Abstract
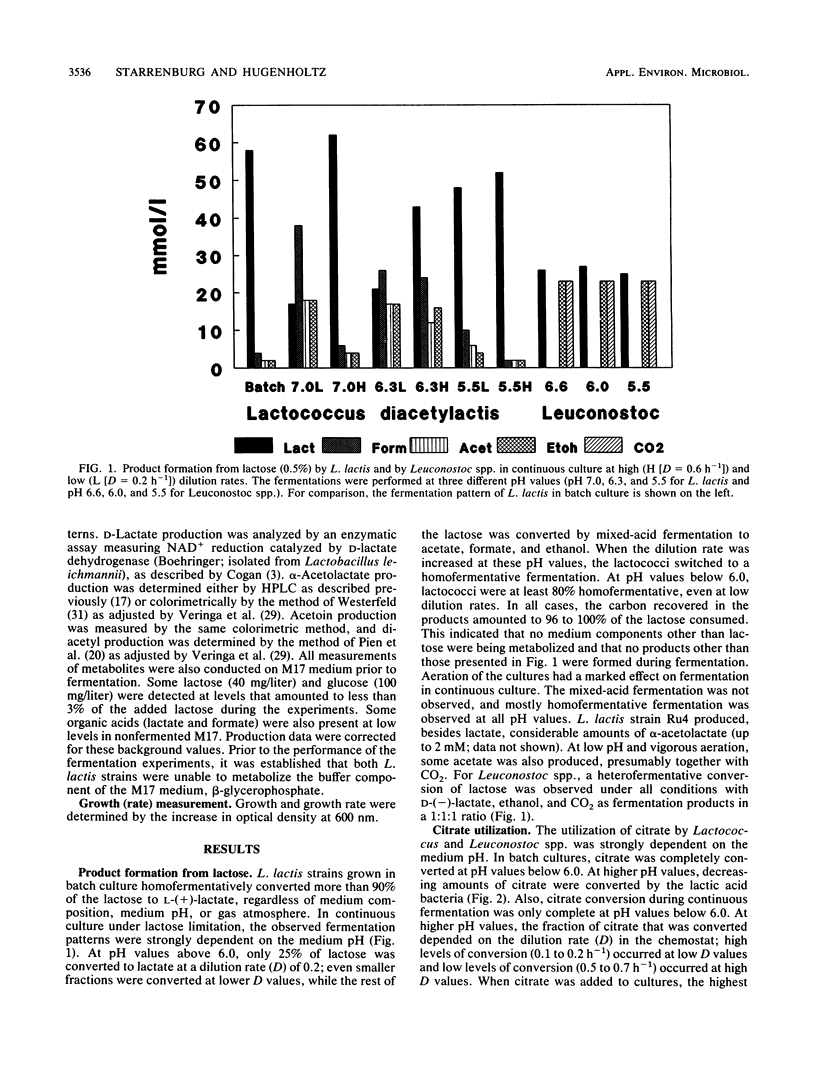
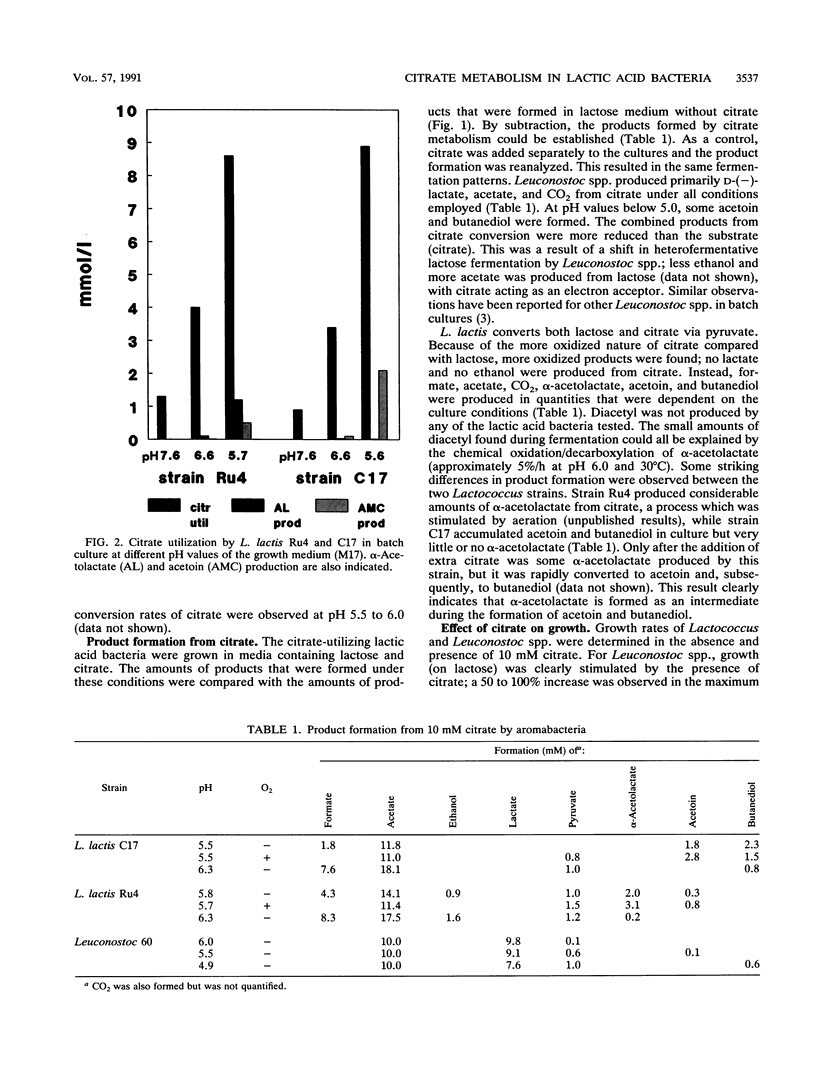
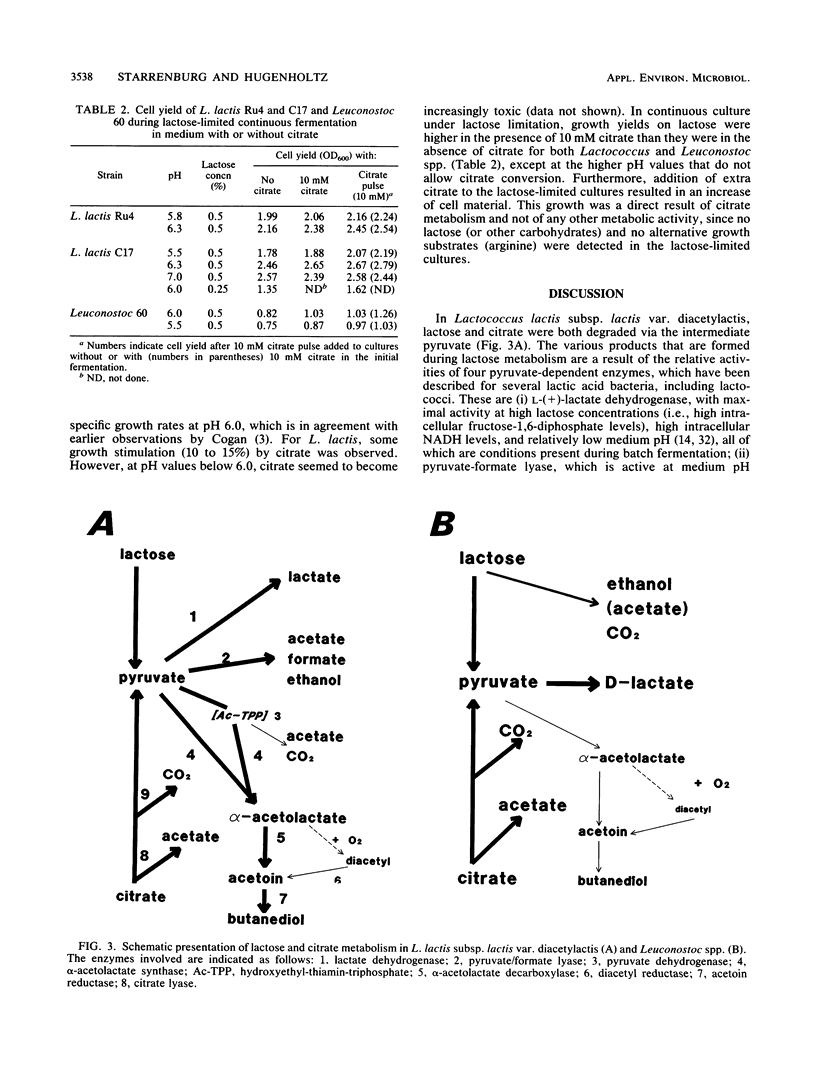
Citrate and lactose fermentation are subject to the same metabolic regulation. In both processes, pyruvate is the key intermediate. Lactococcus lactis subsp. lactis biovar diacetylactis homofermentatively converted pyruvate to lactate at high dilution (growth) rates, low pH, and high lactose concentrations. Mixed-acid fermentation with formate, ethanol, and acetate as products was observed under conditions of lactose limitation in continuous culture at pH values above 6.0. An acetoin/butanediol fermentation with α-acetolactate as an intermediate was found upon mild aeration in continuous culture and under conditions of excess pyruvate production from citrate. Leuconostoc spp. showed a limited metabolic flexibility. A typical heterofermentative conversion of lactose was observed under all conditions in both continuous and batch cultures. The pyruvate produced from either lactose or citrate was converted to d-lactate. Citrate utilization was pH dependent in both L. lactis and Leuconostoc spp., with maximum rates observed between pH 5.5 and 6.0. The maximum specific growth rate was slightly stimulated by citrate, in L. lactis and greatly stimulated by citrate in Leuconostoc spp., and the conversion of citrate resulted in increased growth yields on lactose for both L. lactis and Leuconostoc spp. This indicates that energy is conserved during the metabolism of citrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Involvement of oxygen-sensitive pyruvate formate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982 Oct;152(1):175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome M. C., Thomas M. P., Hillier A. J., Jago G. R. Pyruvate dehydrogenase activity in group N streptococci. Aust J Biol Sci. 1980 Mar;33(1):15–25. [PubMed] [Google Scholar]
- Cogan T. M., O'dowd M., Mellerick D. Effects of pH and Sugar on Acetoin Production from Citrate by Leuconostoc lactis. Appl Environ Microbiol. 1981 Jan;41(1):1–8. doi: 10.1128/aem.41.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., van der Rest M. E., Driessen A. J., Simons G., de Vos W. M. Nucleotide sequence and expression in Escherichia coli of the Lactococcus lactis citrate permease gene. J Bacteriol. 1990 Oct;172(10):5789–5794. doi: 10.1128/jb.172.10.5789-5794.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P. Sodium ion transport decarboxylases and other aspects of sodium ion cycling in bacteria. Microbiol Rev. 1987 Sep;51(3):320–340. doi: 10.1128/mr.51.3.320-340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinan D. F., Robin S., Cogan T. M. Citric acid metabolism in hetero- and homofermentative lactic acid bacteria. Appl Environ Microbiol. 1976 Apr;31(4):481–486. doi: 10.1128/aem.31.4.481-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P. Bacterial Na+ energetics. FEBS Lett. 1989 Jun 19;250(1):106–114. doi: 10.1016/0014-5793(89)80693-3. [DOI] [PubMed] [Google Scholar]
- Strobel H. J., Russell J. B., Driessen A. J., Konings W. N. Transport of amino acids in Lactobacillus casei by proton-motive-force-dependent and non-proton-motive-force-dependent mechanisms. J Bacteriol. 1989 Jan;171(1):280–284. doi: 10.1128/jb.171.1.280-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]


