Abstract
In Neurospora, the circadian rhythm is expressed as rhythmic conidiation driven by a feedback loop involving the protein products of frq (frequency), wc-1 (white collar-1), and wc-2, known as the frq/wc (FWC) oscillator. Although strains carrying null mutations such as frq10 or wc-2Δ lack a functional FWC oscillator and do not show a rhythm under most conditions, a rhythm can be observed in them by the addition of geraniol or farnesol to the media. Employing this altered media as an assay, the effect of other clock mutations in a frq10- or wc-2Δ-null background can be measured. It was found that the existing clock mutations fall into three classes: (1) those, such as prd-3 or prd-4 or frq1, that showed no effect in a clock null background; (2) those, such as prd-1 or prd-2 or prd-6, that did have a measurable effect in the frq10 background; and (3) those, such as the new mutation ult, that suppressed the frq10 or wc-2Δ effect, i.e., geraniol/farnesol was not required for a visible rhythm. This classification suggests that some of the known clock mutations are part of a broader multioscillator system.
NEUROSPORA crassa, an ascomycete fungus, is a model species for studying circadian rhythms due to its readily apparent rhythm in its pattern of conidiation, or asexual spore formation, its haploid nature, and the fact that its genome has been sequenced and methodically mapped. Specifically, when grown on agar media, Neurospora exhibits a growth pattern of alternating regions of conidiation, or “bands,” and regions without conidiation and with less dense mycelial mass.
It is generally held that this conidiation cycle is partially the product of a negative molecular feedback loop involving the rhythmic levels of products of the frq (frequency), wc-1 (white collar), and wc-2 genes, or the frq/wc (FWC) oscillator (Dunlap et al. 1995; He et al. 2005b) . There are also positive feedback mechanisms, as well as an important role played by post-translational modifications (Liu 2005) and protein degradation. More details, in a recent review of the proteins involved in the Neurospora clock function, are found in an up-to-date and clear summary (Dunlap 2006). Other recent articles and reviews provide further information (Brunner and Schafmeier 2006; de Paula et al. 2006; Liu and Bell-Pedersen 2006). In addition, a review of the Neurospora rhythm indicates that its inherent complexities are characteristic of a multioscillator system (Lakin-Thomas and Brody 2004).
Oscillators other than the FWC oscillator, sometimes referred to as frq-less oscillators (FLO), have been proposed on the basis of residual conidiation rhythms in the frq-null strain frq9 (Iwasaki and Dunlap 2000) and in csn-2 mutants defective in FRQ degradation (He et al. 2005a). Additionally, the levels of nitrate reductase in the frq-null strain frq9 (Christensen et al. 2004) and the mRNA levels of some clock-controlled genes (ccgs) in the frq10 strain, in which the frq gene is deleted, have been found to be rhythmic (Correa et al. 2003).
The presence of a FLO (or FLOs) is further suggested by the finding that “clock null” mutants, such as frq10, wc-1Δ, and wc-2Δ (total deletions), that lack conidiation rhythmicity under standard conditions, exhibited rhythmicity when grown on media enriched with farnesol or geraniol (Granshaw et al. 2003) or when combined with certain mutations affecting lipid biosynthesis, such as the cel or chol mutations (Lakin-Thomas and Brody 2000). As these mutants all contain deletions of the noted gene (Aronson et al. 1994; Collett et al. 2002), with frq10 and wc-2Δ expressing very low levels of WC-1 and FRQ, respectively (Lee et al. 2000; Collett et al. 2002), the observation of banding in these strains suggests the presence of an alternate oscillator (or oscillators) that was prompted to action by the addition of farnesol or geraniol or by alteration in lipid biosynthesis. Additional observations about the presence of alternate oscillators can be found in recent articles (Bell-Pedersen et al. 2005; dePaula et al. 2006; Lakin-Thomas 2006) as well as in previous articles (Loros and Feldman 1986; Merrow et al. 1999).
This ability to see a visible rhythm in clock null strains can be used as a means to classify which mutations could act independently of the FWC oscillator and thus which may target another oscillator or oscillators. To examine this hypothesis of a multioscillator system, double mutants with a clock null (frq10, wc-2Δ) and a mutant exhibiting an abnormal period (≠22 hr) were constructed and grown on farnesol- or geraniol-enriched media to see if the second mutation affected the null's observed period. The failure of the second mutation to affect the null's observed period would suggest that the second mutation targets the first oscillator since when this target is deleted, no change in the rhythmicity is then seen. Whereas, a change in the null's observed period due to the presence of the second mutation would indicate that the second mutation has as its target some component other than in the FWC oscillator.
The focus of this investigation was on the behavior of the prd mutants in a null background, but to utilize such an assay, it was imperative to have a “proof of principle,” i.e., to first confirm that known point mutations of one of the three genes of the FWC oscillator had no effect in the null background. Thus, the double mutants bd frq1 wc-2Δ, bd frq7 wc-2Δ, and bd frq10 wc-2 ER24 were constructed and characterized on farnesol- or geraniol-enriched media.
MATERIALS AND METHODS
Strains and growth conditions:
The general growth conditions were as previously described (Granshaw et al. 2003). If the media contained farnesol where farnesol refers to trans, trans-farnesol, or geraniol then a fresh 10% solution in 100% ethanol was made that day and added to cooled (65°) media after autoclaving. The final concentration of farnesol in the media for strains containing frq10 was 5.4 × 10−5 m while for those containing wc-2Δ it was 2.7 × 10−5 m. The final concentration of geraniol for the frq10 strains was 3.9 × 10−4 m. The one exception to these concentrations was the farnesol experiments performed on bd prd-1 frq10; the concentration used was 5.4 × 10−4 m. Farnesol and geraniol were purchased from Sigma (St. Louis), catalog nos. 277541 and G-5135, respectively. Crosses were carried out on synthetic crossing media (Davis and Deserres 1970).
All strains used, unless otherwise noted, contained the bd mutation to allow banding in spite of CO2 accumulation in growth tubes (Sargent and Kaltenborn 1972). Those noted as containing the csp mutation contain the csp-1 mutation to prevent self-inoculation (Brody and Martins 1979). The sources of the strains utilized are indicated in Table 1. The annotation of S.G. used in the supplemental Methods section (supplemental Appendix at http://www.genetics.org/supplemental/) refers to our silica gel number.
TABLE 1.
Mutations employed
| Strain | Comments | Period (hr)a | Source |
|---|---|---|---|
| frq1 | Frequency gene allele | 16.5 | D. D. Perkins |
| frq7 | Frequency gene allele | 29 | FGSC no. 4898b |
| frq10 | Clock null, hygR(hygromycin B resistant) due to gene replacement | Arhythmic | J. C. Dunlap |
| prd-1 | Period | 25–26 | J. F. Feldman |
| prd-2 | 25 | FGSC no. 4904 | |
| prd-3 | 25 | FGSC no. 4906 | |
| prd-4 | 18 | FGSC no. 4907 | |
| prd-6 | 19 | L. W. Morgan | |
| ult | Ultradian | 12 | This laboratory |
| wc-2 ER24 | Temperature sensitive | 29c | J. C. Dunlap, FGSC no. 4405 |
| wc-2Δ | Clock null, hygR(hygromycin B resistant) | Arhythmic | J. C. Dunlap |
At 25°, except for ult, which is at 22°, and wc-2 ER24 at 26°.
Fungal Genetics Stock Center, http://www.fgsc.net.
At permissive temperature of 26° (Degli-Innocenti and Russo 1984; Collett et al. 2001).
Period calculation and double-mutant isolation:
The growth fronts of cultures were marked under red light and, after a strain had finished growing, bands were marked and transferred to paper along with growth marks. The marks on paper were then scanned into a computer and analyzed with Program Tau, developed in this lab by Fred Hajjar and modified by Mike Ferry. Average growth rates and periods (τ) were calculated by averaging sets of five growth tubes per condition. The numbers (n) in the tables are individual periods, not the number of growth tubes. SD is the standard deviation. Growth tubes were of the conventional size, i.e., 27 cm long, 1.1 cm internal diameter width.
Standard genetic techniques for Neurospora crosses were employed and the details for the construction and characterization of the various double mutants are given in a supplemental Methods section (supplemental Appendix at http://www.genetics.org/supplemental/). Putative double mutants were then individually backcrossed to the bd strain to demonstrate segregation of the original markers. Only strains whose genotypes were confirmed by backcrossing or by sequencing were then analyzed.
Imaging/densitometry:
Growth tubes were illuminated from the ends and pictures taken with a digital camera from directly above. The initial pixel densities of the tubes were obtained by employing the Plot Profile function of the Image J program. Pixel density tracings were then analyzed with either the MatLab smoothing programs moving average or with R-loess. The parameters for these programs were: smoothing window of 41, peak finder of 5.
PCR and DNA sequencing:
Genomic DNA of N. crassa was extracted using the Puregene DNA Isolation kit (Gentra Systems, Research Triangle Park, NC). PCR was performed on genomic DNA from strains of interest with Pfu DNA polymerase (Stratagene, La Jolla, CA) and primers designed to the published frq and wc-2 sequences (Aronson et al. 1994; Collett et al. 2001) . PCR products visible as a single band were purified using the QIAquick gel purification kit (QIAGEN, Valencia, CA). Sequencing was performed by Eton Biosciences (San Diego).
RESULTS
The frq1 and frq7 mutations failed to have an effect in wc-2 null:
To test the hypothesis that mutations that affect the first oscillator will not have an effect when combined with a deletion that disables the FWC oscillator, bd frq1 wc-2Δ and bd frq7 wc-2Δ double mutants were constructed and characterized on farnesol- or geraniol-enriched media. These two frq point mutations were selected to assay as their phenotypes represent the extremes of the aberrant periods produced by frq point mutations, with the frq1 mutation producing a reduced period (∼16.5 hr), while the frq7 mutation produces a long period (∼29 hr) (Feldman 1982).
The double mutants were isolated by crossing the frq point mutant to the wc-2Δ null and screening the null progeny for the frq point mutation via sequencing. As predicted, on minimal media, bd wc-2Δ, bd frq1 wc-2Δ, and bd frq7 wc-2Δ strains exhibited arrhythmic conidiation. Upon supplementation with farnesol, both bd frq1 wc-2Δ and bd frq7 wc-2Δ strains had average periods that failed to deviate from the period of the bd wc-2Δ control strain (Table 2, Figure 1A). As shown previously (Granshaw et al. 2003), there were no significant period effects of farnesol/geraniol on the bd or the bdcsp strains.
TABLE 2.
frq1 wc-2Δ and frq7 wc-2Δ double mutants
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd csp frq1 | 22° | Farnesol | 16.3 | 123 | 2.5 | 1.2 |
| bd wc-2Δ | 22° | Farnesol | 21.1 | 137 | 3.5 | 1.4 |
| bd frq1 wc-2Δ#2 | 22° | Farnesol | 20.9 | 123 | 3.7 | 1.4 |
| bd frq1 wc-2Δ#5 | 22° | Farnesol | 21.1 | 65 | 3.5 | 1.4 |
| bd frq7 | 22° | Farnesol | 28.7 | 120 | 2.1 | 1.2 |
| bd wc-2Δ | 22° | Farnesol | 19.8 | 117 | 3.1 | 1.4 |
| bd frq7 wc-2Δ#2 | 22° | Farnesol | 19.7 | 156 | 2.8 | 1.4 |
Figure 1.—
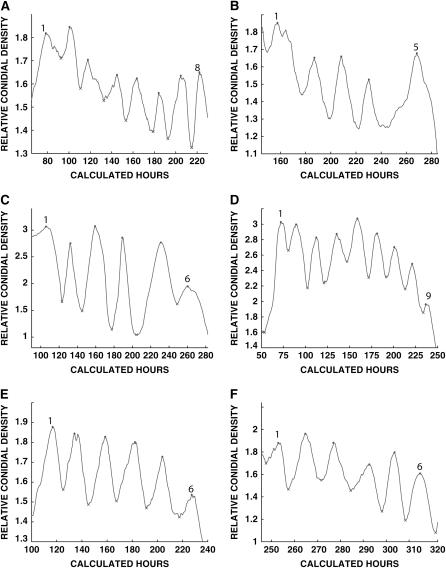
Densitometric tracings of representative growth tube cultures. The x-axis is in calculated hours, and the y-axis is in relative conidial density. (A) bd frq1 wc-2Δ#5 . Peak 1 had its maximum at 78.5 hr, and peak 8 had its maximum at 222 hr. See Table 2. (B) bd frq10 prd-1#49. Peak 1, 157 hr; peak 5, 268 hr. See Table 3. (C) bd frq10 prd-2#22. Peak 1 had its maximum at 106 hr, and peak 6 had its maximum at 260 hr. See Table 4. (D) bd frq10 prd-3#8. Peak 1, 72 hr; peak 9, 238 hr. See Table 6. (E) bd frq10 prd-4#56. Peak 1, 117 hr; peak 6, 227 hr. See Table 7. (F) bd frq 10 prd-6#13. Peak 1, 235 hr; peak 6, 316 hr. See Table 5.
Further, to inspect whether the means of disabling the FWC oscillator would affect the result, in contrast to the previous two double mutants, a frq10 wc-2 ER24 double mutant was constructed. The wc-2 point mutation ER24 has a period at permissive temperatures of ∼29 hr (Collett et al. 2001). The double mutants were isolated by sequencing the wc-2 gene of frq10 (hygromycin B-resistant) progeny. Characterization was performed on several isolates (data not shown) and no statistically significant differences were found between the periods of these isolates and the parental strain, frq10 .
The prd-1, prd-2, and prd-6 mutations affected the period in the absence of a functional FWC oscillator:
The prd-1 mutation has not been cloned and produces a long-period phenotype (∼26 hr at 25°) (Morgan et al. 2001). Three bd frq10 prd-1 double mutants were isolated and all exhibited arrhythmic conidiation on minimal media but long periods upon supplementation. In Table 3 and Figure 1B data are presented for one of the double mutants with farnesol supplementation at 25°, but similar long periods were obtained for the other two bd frq10 prd-1 strains isolated, as well as with geraniol supplementation. The different temperature employed for these strains or the use of a different supplement for others was just to improve the clarity of the banding. The prd-1 mutation clearly lengthens the period of bd frq10 and, hence, maintains the ability to affect the rhythm in the absence of a functional FWC oscillator.
TABLE 3.
frq10 prd-1 double mutant
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd frq10 | 25° | Farnesol | 17.1 | 23 | 2.8 | 1.2 |
| bd prd-1 | 25° | Farnesol | 27.4 | 20 | 3.8 | 0.9 |
| bd frq10 prd-1#49 | 25° | Farnesol | 27.6* | 16 | 4.5 | 0.8 |
P < 0.0001 when compared to frq10.
The prd-2 mutation also had an effect in the frq10 background, as bd frq10 prd-2 double mutants exhibited an average period ∼20% greater than that of bd frq10 upon supplementation with geraniol (Table 4 and Figure 1C). This increase corresponds to the increased period behavior of the prd-2 mutant on minimal media (25 hr) (Feldman 1982).
TABLE 4.
frq10 prd-2 double mutants
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd frq10 | 16° | Geraniol | 25.8 | 54 | 1.4 | 0.8 |
| bd prd-2 | 16° | Geraniol | 29 | 28 | 0.6 | 0.7 |
| bd frq10 prd-2#19 | 16° | Geraniol | 31.1 | 14 | 0.8 | 0.7 |
| bd frq10 prd-2#22 | 16° | Geraniol | 30.5 | 25 | 1.7 | 0.7 |
The prd-6 mutation had a shortening (41%) effect in the frq10 background (Table 5 and Figure 1F). The prd-6 mutation has a shortening (30%) effect in the frq+ background as well. It is not known whether or not the difference between 41 and 30% suggests some type of interaction between the prd-6 gene product and the residual FWC. The ability of the prd-1, prd-2, and prd-6 mutations to alter the period of a frq10 strain indicates that these mutations are able to have an effect on rhythmicity in the absence of a functional FWC oscillator and, therefore, that these mutations possibly affect a separate oscillator (or oscillators).
TABLE 5.
frq10 prd-6 double mutants
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd frq10 | 22° | Geraniol | 21.3 | 21 | 3.0 | 0.9 |
| bd prd-6 | 22° | Geraniol | 19.7 | 62 | 2.3 | 0.9 |
| bd frq10 prd-6#9 | 22° | Geraniol | 14.5 | 62 | 2.2 | 0.9 |
| bd frq10 prd-6#13 | 22° | Geraniol | 15.3 | 41 | 2.5 | 0.9 |
The prd-3 and prd-4 mutations failed to have an effect when the FWC oscillator was disabled:
Unlike the bd frq10 prd-1 and bd frq10 prd-2 double mutants that had average periods that differed significantly from the period of the bd frq10 control strain, bd frq10 prd-3 double mutants and bd frq10 prd-4 double mutants did not show any period deviation upon supplementation (Table 6 and 7; Figure 1, D and E). This indicates that the prd-3 and prd-4 mutations may produce aberrant periods by affecting the FWC oscillator and/or that the mutation does not affect any components of the farnesol/geraniol mechanism.
TABLE 6.
frq10 prd-3 double mutants
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd frq10 | 22° | Geraniol | 19.6 | 33 | 2.9 | 1.1 |
| bd prd-3 | 22° | Geraniol | 25.8 | 25 | 2.5 | 1.0 |
| bd frq10 prd-3#8 | 22° | Geraniol | 19.8 | 23 | 2.5 | 0.9 |
| bd frq10 prd-3#4 | 22° | Geraniol | 19.7 | 9 | 1.8 | 1.0 |
TABLE 7.
frq10 prd-4 double mutants
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd frq10 | 22° | Geraniol | 22.0 | 80 | 2.9 | 1.0 |
| bd prd-4 | 22° | Geraniol | 20.0 | 111 | 2.0 | 1.3 |
| bd frq10 prd-4#56 | 22° | Geraniol | 21.5 | 103 | 4.0 | 1.1 |
| bd frq10 prd-4#66 | 22° | Geraniol | 22.3 | 69 | 4.0 | 1.0 |
The novel ult mutation allowed expression of a conidiation rhythm in null strains without supplementation:
This lab has isolated a novel clock mutant ult (ultradian) that has a conidiation rhythm of ∼12 hr (Table 8 and Figure 2A). This mutation has not been mapped or the gene cloned. The mutant strain has the unusual property of producing small bands between large bands (Figure 2A) to give the 12-hr period. bd frq10 ult and bd ult-1 wc-2Δ double mutants were constructed and tested on farnesol or geraniol or minimal media. In contrast to any known clock null strain, approximately half of the hygromycin B-resistant progeny from each cross exhibited a conidiation rhythm on minimal media. The ult mutation was clearly epistatic to the frq10 mutation, as the average period of the bd frq10 ult double mutants was ∼12 hr on both minimal (Table 8 and Figure 2B) and geraniol-supplemented media (Table 8 and Figure 2C). On farnesol-supplemented media, the average periods for both bd ult and the double mutants increased to ∼13.5 hr (Table 8 and Figure 2D).
TABLE 8.
frq10 ult double mutant
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd frq10 | 22° | Minimal | 0 | — | — | 1.1 |
| bd ult | 22° | Minimal | 11.7 | 35 | 2.0 | 0.9 |
| bd frq10 ult#12 | 22° | Minimal | 12.6 | 34 | 1.7 | 0.8 |
| bd csp-1 | 22° | Minimal | 21.5 | 43 | 2.0 | 1.3 |
| bd frq10 | 22° | Geraniol | 19.9 | 23 | 2.1 | 1.1 |
| bd ult | 22° | Geraniol | 11.4 | 38 | 1.4 | 1.0 |
| bd frq10 ult#12 | 22° | Geraniol | 12.0* | 31 | 1.9 | 0.8 |
| bd frq10 | 22° | Farnesol | 22.2 | 16 | 2.4 | 1.0 |
| bd ult | 22° | Farnesol | 13.9 | 18 | 1.0 | 0.8 |
| bd frq10 ult#12 | 22° | Farnesol | 13.2* | 34 | 1.8 | 0.7 |
P < 0.0001 vs. frq10 on geraniol.
Figure 2.—
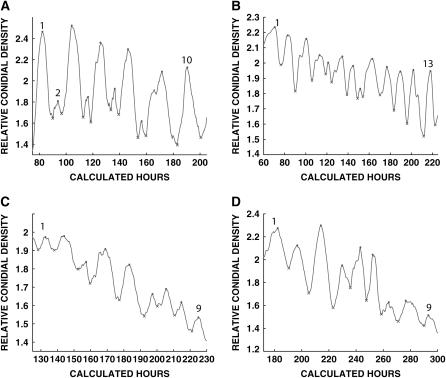
Densitometric tracings, as for Figure 1. (A) bd ult grown on minimal media. Peak 1, 82 hr; peak 10, 190 hr. See Table 8. (B) bd frq10 ult#12 (minimal media). Peak 1, 71 hr; peak 13, 218 hr. See Table 8. (C) bd frq10 ult#12 (geraniol media) . Peak 1, 132 hr; peak 9, 225 hr. See Table 8. (D) bd frq10ult#12 (farnesol media). Peak 1, 182 hr; peak 9, 293 hr. See Table 8.
Without supplementation, the bd ult wc-2Δ double mutants displayed a clear, strong rhythm with an average period of ∼16.5 hr (Table 9 and Figure 3A). With farnesol supplementation, the average period of the double mutants decreased to ∼15 hr (Table 9 and Figure 3, C and D), representing a 32% decrease in period length compared to the period of the bd wc-2Δ control strain. These results demonstrate that the ult mutation requires neither the FWC oscillator nor the possible alternate oscillator (or oscillators) expressed upon enrichment with farnesol/geraniol. That the average periods for the bd frq10 ult and bd ult wc-2Δ double mutants on minimal media differ indicates, though, that the means of disabling the FWC oscillator change the effects of the ult mutation.
TABLE 9.
ult wc-2Δ double mutants
| Strain | Temperature | Supplement | τ (hr) | n | SD | Growth rate (mm/hr) |
|---|---|---|---|---|---|---|
| bd wc-2Δ | 22° | Minimal | 0 | — | — | 1.3 |
| bd ult | 22° | Minimal | 11.7 | 100 | 1.7 | 1.0 |
| bd ult wc-2Δ#29 | 22° | Minimal | 16.6 | 29 | 1.8 | 1.3 |
| bd ult wc-2Δ#31 | 22° | Minimal | 16.4 | 34 | 1.9 | 1.4 |
| bd wc-2Δ | 22° | Farnesol | 21.8 | 13 | 2.7 | 1.2 |
| bd ult | 22° | Farnesol | 12.8 | 67 | 1.8 | 0.9 |
| bd ult wc-2Δ#29 | 22° | Farnesol | 14.8* | 45 | 1.9 | 1.0 |
| bd ult wc-2Δ#31 | 22° | Farnesol | 15.1* | 43 | 1.8 | 1.0 |
P < 0.0001 vs. bd wc-2Δ on farnesol.
Figure 3.—
Densitometric tracings as for Figure 1. (A) bd ult wc-2Δ#31(minimal media). Peak 1, 56 hr; peak 10, 200 hr. See Table 9. (B) bd ult wc-2Δ#31 (farnesol media). Peak 1, 68 hr; peak 11, 221 hr. See Table 9. (C) bd ult wc-2Δ#29 (farnesol media). Peak 1, 162 hr; peak 8, 277 hr. See Table 9. (D) bd csp-1 (minimal media). Peak 1, 44 hr; peak 6, 153 hr. See Table 8.
DISCUSSION
Mutant classification:
The frq1 and frq7 mutations both failed to have an effect on the period when the FWC oscillator was disabled by the wc-2Δ mutation. Similarly, the wc-2 ER24 mutation failed to have an effect when the FWC oscillator was disabled by the frq10 mutation. This evidence would suggest that these second mutations affecting the FWC oscillator cannot have a period effect in the presence of another FWC null mutation even when these null mutants' rhythm is expressed by farnesol or geraniol. This demonstrates that this expression of a rhythm can be used as a valid assay for determining which mutations affect the FWC oscillator and which do not. It can also be inferred that the prd-3 and prd-4 mutations affect the FWC oscillator and should be put into this class as well (Table 10). The data do not allow any inference as to whether the frq1 or frq7 or wc-2 ER24 mutations may, under some conditions, have effects on the oscillator responsible for the farnesol/geraniol effect.
TABLE 10.
Mutant classification
Similarly, those mutations that either allow expression of rhythmicity in a clock null strain, such as ult and, as previously reported, cel and chol (Lakin-Thomas and Brody 2000), or alter the period of the null strain, such as prd-1, prd-2, and prd-6, can be classified as able to have clock effects in the absence of a functional FWC oscillator. It cannot be ruled out that these mutations may also have some effect on the FWC oscillator. Thus, mutations in many genes have been divided into two groups: those that have no effect in the absence of the FWC oscillator and those that are able to have an effect (Table 10). This second group of mutations may affect an alternate oscillator such as the FLO, or they may affect some part of the signaling mechanism between oscillators.
Expanding on the classification outlined above, it is clear that double mutants can then be classified as containing two mutations targeting the FWC oscillator, such as frq7 prd-3, two mutations that can act independently of the FWC oscillator, such as prd-1 cel, or a combination, such as frq7 cel. Approximately 30 of the >100 possible such double mutants have been constructed and characterized (Lakin-Thomas and Brody 1985, 2000; Lakin-Thomas 1998; Morgan and Feldman 2001; Compton and Feldman 2004; L. W. Morgan, personal communication). In this investigation, the prd-1, prd-2, prd-3, prd-4, and prd-6 mutations were each studied in the frq10 background, but these prd mutations could also be assayed in the wc-2Δ background to further address the inherent differences between a frq null and a wc-2 null. It would be particularly interesting if a prd mutation was able to have an effect on the rhythm in one background but not in another, or vice versa, as this might indicate a requirement for expression. This is pertinent due to the role of the WC proteins in activating the transcription of the myriad ccgs (Lewis et al. 2002; Correa et al. 2003) responsible for regulating metabolic and developmental processes (Dunlap and Loros 2004). Additionally, the prd-6 mutation should be studied further as this mutation has been reported to be epistatic to the prd-2 and prd-3 mutations and able to suppress the frq7 mutation (Morgan and Feldman 2001; Morgan et al. 2001).
FWC oscillator mutation effects:
The ability of the prd-1, prd-2, and prd-6 mutations to affect rhythmicity in the absence of a functional FWC oscillator indicates that they affect some alternate oscillatory mechanism. It has previously been found that the membranes of prd-1 mutants have altered fatty acid composition (Cote and Brody 1987). Additionally, the prd-1 mutation is epistatic to the period-lengthening effects of linoleic acid (18:2) on the cel mutation defective in fatty acid synthesis (Lakin-Thomas and Brody 1985), and, as the cel mutation allows expression of rhythmicity in a frq-null strain (Lakin-Thomas and Brody 2000), it is possible that these mutations affect a lipid oscillatory mechanism. Oscillations of 18:2 and 18:3 levels have been previously reported as having periods of ∼20 hr (Roeder et al. 1982).
It has been found that the prd-4 mutation results in a checkpoint kinase 2 (CHK2) gain of function that causes an increased phosphorylation of FRQ (Pregueiro et al. 2006). This could explain the period-shortening effect of the prd-4 mutation in frq+ strains and is consistent with the lack of period effect in frq-null strains.
The prd-6 mutation has been reported to be a mutation in a gene coding for a type 1 RNA helicase involved in translational termination and RNA metabolism (Compton and Feldman 2004). Numerous double mutants between prd-6 and other clock mutations have been constructed and their properties with respect to temperature compensation reported (Compton and Feldman 2004).
Rhythm expression without supplementation:
Introduction of the ult mutation allowed the expression of a conidiation rhythm on minimal media in both frq10 and wc-2Δ. This rhythm still had many of the properties found in the null strains in that it was neither temperature compensated nor light sensitive (data not shown), so it was not a complete restoration of a circadian rhythm. The bd ult frq10 period differs from the bd ult wc-2Δ period, suggesting that the disruption of the FWC oscillator by different mutations can give different results. This could be due to fact that in the wc-2Δ strain there is still a small amount of FRQ protein made (Collett et al. 2002), whereas in the frq10 null there is none.
In summary, the finding that the prd-1, prd-2, prd-6, and ult mutations can have a period effect in the absence of a functional FWC oscillator mutation provides identification of mutations affecting possible alternate oscillators in Neurospora. These results raise a number of questions for further work, including how farnesol and geraniol act to allow the expression of a rhythm, how the prd-1, prd-2, and prd-6 mutations possibly affect this pathway, and how the ult mutation is able to allow the expression of a rhythm independent of farnesol/geraniol supplementation. It is anticipated that further genetic dissection of the Neurospora multioscillator system will yield more useful information and uncover more novel interactions.
It is also anticipated that this approach could be applied, in a general way, to other situations where conditional clock mutations have been described. For instance, mutations that lead to an arhythmic phenotype in constant darkness (D/D), but are rhythmic in constant light (L/L) (Spoelstra et al. 2002), could be combined with other mutations and the periodicity of the double mutant assayed in D/D and L/L. In this way, the status of the second mutation vs. the first one could then be worked out. Likewise, phase-shifting treatments could be characterized as to which oscillator they affect in a multioscillator system by employing conditional clock mutants and assaying the effects of the phase-shifting treatments in a clock null strain whose rhythm is now expressed.
Acknowledgments
We are grateful to Pepper Stockton, Avi Pekurovsky, Qian Xiao, and Mike Ferry for their contributions and to Pat Lakin-Thomas for helpful suggestions on this manuscript. This work was supported by funds from the National Science Foundation (MCB0212190) and the Arnold O. and Mabel Beckman Foundation.
References
- Aronson, B. D., K. A. Johnson and J. C. Dunlap, 1994. Circadian clock locus frequency: protein encoded by a single open reading frame defines period length and temperature compensation. Proc. Natl. Acad. Sci. USA 91: 7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Pedersen, D., V. M. Cassone, D. J. Earnest, S. S. Golden, P. E. Hardin et al., 2005. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 6: 544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, S., and S. A. Martins, 1979. Circadian rhythms in Neurospora crassa: effects of unsaturated fatty acids. J. Bacteriol. 137: 912–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner, M., and T. Schafmeier, 2006. Transcriptional and post-transcriptional regulation of the circadian clock of cyanobacteria and Neurospora. Genes Dev. 20: 1061–1074. [DOI] [PubMed] [Google Scholar]
- Christensen, M. K., G. Falkeid, J. J. Loros, J. C. Dunlap, C. Lillo et al., 2004. A nitrate-induced frq-less oscillator in Neurospora crassa. J. Biol. Rhythms 19: 280–286. [DOI] [PubMed] [Google Scholar]
- Collett, M. A., J. C. Dunlap and J. J. Loros, 2001. Circadian clock-specific roles for the light response protein WHITE COLLAR-2. Mol. Cell. Biol. 21: 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett, M. A., N. Garceau, J. C. Dunlap and J. J. Loros, 2002. Light and clock expression of the Neurospora clock gene frequency is differentially driven by but dependent on WHITE COLLAR-2. Genetics 160: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa, A., Z. A. Lewis, A. V. Greene, I. J. March, R. H. Gomer et al., 2003. Multiple oscillators regulate circadian gene expression in Neurospora. Proc. Natl. Acad. Sci. USA 100: 13597–13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton, J. E., and J. F. Feldman, 2004. Many genetic loci contribute to circadian rhythms in Neurospora crassa, pp. 48–66 in Circadian Clocks in Eukaryotic Microbes, edited by F. Kippert. Landes Bioscience, Georgetown, TX.
- Cote, G. G., and S. Brody, 1987. Circadian rhythms in Neurospora crassa: a clock mutant, prd-1, is altered in membrane fatty acid composition. Biochim. Biophys. Acta 904: 131–139. [DOI] [PubMed] [Google Scholar]
- Davis, R. H., and F. J. Deserres, 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 17A: 79–143. [Google Scholar]
- Degli-Innocenti, F., and V. E. Russo, 1984. Isolation of new white collar mutants of Neurospora crassa and studies on their behavior in the blue light-induced formation of protoperithecia. J. Bacteriol. 159: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dePaula, R., Z. A. Lewis, A. V. Greene, K. S. Seo, L. W. Morgan et al., 2006. Two circadian timing circuits in Neurospora crassa cells share components and regulate distinct rhythmic processes. J. Biol. Rhythms 21: 159–168. [DOI] [PubMed] [Google Scholar]
- Dunlap, J. C., 2006. Proteins in the Neurospora circadian clockworks. J. Biol. Chem. 281: 28489–28493. [DOI] [PubMed] [Google Scholar]
- Dunlap, J. C., and J. J. Loros, 2004. The Neurospora circadian system. J. Biol. Rhythms 19: 414–424. [DOI] [PubMed] [Google Scholar]
- Dunlap, J. C., J. J. Loros, B. D. Aronson, M. Merrow, S. Crosthwaite et al., 1995. The genetic basis of the circadian clock: identification of frq and FRQ as clock components in Neurospora. Ciba Found. Symp. 183: 3–17, 17–25. [DOI] [PubMed] [Google Scholar]
- Feldman, J. F., 1982. Genetic approaches to circadian clocks. Annu. Rev. Plant Physiol. 33: 583–608. [Google Scholar]
- Granshaw, T., M. Tsukamoto and S. Brody, 2003. Circadian rhythms in Neurospora crassa: farnesol or geraniol allow expression of rhythmicity in the otherwise arrhythmic strains frq10, wc-1, and wc-2. J. Biol. Rhythms 18: 287–296. [DOI] [PubMed] [Google Scholar]
- He, Q., P. Cheng and Y. Liu, 2005. a The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev. 19: 1518–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q., H. Shu, P. Cheng, S. Chen, L. Wang et al., 2005. b Light-independent phosphorylation of WHITE COLLAR-1 regulates its function in the Neurospora circadian negative feedback loop. J. Biol. Chem. 280: 17526–17532. [DOI] [PubMed] [Google Scholar]
- Iwasaki, H., and J. C. Dunlap, 2000. Microbial circadian oscillatory systems in Neurospora and Synechococcus: models for cellular clocks. Curr. Opin. Microbiol. 3: 189–196. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas, P. L., 1998. Choline depletion, frq mutations, and temperature compensation of the circadian rhythm in Neurospora crassa. J. Biol. Rhythms 13: 268–277. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas, P. L., 2006. Circadian clock genes frequency and white collar-1 are not essential for entrainment to temperature cycles in Neurospora crassa. Proc. Natl. Acad. Sci. USA 103: 4469–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas, P. L., and S. Brody, 1985. Circadian rhythms in Neurospora crassa: interactions between clock mutations. Genetics 109: 49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas, P. L., and S. Brody, 2000. Circadian rhythms in Neurospora crassa: lipid deficiencies restore robust rhythmicity to null frequency and white-collar mutants. Proc. Natl. Acad. Sci. USA 97: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas, P. L., and S. Brody, 2004. Circadian rhythms in microorganisms: new complexities. Annu. Rev. Microbiol. 58: 489–514. [DOI] [PubMed] [Google Scholar]
- Lee, K., J. J. Loros and J. C. Dunlap, 2000. Interconnected feedback loops in the Neurospora circadian system. Science 289: 107–110. [DOI] [PubMed] [Google Scholar]
- Lewis, Z. A., A. Correa, C. Schwerdtfeger, K. L. Link, X. Xie et al., 2002. Overexpression of White Collar-1 (WC-1) activates circadian clock-associated genes, but is not sufficient to induce most light-regulated gene expression in Neurospora crassa. Mol. Microbiol. 45: 917–931. [DOI] [PubMed] [Google Scholar]
- Liu, Y., 2005. Analysis of posttranslational regulations in the Neurospora circadian clock. Methods Enzymol. 393: 379–393. [DOI] [PubMed] [Google Scholar]
- Liu, Y., and D. Bell-Pedersen, 2006. Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot. Cell 5: 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros, J. J., and J. F. Feldman, 1986. Loss of temperature compensation of circadian period length in the frq-9 mutant of Neurospora crassa. J. Biol. Rhythms 1: 187–198. [DOI] [PubMed] [Google Scholar]
- Merrow, M., M. Brunner and T. Roenneberg, 1999. Assignment of circadian function for the Neurospora clock gene frequency. Nature 399: 584–586. [DOI] [PubMed] [Google Scholar]
- Morgan, L. W., and J. F. Feldman, 2001. Epistatic and synergistic interactions between circadian clock mutations in Neurospora crassa. Genetics 159: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, L. W., J. F. Feldman and D. Bell-Pedersen, 2001. Genetic interactions between clock mutations in Neurospora crassa: Can they help us to understand complexity? Philos. Trans. R. Soc. Lond. B Biol. Sci. 356: 1717–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregueiro, A. M., Q. Liu, C. L. Baker, J. C. Dunlap and J. J. Loros, 2006. The Neurospora checkpoint kinase 2: a regulatory link between the circadian and cell cycles. Science 313: 644–649. [DOI] [PubMed] [Google Scholar]
- Roeder, P. E., M. L. Sargent and S. Brody, 1982. Circadian rhythms in Neurospora crassa: oscillations in fatty acids. Biochemistry 21: 4909–4916. [DOI] [PubMed] [Google Scholar]
- Sargent, M. L., and S. H. Kaltenborn, 1972. Effects of medium composition and carbon dioxide on circadian conidiation in Neurospora. Plant Physiol. 50: 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra, K., M. Oklejewicz and S. Daan, 2002. Restoration of self-sustained circadian rhythmicity by the mutant clock allele in constant illumination. J. Biol. Rhythms 17(6): 520–525. [DOI] [PubMed] [Google Scholar]