–132. [DOI] [PubMed] [Google Scholar]
- Crusio, W. E., and J. H. van Abeelen, 1986. The genetic architecture of behavioural responses to novelty in mice. Heredity 56: 55–63. [DOI] [PubMed] [Google Scholar]
- Ewing, A. W., 1963. Attempts to select for spontaneous activity in Drosophila. Anim. Behav. 14: 444–449. [Google Scholar]
- Friggi-Grelin, F., H. Coulom, M. Meller, D. Gomez, J. Hirsh et al., 2003. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54: 618–627. [DOI] [PubMed] [Google Scholar]
- Ge, H., P. Krishnan, L. Liu, B. Krishnan, R. L. Davis et al., 2006. A Drosophila nonvisual arrestin is required for the maintenance of olfactory sensitivity. Chem. Senses 31(1): 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman, S. E., and K. E. Hartz, 1964. Exploratory behavior in several species of rodents. J. Comp. Physiol. Psychol. 58: 101–104. [DOI] [PubMed] [Google Scholar]
- Götz, K. G., and R. Biesinger, 1985. Centrophobism in Drosophila melanogaster II. A physiological approach to search and search control. J. Comp. Physiol. A 156: 329–337. [Google Scholar]
- Grange-Midroit, M., J. A. Garcia-Sevilla, M. Ferrer-Alcon, R. La Harpe, P. Huguelet et al., 2003. Regulation of GRK 2 and 6, beta-arrestin-2 and associated proteins in the prefrontal cortex of drug-free and antidepressant drug-treated subjects with major depression. Brain Res. Mol. Brain Res. 111(1–2): 31–41. [DOI] [PubMed] [Google Scholar]
- Hall, C. S., 1936. Motional behavior in the rat: III. The relationship between emotionality and ambulatory activity. J. Comp. Psychol. 22: 345–352. [Google Scholar]
- Harris, W. A., and W. S. Stark, 1977. Hereditary retinal degeneration in Drosophila melanogaster: a mutant defect associated with the phototransduction process. J. Gen. Physiol. 69: 261–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, E. M., and R. L. Garber, 1987. Function and misfunction of the two promoters of the Drosophila Antennapedia gene. Genes Dev. 11: 544–555. [DOI] [PubMed] [Google Scholar]
- Kalidas, S., and D. P. Smith, 2002. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron 33(2): 177–184. [DOI] [PubMed] [Google Scholar]
- Kalmus, H., 1943. The optomotor responses of some eye mutants in Drosophila. J. Genet. 45: 206–213. [Google Scholar]
- Kankel, M. W., D. M. Duncan and I. Duncan, 2004. A screen for genes that interact with the Drosophila pair-rule segmentation gene fushi tarazu. Genetics 168: 161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout, T. A., F. S. Lin, S. J. Perry, D. A. Conner and R. J. Lefkowitz, 2001. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc. Natl. Acad. Sci. USA 98(4): 1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivshenko, J. D., 1954. New mutants report. Dros. Inf. Serv. 28: 74–75. [Google Scholar]
- Kume, K., S. Kume, S. K. Park, J. Hirsh, F. R. Jackson, 2005. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25(32): 7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, M. C., A. I. Domingos, W. D. Jones, M. E. Chiappe, H. Amrein et al., 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43: 703–714. [DOI] [PubMed] [Google Scholar]
- Lát, J., and E. Gollová-Hemon, 1969. Permanent effects of nutritional and endocrinological intervention in early ontogeny on the level of nonspecific excitability and on lability (emotionality). Ann. NY Acad. Sci. 159(3): 710–720. [DOI] [PubMed] [Google Scholar]
- Lefkowitz, R. J., and E. J. Whalen, 2004. beta-arrestins: traffic cops of cell signaling. Curr. Opin. Cell Biol. 16(2): 162–168. [DOI] [PubMed] [Google Scholar]
- Lewis, H. W., and H. S. Lewis, 1963. Genetic regulation of dopa oxidase activity in Drosophila. Ann. NY Acad. Sci. 100: 827–839. [Google Scholar]
- Lima, S. Q., and G. Miesenböck, 2005. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121(1): 141–152. [DOI] [PubMed] [Google Scholar]
- Lister, R. G., 1990. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 46(3): 321–340. [DOI] [PubMed] [Google Scholar]
- Martin, J.-R., 2004. A portrait of locomotor behaviour in Drosophila determined by a video-tracking paradigm. Behav. Processes 67: 207–219. [DOI] [PubMed] [Google Scholar]
- Martin, J.-R., R. Ernst and M. Heisenberg, 1999. Temporal pattern of locomotor activity in Drosophila melanogaster. J. Comp. Physiol. A 184(1): 73–84. [DOI] [PubMed] [Google Scholar]
- McClung, C., and J. Hirsh, 1998. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr. Biol. 8(2): 109–112. [DOI] [PubMed] [Google Scholar]
- McGuire, S. E., P. T. Le, A. J. Osborn, K. Matsumoto and R. L. Davis, 2003. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302(5651): 1765–1768. [DOI] [PubMed] [Google Scholar]
- McKenna, M., P. Monte, S. L. Helfand, C. Woodard and J. Carlson, 1989. A simple chemosensory response in Drosophila and the isolation of acj mutants in which it is affected. Proc. Natl. Acad. Sci. USA 86: 8118–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan, M. J., and R. Wilson, 1987. Locomotor activity in the Tyr-1 mutant of Drosophila melanogaster. Behav. Genet. 17: 503–512. [DOI] [PubMed] [Google Scholar]
- Miller, A., 1950. The internal anatomy and histology of the imago of Drosophila melanogaster, pp. 420–534 in Biology of Drosophila, edited by M. Demerec. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Moses, K., M. C. Ellis and G. M. Rubin, 1989. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature 340: 531–536. [DOI] [PubMed] [Google Scholar]
- Niculescu, A. B., III, D. S. Segal, R. Kuczenski, T. Barrett, R. L. Hauger et al., 2000. Identifying a series of candidate genes for mania and psychosis: a convergent functional genomics approach. Physiol. Genomics 4(1): 83–91. [DOI] [PubMed] [Google Scholar]
- Prut, L., and C. Belzung, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463: 3–33. [DOI] [PubMed] [Google Scholar]
- Ramos, A., and P. Mormede, 1998. Stress and emotionality: a multidimensional and genetic approach. Neurosci. Biobehav. Rev. 22: 33–57. [DOI] [PubMed] [Google Scholar]
- Roman, G., V. Meller, K. H. Wu and R. L. Davis, 1998. The opt1 gene of Drosophila melanogaster encodes a proton-dependent dipeptide transporter. Am. J. Physiol. 275: C857–C869. [DOI] [PubMed] [Google Scholar]
- Roman, G., J. He and R. L. Davis, 2000. kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics 155: 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, M. P., A. J. Weiner, T. I. Hazelrigg, B. A. Polisky, V. Pirrotta et al., 1983. The molecular organization of the Antennapedia locus of Drosophila. Cell 35: 763–776. [DOI] [PubMed] [Google Scholar]
- Sweeney, S. T., K. Broadie, J. Keane, H. Niemann and C. J. O'Kane, 1995. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14(2): 341–351. [DOI] [PubMed] [Google Scholar]
- Tinbergen, N., 1963. On aims and methods of ethology. Z. Tierpsychol. 20: 410–433. [Google Scholar]
- Walsh, R. N., and R. A. Cummins, 1976. The open-field test: a critical review. Psychol. Bull. 83: 482–504. [PubMed] [Google Scholar]
- Whimbey A. E., and V. H. Denenberg, 1967. Two independent behavioral dimensions in open-field performance. J. Comp. Physiol. Psychol. 63(3): 500–504. [DOI] [PubMed] [Google Scholar]
- Wolfer, D. P., R. Madani, P. Valenti and H. P. Lipp, 2001. Extended analysis of path data from mutant mice using the public domain software Wintrack. Physiol. Behav. 73: 745–53. [DOI] [PubMed] [Google Scholar]