Abstract
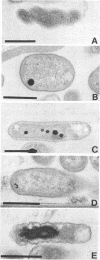
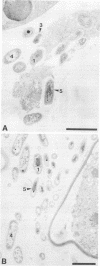
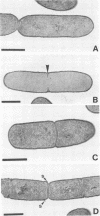
The occurrence and ultrastructure of bacteria in leaf cavities of symbiotic Azolla caroliniana were examined by transmission electron microscopy. Bacteria were observed in all leaf cavities of Azolla cultures. Five ultrastructurally distinct types of bacteria were observed in each individual leaf cavity. Features used to characterize the bacteria included morphology, cell wall structure, and cytoplasmic organization. At least one gram-positive and as many as four gram-negative types of bacteria reside in leaf cavities of A. caroliniana. The morphological and ultrastructural characteristics of the gram-positive bacterium suggest that it is an Arthrobacter sp. The gram-negative bacteria could not be cultured; therefore, they have not been classified further. Bacterial cell shape and cell wall structure were similar in leaf cavities of different ages, but cell size and cytoplasmic composition varied. The relative contributions of each bacterial type to the total community within individual leaves was determined. Ultrastructural characteristics of bacterial isolates cultured from A. caroliniana in a free-living state were also examined.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Boylen C. W., Pate J. L. Fine structure of Arthrobacter crystallopoietes during long-term starvation of rod and spherical stage cells. Can J Microbiol. 1973 Jan;19(1):1–5. doi: 10.1139/m73-001. [DOI] [PubMed] [Google Scholar]
- Chao L., Bowen C. C. Purification and properties of glycogen isolated from a blue-green alga, Nostoc muscorum. J Bacteriol. 1971 Jan;105(1):331–338. doi: 10.1128/jb.105.1.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Pate J. L. Ultrastructural explanation for snapping postfission movements in Arthrobacter crystallopoietes. J Bacteriol. 1971 Jan;105(1):408–412. doi: 10.1128/jb.105.1.408-412.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A., Mayne B. C. The Azolla, Anabaena azollae Relationship: I. Initial Characterization of the Association. Plant Physiol. 1974 Jun;53(6):813–819. doi: 10.1104/pp.53.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A., Mayne B. C. The Azolla, Anabaena azollae Relationship: II. Localization of Nitrogenase Activity as Assayed by Acetylene Reduction. Plant Physiol. 1974 Jun;53(6):820–824. doi: 10.1104/pp.53.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A. The Azolla-Anabaena azzolae symbiosis. Basic Life Sci. 1977;9:231–258. doi: 10.1007/978-1-4684-0880-5_16. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. B., Mayne B. C., Toia R. E., Peters G. A. Azolla-Anabaena Relationship: VIII. Photosynthetic Characterization of the Association and Individual Partners. Plant Physiol. 1979 Nov;64(5):791–795. doi: 10.1104/pp.64.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. B., Peters G. A., Toia R. E., Mayne B. C. Azolla-Anabaena Relationships: VII. Distribution of Ammonia-assimilating Enzymes, Protein, and Chlorophyll between Host and Symbiont. Plant Physiol. 1978 Sep;62(3):463–467. doi: 10.1104/pp.62.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Paone D. A. Accumulation of Cyanophycin Granules as a Result of Phosphate Limitation in Agmenellum quadruplicatum. Plant Physiol. 1981 Apr;67(4):716–719. doi: 10.1104/pp.67.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L. The fine structure of Arthrobacter pascens and the development of mesosomes during the growth cycle. Can J Microbiol. 1968 Oct;14(10):1029–1034. doi: 10.1139/m68-173. [DOI] [PubMed] [Google Scholar]
- Wallace W. H., Gates J. E. Identification of eubacteria isolated from leaf cavities of four species of the N-fixing azolla fern as arthrobacter conn and dimmick. Appl Environ Microbiol. 1986 Sep;52(3):425–429. doi: 10.1128/aem.52.3.425-429.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. M., Jr, Claus G. W. Gram characteristics and wall ultrastructure of Arthrobacter crystallopoietes during coccus-rod morphogenesis. J Bacteriol. 1973 Apr;114(1):378–389. doi: 10.1128/jb.114.1.378-389.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P. Formation and function of the glycogen-like polysaccharide of Arthrobacter. Antonie Van Leeuwenhoek. 1966;32(4):356–372. doi: 10.1007/BF02097485. [DOI] [PubMed] [Google Scholar]