Abstract
In facultatively sexual species, lineages that reproduce asexually for a period of time can accumulate mutations that reduce their ability to undergo sexual reproduction when sex is favorable. We propagated Saccharomyces cerevisiae asexually for ∼800 generations, after which we measured the change in sexual fitness, measured as the proportion of asci observed in sporulation medium. The sporulation rate in cultures propagated asexually at small population size declined by 8%, on average, over this time period, indicating that the majority of mutations that affect sporulation rate are deleterious. Interestingly, the sporulation rate in cultures propagated asexually at large population size improved by 11%, on average, indicating that selection on asexual function effectively eliminated most of the mutations deleterious to sporulation ability. These results suggest that pleiotropy between mutations' effects on asexual fitness and sexual fitness was predominantly positive, at least for the mutations accumulated in this experimental evolution study. A positive correlation between growth rate and sporulation rate among lines also provided evidence for positive pleiotropy. These results demonstrate that, at least under certain circumstances, selection acting on asexual fitness can help to maintain sexual function.
THE vast majority of eukaryotic organisms reproduce sexually, yet the origin and maintenance of sexual reproduction are not well understood, particularly in light of the costs of sex including the twofold cost of sex and the breakdown of favorable genetic combinations (Michod and Levin 1988; Barton and Charlesworth 1998; Otto and Lenormand 2002). For sexuality to be maintained within a population, the advantages of sex must overcome these costs. Several hypotheses have been put forward, describing the advantages of sex (Kondrashov 1993). To evaluate the plausibility of these different hypotheses, it would be useful to estimate the rate at which mutations appear, causing sex to be lost. This provides an estimate for the time frame over which the advantages of sex must act to maintain sexuality, which is particularly important for theories that advocate accelerated adaptation of sexual populations to occasional changes in the environment. For example, if sex is advantageous in a new environment, then we need to know how often environments must change to ensure that sex is not lost in intervening periods with a constant environment.
We have determined the proportion of mutations that adversely affect sexual function in the facultatively sexual budding yeast Saccharomyces cerevisiae. While some organisms can be classified as either fully sexual or asexual, many, including several groups of fungi, invertebrates, and protists (Bell 1982), are not obligately sexual or asexual but can reproduce through either means.
In nonstressful environments, budding yeast typically reproduce asexually in the diploid state. When yeast are nitrogen stressed, they undergo meiosis, forming four haploid ascospores (an ascus) in a process known as sporulation. They remain in this state until conditions become suitable for a return to vegetative growth. At this point, haploid cells of opposite mating type (α and a) fuse, returning the yeast to the diploid state. In the absence of such stress, yeast can propagate asexually indefinitely. There is anecdotal evidence that laboratory yeast strains can lose the ability to reproduce sexually after many generations of asexual reproduction in the lab (e.g., Zeyl 2005), yet there has been no quantitative assessment of how frequently or easily sexuality can be lost in S. cerevisiae.
To estimate the total rate of mutations that are deleterious to sexual function, we performed a mutation-accumulation experiment (small mutation accumulation, SMA) (as in Kibota and Lynch 1996). Small, replicate populations of diploid yeast were propagated asexually for 800 generations using regular bottlenecks to single cells to reduce the genetic variance and efficacy of selection within each line. Consequently, all but the most deleterious mutations were maintained in the SMA lines. If the lines are initially well adapted to their environment, the mean fitness is expected to decline, and the genetic variance among lines is expected to increase over time, as different mutations accumulate in the various lines (Bateman 1959; Mukai 1964; Mukai et al. 1972). These changes in fitness can be used to estimate the deleterious mutation rate and the average fitness effect of a mutation, using either the Bateman–Mukai method (Bateman 1959; Mukai 1964) or a maximum-likelihood algorithm (Keightley 1994). Here, we use both methods to estimate the rate and average effect of mutations on sexual fitness, specifically on sporulation rate, by measuring the proportion of cells that form asci under sporulation conditions.
When yeast are propagated asexually, mutations that are deleterious only to sexual function are effectively neutral, regardless of the population size. Thus, if mutations affecting sexual function had no effect on asexual growth (no pleiotropy), then the patterns observed in the SMA lines should be similar in populations propagated asexually at large size. To test this, we measured the decline over time in sporulation ability of large, asexually propagated populations (large mutation accumulation, LMA). By comparing the fate of mutations reducing sexual function between the LMA and the SMA lines, we were able to assess the extent to which sexual function is affected by pleiotropic selection on asexual function.
In addition, we assessed vegetative growth rate, a measure of asexual fitness, as an alternative means of examining the relationship between asexual and sexual function. These data allow us to assess two different ways in which sexual and asexual function are pleiotropically linked. Pleiotropy between sporulation and growth rate among virtually all mutations (excluding near lethals) is assessed by measuring the correlation between growth rate and sporulation rates among all of the lines. In contrast, pleiotropy among those mutations that can accumulate in large populations (only those beneficial or nearly neutral to asexual growth) is assessed by measuring the sporulation rate after a period of time during which selection has acted to improve asexual fitness (comparing the initial to final LMA lines).
From our SMA experiment, we have estimated mutational parameters Usex (deleterious mutation rate per diploid genome per generation) and ssex (the average reduction in fitness of heterozygous mutants) with respect to sexual function in yeast. Previous mutation-accumulation studies have determined the genomic mutation rate for mutations affecting maximum growth rate in asexually reproducing S. cerevisiae (Korona 1999; Zeyl and DeVisser 2001; Joseph and Hall 2004), yet the rate of mutations affecting sexual function has not been examined. Estimates of mutational parameters with respect to either vegetative or sexual growth (growth rate or sporulation rate, respectively) are underestimates of the total deleterious mutation rate as both are only components of total fitness (e.g., ∼50% of gene disruptions have no apparent growth deficiency (Hampsey 1997); sporulation rate is a component of sexual fitness that does not include mating ability, for example). By comparing estimates of Uasex and sasex derived from growth rates with our estimates of Usex and ssex, a more comprehensive picture of mutational parameters can be obtained.
The rate of loss of sex has been examined in the distantly related fungus Cryptococcus neoformans (Cryptococcus, phylum Basidiomycota; Saccharomyces, phylum Ascomycota) (Xu 2002). Sexual function in C. neoformans was estimated for two traits, haploid mating ability and filamentation after 600 generations of SMA. In addition, Zeyl et al. (2005) have examined the nature of pleiotropy between asexual fitness and sexual fitness (as mating efficiency in haploids and sporulation rates in diploids) in different strains of S. cerevisiae propagated in minimal media (a nutrient-limiting environment) or in mouse brains (a heterogeneous environment). We can thus contrast aspects of our results with results from a different fungus (estimating Usex and ssex; Xu 2002) and with results from the same species but using different environments (estimating pleiotropy; Zeyl et al. 2005) to speculate on the generality of our findings.
MATERIALS AND METHODS
Founding strain:
The founding strain in this study is a diploid Y55 derivative of genotype leu2Δ (Zeyl and DeVisser 2001). The ancestral lines for both the replicate SMA and the LMA experiments were established from single colonies. Samples from these ancestral lines were frozen in 15% glycerol.
Mutation-accumulation environment:
Strains were propagated in rich media (YPD: 2% yeast extract, 1% peptone, 2% dextrose plus 2% agar for solid media). All cultures were maintained at 30°. Liquid cultures were shaken at 200 rpm in 18 × 150-mm borosilicate tubes. Testing for contamination was conducted by plating yeast on synthetic complete media lacking leucine (SC − leu: 0.67% yeast nitrogen base without amino acids, 2% glucose, 0.2% leucine dropout mix), which prevents growth of leucine-deficient strains like the strains used in this study. Tests for contamination sampled at least 20 randomly selected SMA lines and 10 LMA lines at intervals of ∼2 weeks. Growth was observed on media lacking leucine three times in ∼750 tests, which could be due to contamination either from an outside source (yeast or otherwise) or from a compensatory mutation allowing leucine synthesis; in either case, we regrew the culture from a recent uncontaminated culture (one LMA line) when we had a recent uncontaminated culture or eliminated the strain from the experiment otherwise (two SMA lines). Undetected contamination among lines would serve to reduce the variance among lines and/or increase the mean fitness, making it difficult to determine the direction of bias in our estimated mutation rates and selective effects.
Small mutation accumulation:
To perform the SMA, 100 initially isogenic strains were propagated by single-colony transfer for ∼800 generations. To propagate lines, a colony was randomly selected for transfer by choosing the isolated colony nearest to a previously applied dot on the plate. The selected colony was then streaked on to a new YPD plate with a sterile toothpick. The plates were incubated for 48 hr at 30°, after which the next transfer occurred. The SMA consisted of a total of 35 transfers. A sample colony from each plate was frozen in 15% glycerol at transfers 0, 6, 16, 22, 26, and 35.
Before freezing, lines were tested for contamination and for petite mutations. Petites are unable to sporulate due to a respiration deficiency that results primarily from mitochondrial mutations, which occur at a relatively high frequency. Petites were detected by streaking a colony from each SMA line on a medium that does not support anaerobic growth (YPG: 1% yeast extract, 2% peptone, 3% glycerol plus 2% agar). Testing for petites occurred at regular intervals (approximately every 100 generations). Over the course of the transfers, 20 SMA strains exhibited a petite phenotype. By eliminating these lines, we created a selective environment in which petites were effectively lethal. We performed this procedure to avoid having our results dominated by mitochondrial mutations, so that we could focus on the less well-understood effects of nuclear mutations.
The number of generations occurring between transfers was determined by estimating the number of cells in a 48-hr colony using a hemacytometer (Spencer Bright-Line). Six cultures from transfers 0, 6, 16, 22, and 26 were grown from frozen stock, and single colonies from each culture were randomly selected for counting. On average, each colony contained 9.37 × 106 cells with little heterogeneity. Thus, ∼23 generations occurred over 48 hr (223.15 = 9.37 × 106). The effective population size of each SMA line is thus ∼16 cells (Wahl and Gerrish 2001).
Large mutation accumulation:
The LMA experiment consisted of 50 lines propagated in batch culture. Every 24 hr, cultures were vortexed thoroughly and 0.1 ml was transferred to 10 ml of fresh YPD. Approximately 6.64 generations occurred between transfers (26.64 = 100), so that 800 generations accumulated after 121 transfers. The mean number of inoculating cells was 1.45 × 106 cells, estimated using a hemacytometer from the density of cells in five 24-hr cultures that had evolved for 800 generations. Cultures reached stationary phase after ∼10–11 hr. The effective population size of the LMA lines was 6.9 × 106 (Wahl and Gerrish 2001), >104 times greater than that in the SMA experiment. Lines were frozen at transfers 0, 12, 26, 73, 90, and 121 by adding 0.75 ml of culture to 0.75 ml of 15% glycerol. Prior to freezing, LMA lines were bottlenecked to a single colony, which was regrown in liquid YPD for 24 hr and then frozen. LMA lines were bottlenecked to reduce genetic variation within lines, thereby allowing a direct comparison of fitness in LMA and SMA lines.
LMA lines were tested for contamination by plating on SC − leu plates and tested for petites by plating on YPG before freezing (as described above). Petite mutations are only ∼70% as fit as their wild-type counterparts (Zeyl and DeVisser 2001) and should be swiftly eliminated from the LMA lines. As expected, no petite LMA lines were observed.
Sexual fitness measure:
A component of sexual fitness was measured by counting the proportion of asci per line, observed after inducing sporulation. Frozen strains of the ancestral and the SMA and LMA evolved lines (t ≈ 800 cell generations) were reacclimated by growing on YPD plates for 24 hr, after which a single, randomly selected colony was transferred for growth in 10 ml of liquid YPD for 24 hr. To facilitate sporulation, 0.1 ml of culture was added to 10 ml of presporulation media (6% YPD: 1% yeast extract, 2% peptone, 6% dextrose) and grown for 24 hr. Subsequently, 1 ml of culture was washed by centrifuging at 6000 rpm, removing the supernatant, adding 1 ml of dH2O, repeating these steps, and then resuspending in 0.2 ml of dH2O. From the washed culture, 0.1 ml was transferred to 10 ml of sporulation media (SPM plus leu: 0.3% KAc, 0.02% raffinose, 0.003% leucine) and incubated for 4 days. After 4 days had elapsed, each culture was examined under a light microscope (Zeiss Axioskop) at 400× magnification. A minimum of 200 cells and, when the proportion of asci was low, up to 600 cells were counted per culture to determine the proportion of asci relative to vegetative cells. Two replicate measurements were conducted, with cultures randomized with respect to time and experiment type to minimize block effects. The second replicate measurement was conducted blindly. The replicate measurements were significantly and positively correlated (Spearman's rank-order correlation: ρ = 0.26, P < 0.0001; repeatability = 0.59). For all subsequent analyses, the mean sporulation rate across the two replicates was used.
Asexual fitness measure:
A component of asexual fitness of strains was estimated by determining the growth rates using the microbiology workstation Bioscreen C (ThermoLabsystems), which samples the optical density of cultures over time. Lines were grown from frozen culture on YPD plates for 24 hr, after which a single colony was transferred to 10 ml liquid YPD for 24 hr. From these stationary phase cultures, with cell densities of ∼108 cells/ml, 1.5 μl of culture was added to 150 μl YPD in a well on a Bioscreen plate. Two replicate measurements were performed per line, one on each of two 100-well Bioscreen plates. Evolved cultures were grown next to their ancestors, and these “culture pairs” were randomly assigned to the wells of the plate, excluding the outer perimeter as controls. The two Bioscreen plates were cultured side-by-side for 48 hr in the Bioscreen, while continuously shaking at 30°. Optical density (OD) of cultures was measured automatically at set intervals. Growth curves were created by plotting OD measurements. A sliding-window program was created in Mathematica 5.0 (Wolfram Research 2003) to estimate growth rate from log-transformed OD data. The sliding-window program calculates the least-squares regression for a set of log(OD) measurements within a set window length. Several different sliding-window lengths were tested (180, 360, 420, and 480 min). A 420-min window was selected for analysis because it minimized the variance within replicates relative to the variance between replicates. Across the 48 hr over which the cultures grew, the slopes were calculated for each window, and the 98th percentile slope was used as the measure of growth rate. We used the 98th percentile slope rather than the maximum growth rate to avoid the influence of outliers. We found that this sliding-window program gave growth rate estimates that had lower variance within replicates than fitting either a logistic growth model or a model involving growth on two resources. Consequently, these other fitting procedures were not pursued further. The replicate measurements on the two Bioscreen plates were significantly and positively correlated (Spearman's rank-order correlation: ρ = 0.77, P < 0.0001; repeatability = 41%). For all subsequent analyses, the mean growth rate across the replicates was used.
Asexual fitness measurements were conducted on all MA lines, which required measurements to be conducted in four blocks. In each block, the growth rates of the same two standard lines (an ancestral line and an LMA line) were measured. Using only the growth rate data from the standard lines, we performed a two-way ANOVA with replication to determine if there was a block effect and/or an interaction between block and type of line. While a significant block effect was found (ANOVA, F3,8 = 8.96, P < 0.05), there was no significant interaction between block and line (ANOVA, F3,8 = 0.62, P > 0.05). To compensate for the block effect, each growth rate measurement was corrected by dividing by the mean growth rate of the two standard lines within the same block and then multiplying by the mean growth rate of the two standard lines across all four blocks. All data presented are based on these standardized growth rate measurements.
In three wells, growth was negligible (growth rates of <0.01/hr). Lack of growth in these cases most likely represents experimental error (e.g., using an insufficient number of cells to inoculate the cultures), because subsequent Bioscreen analyses of the same lines exhibited normal growth. We excluded these three wells from our data analyses.
Analysis of fitness measures:
Differences in sexual and asexual fitness were tested using t-tests comparing means and Levene's tests comparing variances for three cases: (1) between SMA lines and ancestral lines, (2) between LMA lines and ancestral lines, and (3) between SMA and LMA lines. In addition, we tested for correlations between vegetative and sexual fitness of lines. Because the sporulation rates of the SMA t = 800 lines were not consistent with a normal distribution (Shapiro–Wilk, W = 941, P = 0.03), we evaluated the significance of test statistics using randomization tests, randomly permuting the data across categories and repeating this process 10,000 times. Throughout, we present two-tailed probability values, P, describing the fraction of randomized data sets with an absolute difference (e.g., between the means of two groups) greater than or equal to the observed difference.
As expected, no significant difference was found between the mean fitnesses of the ancestral lines used for the LMA and the SMA lines (randomization test; sporulation rate, P = 0.8170; growth rate, P = 0.8071), so the ancestral lines were grouped for comparison. All statistics were performed in Mathematica 5.0 and/or JMP IN 5.1 (SAS Institute, Cary, NC).
Mutational parameter estimates:
The mutation rate per diploid genome per generation (U) and the heterozygous mean fitness effect per mutation (s) were estimated using two methods: the Bateman–Mukai (BM) method and Keightley's maximum-likelihood (ML) algorithm. The Bateman–Mukai method (Bateman 1959; Mukai 1964) is based on theory describing how the mean and variance in fitness should change over time, allowing one to derive a minimum point estimate of U (Umin) and a maximum point estimate of s (smax). To implement the BM method, we assumed that mutations were deleterious and had equal effects. The ML algorithm assumes a Poisson distribution of the number of mutations, a gamma distribution of mutational effects, and a proportion P of beneficial mutations to generate the log-likelihood of the data given the mutational parameters. The gamma distribution requires values for β and α, the shape and scale parameters, where the mean mutational effect is equal to β/α. We used the ML program mlgenomeu.c kindly provided by Peter Keightley (Keightley 1994). A profile likelihood for the shape parameter β was generated by searching for values of U and s yielding the highest log-likelihood of explaining the data, using starting values of U = 10−3, 10−1 and s = 0.003, 0.3 and with the proportion of beneficial mutations set initially to P = 0. Approximate 95% confidence intervals (C.I.) were generated for U, s, and P by determining the range of values of the parameter of interest that generated log-likelihood values within 2 units of the maximum log-likelihood value (Keightley 1994). Confidence intervals were obtained both by holding β fixed at its ML value and by allowing β to vary. ML is thought to provide a more accurate estimate of mutational parameters than BM because it uses all of the data, not just the mean and variance across lines, and can infer the shape of the distribution of mutational effects (Keightley 1998).
RESULTS
Sexual fitness:
The change in mean number of asci (sporulation rate or “sexual fitness”) in both mutation-accumulation experiments was determined by comparing the sexual fitness of each type of evolved strain to that in the ancestral lines (Table 1).
TABLE 1.
Mean (SE) and variance of sexual fitness (sporulation rate) and asexual fitness (growth rate) among ancestral, SMA, and LMA lines of Saccharomyces cerevisiae
| Sexual
|
Asexual
|
|||
|---|---|---|---|---|
| Mean | Variancea | Mean | Variance | |
| t = 0 | 0.6754 [a] (0.004) | 0.0020 [a] | 0.2773 [a] (0.0009) | 1.12 × 10−4 [a] |
| SMA t = 800 | 0.6245 [b] (0.016) | 0.0210 [b] | 0.2734 [b] (0.0015) | 1.72 × 10−4 [b] |
| LMA t = 800 | 0.7484 [c] (0.005) | 0.0013 [a, b] | 0.2809 [c] (0.0015) | 1.15 × 10−4 [a, b] |
Results with different letters [a, b, c] are significantly different according to randomization tests on the difference between means or the difference between variances.
Variance of the raw data. Randomization tests were performed on arcsine-transformed proportions.
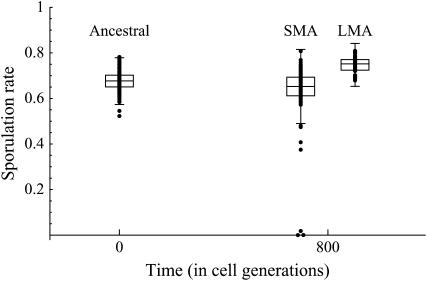
The distributions of the 128 ancestral lines, the 78 SMA evolved lines, and the 50 LMA evolved lines are displayed in Figure 1. The mean sporulation rate of the SMA evolved lines is significantly lower than that of the ancestral lines (randomization test, P < 0.0001). Indeed, three SMA lines lost the ability to sporulate almost entirely. Even when the three nonsporulating lines are removed, the sporulation rate of the SMA evolved lines remains significantly lower than that of the ancestral lines (randomization test, P = 0.0028). The mean sporulation rate of the LMA evolved lines is significantly greater than that of the ancestral lines (randomization test, P < 0.0001), as well as significantly greater than that of the SMA evolved lines (randomization test, P < 0.0001).
Figure 1.—
Mean proportion of asci for 128 ancestral (t = 0), 78 SMA evolved (t = 800), and 50 LMA evolved (t = 800) lines. Dots show all of the data. Box plots represent the 25th, median, and 75th quartiles, with whiskers extending an additional 1.5 times the interquartile range in each direction.
The data were arcsine square-root transformed to examine differences in variance. This transformation was used because the data are proportions confined to lie between 0 and 1, which underrepresents variance differences. The variance among the SMA evolved lines is significantly greater than the variance among ancestral lines (randomization test, P = 0.0011), even when the three nonsporulating lines are removed from the data set (randomization test, P = 0.0018). The variance among the evolved LMA strains is not significantly different from the variance among ancestral lines (randomization test, P = 0.4154) or among the SMA lines (randomization test, P = 0.1186).
Asexual fitness:
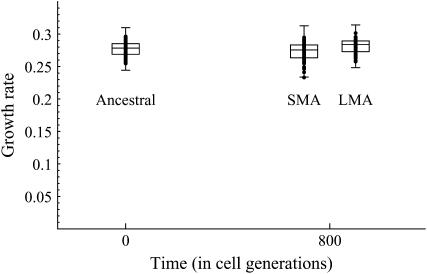
Growth rate measurements were conducted on ancestral, SMA, and LMA lines (Table 1 and Figure 2). Similar patterns emerged as observed with sexual fitness. The mean growth rate of the SMA lines was significantly lower than that of the ancestral lines (randomization test, P = 0.0208) and the LMA lines (randomization test, P = 0.0006). In contrast, the growth rate of the LMA lines was significantly higher than that of the ancestral lines (randomization test, P = 0.0433). While the general patterns were the same, growth rates differed proportionately less among treatments than sporulation rates (Table 1), and the P-values for growth rates were only modestly significant.
Figure 2.—
Mean growth rates for 128 ancestral (t = 0), 78 SMA evolved (t = 800), and 50 LMA evolved (t = 800) lines. Dots show all of the data. Box plots represent the 25th, median, and 75th quartiles, with whiskers extending an additional 1.5 times the interquartile range in each direction.
There was also a significant increase in variance among the SMA lines when compared to the ancestral lines (randomization test, P = 0.0248). No significant difference was observed, however, in among-line variance between the LMA lines and the ancestral lines (randomization test, P = 0.8753) or between the LMA lines and the SMA lines (randomization test, P = 0.2029).
Nongenetic changes:
Some of the observed changes in sexual and asexual fitness over time might have been caused by physiological or epigenetic changes, in addition to mutational changes. The ancestral lines were propagated under the experimental conditions for one cycle of clonal growth (∼23 cell generations), so that immediate physiological adaptation should have already occurred in both the ancestral and the evolved lines analyzed in this study. Nevertheless, it remains a possibility that some of the fitness differences were caused by physiological or epigenetic changes, which we did not test. The fact that both the sporulation rate and the mean growth rate increased in the LMA lines but decreased in the SMA lines indicates, however, that nongenetic changes were not so large that they overwhelmed the effects of mutation.
Correlations between sexual and asexual function:
The correlation between the mean proportion of asci and the mean growth rate for all lines was significant and positive (Figure 3; Spearman's rank-order correlation, ρ = 0.12, P = 0.0244 by randomization). This is partially driven by the three lines with poor sporulation ability, yet a significant result is found even when these three lines are excluded from analysis (ρ = 0.11, P = 0.0365 by randomization).
Figure 3.—
Correlation between sexual fitness on the y-axis and asexual fitness on the x-axis using all lines. The correlation is significant when all data are included (solid line, Spearman's rank-order correlation, ρ = 0.12, P = 0.0244) and when the poorly sporulating lines (open circles) are excluded (dashed line, ρ = 0.11, P = 0.0365). This positive correlation remains, but is not significant, when LMA lines or SMA lines are analyzed separately.
Estimates of mutational parameters:
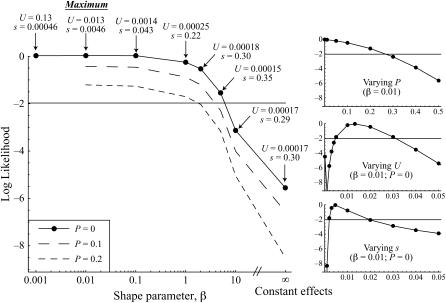
Applying the Bateman–Mukai method to the observed changes in the SMA lines and assuming equal effects of mutations, the mutation rate affecting sporulation rate, Umin,sex, was estimated to be 1.6 × 10−4, and smax,sex was estimated to be 0.38. Maximum-likelihood analyses indicated that the distribution of mutations affecting sexual fitness was most likely L-shaped (β = 0.01). Although we could not reject even more L-shaped distributions, an exponential distribution, or a slightly bell-shaped distribution (95% confidence interval for β: 0–7), an equal-effects model provided a significantly poorer fit to the data by 5.6 log-likelihood units (Figure 4). The maximum-likelihood point estimate for the rate of mutations affecting sporulation ability was Usex = 1.3 × 10−2 with a mean effect of ssex = 0.0046. Confidence intervals for Usex and ssex are reported in Table 2, both when β is held fixed at its maximum-likelihood value (β = 0.01) for comparison to previous results and when the uncertainty in β is incorporated (β-variable). The maximum-likelihood estimate for the proportion of beneficial mutations was Psex = 0, although the possibility that some beneficial mutations arose cannot be rejected (95% C.I. for Psex: 0–0.27 with β = 0.01).
Figure 4.—
Likelihood estimation of mutational parameters affecting sexual fitness. The program mlgenomeu.c was used to compare the sporulation rates of the 78 SMA evolved lines to those of the 128 ancestral lines. The log-likelihood associated with the ML values of Usex and ssex is plotted as a function of the shape parameter β for the gamma distribution (β < 1 corresponds to L-shaped distributions, and β > 1 corresponds to bell-shaped distributions). The straight line lies 2 log-likelihood units below the maximum-likelihood point at β = 0.01. Plots on the right show likelihood profiles for the proportion of beneficial mutations Psex (top), the mutation rate Usex (middle), and the average effect on sporulation rate ssex (bottom), holding β fixed at 0.01.
TABLE 2.
Maximum-likelihood estimates of deleterious mutation rate per diploid genome, U, and mean heterozygous effect, s, for fitness traits in Saccharomyces cerevisiae derived from MA experiments on nonmutator strains
| U estimate (95% C.I.) | s estimate (95% C.I.) | Fitness trait | Reference |
|---|---|---|---|
| 5.5 × 10−5a | 0.217a | Growth rate via competition experiments | Zeyl and DeVisser (2001) |
| (3.5 × 10−5–1.5 × 10−4)a | (0.208–0.236)a | ||
| 1.2 × 10−4b | 0.061b | Maximum growth rate via sliding-window program | Joseph and Hall (2004) |
| (4.6 × 10−5–∞)b | (0–0.077)b | ||
| 1.3 × 10−2c | 0.0046c | Sporulation rate via the proportion of asci | This study |
| (4.8 × 10−3–3.2 × 10−2)d | (0.0019–0.020)d | ||
| (8 × 10−5–∞)c | (0–0.50)c | ||
| 3 × 10−4c | 0.017c | Maximum growth rate via sliding-window program | This study |
| (6 × 10−5–1.1 × 10−3)e | (0.006–0.033)e | ||
| (6 × 10−5–∞)c | (0–0.033)c |
These estimates have not been corrected for selection during colony growth; see Zeyl and DeVisser (2001) and Joseph and Hall (2004) for corrections.
Estimated with β = 60, which yielded the ML fit.
Estimated with β and P allowed to vary; includes petite mutations.
Estimated with β and P allowed to vary.
Estimated with β = 0.01 and P = 0, which yielded the ML fit.
Estimated with β = ∞ and P = 0, which yielded the ML fit.
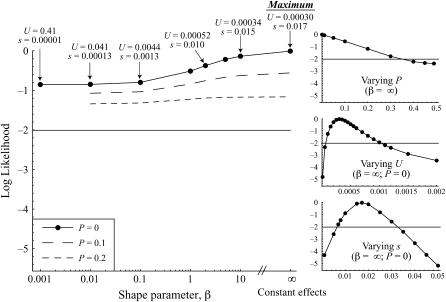
In addition, mutation rate and average effect size per mutation were determined with respect to asexual fitness (growth rate). The BM estimates for Umin,asex and smax,asex were determined to be 3.1 × 10−4 and 0.016, respectively. The ML point estimates were Uasex = 3.0 × 10−4 and sasex = 0.017. There was, however, no statistical support for any value of β over any other (Figure 5), and the confidence intervals for Uasex and sasex are very broad when uncertainty in the shape parameter is incorporated (Table 2). Again, there was no evidence for beneficial mutations affecting growth rate in the SMA lines, as the most likely estimate for Pasex was 0 (95% C.I. for Pasex: 0–0.35, with β set to its most likely value of ∞, corresponding to an equal-effects model). Our estimates are compared to previous estimates of U and s from S. cerevisiae in Table 2.
Figure 5.—
Likelihood estimation of mutational parameters affecting asexual fitness. The program mlgenomeu.c was used to compare the growth rates of the 78 SMA evolved lines to those of the 128 ancestral lines. The log-likelihood associated with the ML values of Uasex and sasex is plotted as a function of the shape parameter β for the gamma distribution. The straight line lies 2 log-likelihood units below the maximum-likelihood point at β = ∞. Plots on the right show likelihood profiles for the proportion of beneficial mutations Pasex (top), the mutation rate Uasex (middle), and the average effect on sporulation rate sasex (bottom), holding β fixed at ∞.
An important caveat that must be remembered when inferring mutational parameters is that the parameter estimates apply only to the component of fitness as measured in the particular environment of the experiment. Given that we detected a block effect when measuring growth rate, the potential exists for environmental heterogeneity that would alter the estimated values of U and s if we were to repeat the fitness assays.
DISCUSSION
To date, no comprehensive explanation for the near ubiquity of sex exists. Many theories have been proposed (Kondrashov 1993), the relevance of which can be evaluated by knowing the time frame over which sexual function can be lost. We have determined the rate at which sporulation rate (an essential component of sexual fitness) changes following a period of asexual growth in a facultatively sexual organism, S. cerevisiae, both when drift dominates selection (SMA) and when selection dominates drift (LMA).
Over a period of 800 generations at low population size so that selection was ineffective (SMA lines), sporulation rate decreased significantly and the variance in sexual ability increased significantly (Figure 1). Three of 78 SMA lines completely lost the ability to sporulate, while the remaining lines had sporulation rates slightly but significantly lower than the ancestral lines. This distribution is consistent with a fairly high mutation rate, a moderate average effect size per mutation, and a broad distribution of effect sizes (Table 2). On average, sexual ability decreased at a rate of 8% over 800 generations. At this rate, it would take ∼44,000 generations for the frequency of sex to drop to 1%. If environmental shifts are required for the maintenance of sex, they need occur only once every 44,000 generations (ignoring, for the moment, pleiotropy with asexual function). This estimate does not take into account other components of sexual function like spore viability, mating ability, or pheromone production, which would likely increase the estimated rate of loss of sex.
Although our maximum-likelihood estimate of the average effect is similar to some previous estimates, our maximum-likelihood estimate of the mutation rate affecting sporulation rate is two orders of magnitude higher than any of the estimates based on asexual growth rate in S. cerevisiae (Table 2). The confidence intervals are broad, however, and overlap when we account for the uncertainty in the shape parameter, β. Nevertheless, there are reasons to suspect that previous estimates of the genomewide mutation rate in yeast might have underestimated the genomewide deleterious mutation rate. By dissecting tetrads and measuring the growth rate of the resulting haploid colonies, Wloch et al. (2001) also reported high mutation rates in S. cerevisiae (0.0011 per cell generation per diploid genome, measuring only those mutations with >1% effect on growth rate). Measuring declines in sexual fitness rather than asexual fitness may provide a more accurate estimate of the total rate of deleterious mutations, Utot, for two reasons. First, sporulation might represent a larger mutational target if many of the genes active during normal cell growth and division are also important during sporulation, in addition to meiosis-specific genes. Second, mutations having a large effect on sporulation are maintained more readily than mutations having a large effect on growth rate in mutation-accumulation experiments, allowing a more complete picture of the distribution of mutational effects. Using sporulation rate as a fitness measure, we inferred that the distribution of mutational effects was L-shaped (ML value of β = 0.01), which allows for a large number of minor-effect mutations as well as a few large-effect mutations (such as the three poor sporulators detected in our study). In contrast, the shape of the mutational distribution based on growth rates tends to be bell shaped [ML values of β = ∞ (this study), β = 60 (Zeyl and DeVisser 2001), and β = 2 (Joseph and Hall 2004)], as expected if there is a bias against accumulating mutations with a major effect on growth rate. As can be seen in Figures 4 and 5, a bias causing β to be overestimated tends to drive down the maximum-likelihood estimate of U.
In an SMA experiment in the haploid fungus C. neoformans, Xu (2002) also observed a significant decrease in sexual fitness over 600 generations in both mating efficiency (a general sexual trait) and filamentation (an organism-specific sexual trait). The amount of decline, however, was much greater (67% decrease in mating efficiency and 24% decrease in filamentation) than the 8% decrease in sporulation ability observed over the 800 generations of our experiment. Using the Bateman–Mukai method, Xu estimated a high genomewide mutation rate (2 × 0.047 for mating efficiency and 2 × 0.013 for filamentation, averaged across strains and doubled to give diploid estimates), which is similar to our estimate for the rate of mutations affecting sporulation ability (Table 2). Xu's estimate for the average effect of a mutation was, however, much higher (0.036 for mating efficiency and 0.073 for filamentation), explaining the more dramatic decline in sexual fitness observed in C. neoformans. Note, however, that these fitness measures are estimated from haploid cells and so directly estimate the strength of selection, whereas the estimates from S. cerevisiae are obtained from diploids where the effects of selection are partially masked by dominance interactions.
When maintained asexually at a large population size, our LMA lines evolved higher sporulation rates (Figure 1). Unlike the distribution of phenotypes in the SMA experiment, the majority of the LMA evolved lines exhibit increased sporulation ability relative to the ancestors, suggesting that selection, not mutation, is driving the improvement. Although possible, we doubt that selection acted directly on sporulation rate during the LMA experiment for two reasons. First, sporulation is induced primarily by nitrogen starvation (specifically, a lack of GTPs), not by the growth conditions of this study (Varma et al. 1985). Indeed, it is often difficult to induce sporulation even when starvation conditions are imposed (Codon et al. 1995). Second, when we examined LMA cultures under the microscope, no spores were ever observed. We thus argue that the rise in sporulation rate in the LMA lines was an indirect response to selection on asexual fitness.
The significantly higher sporulation rates in the evolved LMA lines than in the ancestral lines strongly indicate positive pleiotropy between sporulation and asexual fitness among the mutations accumulated in our LMA experiment. This conclusion is supported by our analysis of the pleiotropic relationship between one aspect of asexual fitness, growth rate, and sporulation rate using all of the lines (Figure 3). A significant positive relationship was found between these two traits, a relationship that remained significant even when the three worst sporulators were eliminated.
That pleiotropy occurs between sexual and asexual fitness is not unexpected. Using a comprehensive library of single-gene deletion mutants of S. cerevisiae, Enyenihi and Saunders (2003) found that only 17% of genes deemed necessary for full sporulation were sporulation-specific genes, the remainder being genes involved in some aspect of vegetative growth.
It is also not too surprising that lines with low sporulation ability exhibited lower growth rates (Figure 3), as positive pleiotropy among deleterious mutations might reflect simply the disruption of genes fundamental to normal cellular functioning during both mitosis and meiosis.
What is surprising is the observation that the adaptive changes occurring in the LMA lines exhibited positive pleiotropy between asexual growth and sporulation rate, as demonstrated by the fact that sporulation rates increased over 800 generations without selection on sporulation ability. This result suggests, quixotically, that sexual reproduction can be maintained, in part, by asexual reproduction. That is, purely asexual growth can improve sexual fitness even in the absence of sex.
Yet there are several caveats to this result. First, previous studies have found negative pleiotropy between sexual and asexual fitness. In C. neoformans, Xu (2002) found a significant negative correlation between growth rate and mating efficiency, although this result was driven by one data point (no significant correlation was observed between growth rate and filamentation). Similarly, Zeyl et al. (2005) found that increases in asexual fitness in evolutionary experiments conducted at large population size were typically accompanied by decreased sexual fitness, both in haploid mating efficiency and in diploid sporulation rates. These experiments in S. cerevisiae were conducted, however, under different environmental conditions (in minimal media or in mice rather than under permissive conditions) and using different yeast strains (the 875 lab strain derived from S288c and a pathogenic strain isolated from humans) than our experiments. Second, it is possible that some mutations with negative pleiotropy did arise in our experiment but were masked by more numerous or more strongly pleiotropic mutations exhibiting positive pleiotropy (Baatz and Wagner 1997). Finally, as suggested to us by Michael Whitlock, our LMA lines might not yet have accumulated mutations that disrupt genes essential for meiosis; such mutations might exhibit especially strong negative pleiotropy. In fact, if ever a mutation disrupting a gene essential to meiosis spreads to fixation within an LMA line (by selection, as drift would be too slow and unlikely in such large populations), then that line will appear to exhibit negative pleiotropy because sporulation will not be recovered no matter how many other mutations accumulate with positive pleiotropic effects (assuming that these cannot compensate for the loss of the essential function). Indeed, whether one observes positive or negative pleiotropy between asexual and sexual fitness in an LMA experiment might well depend on the time frame, with positive pleiotropy being more likely to be observed early when few mutations have accumulated (most of which affect general cell functioning) and negative pleiotropy being more likely to be observed later after the accumulation of several mutations (a few of which disrupt genes essential to meiosis). Indeed, Zeyl et al. (2005) observed just such a temporal trend in one of their experiments.
Because the nature of pleiotropy is obscured by the accumulation of multiple mutations, future studies should focus on the distribution of pleiotropic effects using lines bearing single mutations. Such studies would provide much more concrete information about how often mutations affect sexual and asexual function in the same direction vs. in opposite directions. Future studies should also explore the extent to which the sign of pleiotropy depends on the environment. Such studies would allow more general conclusions about how often selection on asexual function promotes the maintenance of sexual function, as observed in this study.
Acknowledgments
The authors gratefully acknowledge the comments and suggestions of A. Gerstein, C. Jordan, R. Redfield, C. Spencer, M. Uyenoyama, M. Whitlock, the Otto lab, the Schluter–Otto–Whitlock–Doebeli (SOWD) lab group, and two anonymous reviewers, as well as the technical support of L. Glaubach and P. Wu. We thank P. Keightley for providing his maximum-likelihood program and for his personal assistance in running the program and C. Zeyl for providing the strains used in this study and for assistance on yeast experimental techniques. Funding was provided by a Natural Sciences and Engineering Research Council (Canada) discovery grant to S.P.O. and by a University of British Columbia graduate fellowship to J.A.H.
References
- Baatz, M., and G. P. Wagner, 1997. Adaptive inertia caused by hidden pleiotropic effects. Theor. Popul. Biol. 51: 49–66. [Google Scholar]
- Barton, N. H., and B. Charlesworth, 1998. Why sex and recombination? Science 281: 1986–1990. [PubMed] [Google Scholar]
- Bateman, A. J., 1959. The viability of near-normal irradiated chromosomes. Int. J. Radiat. Biol. 1: 170–180. [Google Scholar]
- Bell, G., 1982. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. University of California Press, Berkeley, CA.
- Codon, A. C., J. M. Gasent-Ramirez and T. Benitez, 1995. Factors which affect the frequency of sporulation and tetrad formation in Saccharomyces cerevisiae baker's yeasts. Appl. Environ. Microbiol. 61: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyenihi, A. H., and W. S. Saunders, 2003. Large-scale functional genomic analysis of sporulation and meiosis in Saccharomyces cerevisiae. Genetics 163: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey, M., 1997. A review of phenotypes in Saccharomyces cerevisiae. Yeast 13: 1099–1133. [DOI] [PubMed] [Google Scholar]
- Joseph, S. B., and D. W. Hall, 2004. Spontaneous mutations in diploid Saccharomyces cerevisiae: more beneficial than expected. Genetics 168: 1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley, P. D., 1994. The distribution of mutation effects on viability in Drosophila melanogaster. Genetics 138: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley, P. D., 1998. Inference of genomewide mutation rates and distributions of mutation effects for fitness traits: a simulation study. Genetics 150: 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibota, T. T., and M. Lynch, 1996. Estimate of the genomic mutation rate deleterious to overall fitness in E. coli. Nature 381: 694–696. [DOI] [PubMed] [Google Scholar]
- Kondrashov, A. S., 1993. Classification of hypotheses on the advantage of amphimixis. J. Hered. 84: 372–387. [DOI] [PubMed] [Google Scholar]
- Korona, R., 1999. Unpredictable fitness transitions between haploid and diploid strains of the genetically loaded yeast Saccharomyces cerevisiae. Genetics 151: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michod, R. E., and B. R. Levin, 1988. The Evolution of Sex. Sinauer Associates, Sunderland, MA.
- Mukai, T., 1964. The genetic structure of natural populations of Drosophila melanogaster. I. Spontaneous mutation rate of polygenes controlling viability. Genetics 50: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai, T., S. J. Chigusa, L. E. Mettler and J. F. Crow, 1972. Mutation rate and dominance of genes affecting viability in Drosophila melanogaster. Genetics 72: 335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto, S. P., and T. Lenormand, 2002. Resolving the paradox of sex and recombination. Nat. Rev. Genet. 3: 252–261. [DOI] [PubMed] [Google Scholar]
- Varma, A., E. B. Freese and E. Freese, 1985. Partial deprivation of GTP initiates meiosis and sporulation in Saccharomyces cerevisiae. Mol. Gen. Genet. 201: 1–6. [DOI] [PubMed] [Google Scholar]
- Wahl, L. M., and P. J. Gerrish, 2001. The probability that beneficial mutations are lost in populations with periodic bottlenecks. Evolution 55: 2606–2610. [DOI] [PubMed] [Google Scholar]
- Wloch, D. M., K. Szafraniec, R. H. Borts and R. Korona, 2001. Direct estimate of the mutation rate and the distribution of fitness effects in the yeast Saccharomyces cerevisiae. Genetics 159: 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram Research, 2003. Mathematica, Version 5.0. Wolfram Research, Champaign, IL.
- Xu, J., 2002. Estimating the spontaneous mutation rate of loss of sex in the human pathogenic fungus Cryptococcus neoformans. Genetics 162: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyl, C., 2005. The number of mutations selected during adaptation in a laboratory population of Saccharomyces cerevisiae. Genetics 169: 1825–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyl, C., and J. A. G. M. DeVisser, 2001. Estimates of the rate and distribution of fitness effects of spontaneous mutation in Saccharomyces cerevisiae. Genetics 157: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyl, C., C. Curtin, K. Karnap and E. Beauchamp, 2005. Antagonism between sexual and natural selection in experimental populations of Saccharomyces cerevisiae. Evolution 59: 2109–2115. [PubMed] [Google Scholar]