Abstract
We previously have demonstrated that oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC), a component of minimally modified low density lipoprotein (MM-LDL), activates endothelial cells to bind monocytes. 1-Palmitoyl-2- (5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC) and 1- palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC), which are present in OxPAPC, MM-LDL, and atherosclerotic lesions, were shown to have a major role in the activation of endothelial cells. We now demonstrate that these two highly similar molecules have dramatically different effects on leukocyte endothelial interactions. POVPC is a potent regulator of monocyte-specific endothelial interactions. Treatment of endothelial cells with POVPC increased monocyte binding by inducing the surface expression of the connecting segment 1 domain of fibronectin; no increase in neutrophil binding was observed. In addition, POVPC strongly inhibited lipopolysaccharide-mediated induction of neutrophil binding and expression of E-selectin protein and mRNA. This inhibition was mediated by a protein kinase A-dependent pathway, resulting in down-regulation of NF-κB-dependent transcription. In contrast, PGPC induced both monocyte and neutrophil binding and expression of E-selectin and vascular cell adhesion molecule 1. We present evidence to suggest that the two phospholipids act by different novel receptors present in Xenopus laevis oocytes and that POVPC, but not PGPC, stimulates a cAMP-mediated pathway. At concentrations equal to that present in MM-LDL, the effect of POVPC dominates and inhibits PGPC-induced neutrophil binding and E-selectin expression in endothelial cells. In summary, our data provide evidence that both POVPC and PGPC are important regulators of leukocyte-endothelial interactions and that POVPC may play a dominant role in a number of chronic inflammatory processes where oxidized phospholipids are known to be present.
In atherosclerosis, as in other chronic inflammatory diseases, monocyte/macrophages are the predominant cell type present in the vessel wall. Monocyte recruitment was shown to be a rate-limiting step in the development of atherosclerotic lesions and observations in human tissue and animal models suggest that monocytes play an important role in all stages of atherogenesis (reviewed in refs. 1 and 2). In atherosclerosis, even in the early lesion, neutrophils are not present (2). There is growing evidence to suggest that oxidized lipids may play an important role in atherogenesis (3). Our group has demonstrated that minimally oxidized low density lipoprotein (MM-LDL) stimulates endothelial cells to bind monocytes, but not neutrophils (4) by a mechanism involving connecting segment 1 (CS-1) fibronectin and α4β1 integrin (5). There are indications that the effects of MM-LDL on endothelial cells are mediated by a receptor, increasing levels of cAMP (6, 7).
The biological activity of MM-LDL is attributable to biologically active oxidized phospholipids that also can be derived from oxidation of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC) (8). Furthermore, the presence of antibodies to oxidized phospholipids in patients with atherosclerosis, diabetes, hypertension, antiphospholipid syndrome, preeclampsia, and other chronic diseases underlines the potential importance of these molecules (9–11). We previously have identified two biologically active oxidized phospholipids present in MM-LDL and oxidized PAPC (OxPAPC) as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC) and 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC) that activate endothelial cells to bind monocytes (12). We also have shown these phospholipids to be increased in atherosclerotic lesions (12) and livers of fat-fed mice (13, 14).
The present studies have focused on the effects of POVPC and PGPC on the binding of monocytes and neutrophils to the endothelium, an important aspect of the determination of monocyte entry into the vessel wall. We demonstrate that POVPC and PGPC differ in their effects on leukocyte binding and suggest that they act via different receptors. Although PGPC induced both neutrophil and monocyte binding, POVPC specifically induced monocyte binding and inhibited the induction of neutrophil binding by PGPC and other activators. We further demonstrate that the two lipids stimulated human aortic endothelial cells to express different monocyte ligands: POVPC up-regulated the expression of CS-1 fibronectin, whereas PGPC up-regulated vascular cell adhesion molecule 1 (VCAM-1) and E-selectin expression. These studies suggest that both POVPC and PGPC are powerful regulators of leukocyte endothelial interactions.
Materials and Methods
Phospholipid Preparations.
OxPAPC was prepared as described (12). POVPC and PGPC were prepared by total synthesis as described (12). Alternatively, a preparative method was used involving ozonolysis of PAPC (obtained from Avanti Polar Lipids or Sigma). This method is a modification of a previously published method (15). Briefly, 50 mg of PAPC in hexane were cooled to −78°C by using a dry ice-acetone bath. Oxygen containing 2% ozone was bubbled through the solution at a rate of 100 ml/min until the solution turned faint blue in color. The hexane was evaporated under argon, and the residue was redissolved in chloroform (4 ml) and treated with triphenylphosphine (50 mg) at room temperature. The completion of ozonide reduction (about 1–2 h) was monitored by TLC, and the crude product was loaded onto preparative TLC (20 × 20 cm, 250 μm thick) and developed with chloroform/methanol/acetic acid/water (75:45:12:6). TLC was viewed by using p-anisaldehyde as developing agent and the POVPC region (Rƒ = 0.35) of the silica was removed. The product was recovered from silica by using chloroform/methanol/water (65:25:4). Individual phospholipids were purified by using HPLC/MS as described (12). Individual phospholipids were quantitated by flow injection analysis-electrospray ionization MS using dimyristoyl-phosphatidylcholine as an internal standard (16). The concentration of endotoxin in the phospholipid solutions was less than 30 pg/ml (determined by a chromogenic assay), which is 30 times less than that required to induce endothelial cells to bind leukocytes.
ELISA, Cell Adhesion, and Cell Survival Studies.
Human aortic endothelial cells (HAEC) were cultured as described (4). For determination of E-selectin, VCAM-1 and CS-1 expression HAEC were plated into 96-well dishes, exposed to activators, and then incubated with antibodies to E-selectin (BioDesign, New York), VCAM-1 (BioDesign), or CS-1 (a gift from Cytel, San Diego). Peroxidase-labeled second antibody was used and detected with o-phenylene-diamine (Sigma). For leukocyte binding studies endothelial cells were cultured in 48-well dishes and incubated with or without activators for 4 h at 37°C. The test medium was removed, and a suspension of human monocytes or polymorphonuclear neutrophils was added for 12–15 min, then nonadherent cells were removed. Bound monocytes or neutrophils were visually counted. Monocytes were obtained from a large pool of healthy donors by modification of the Recalde procedure as described (17). Neutrophils were isolated by previously described methods (18). Cell survival experiments were performed by using a propidium iodide assay (19).
Measurement of cAMP.
For measurement of cAMP, HAEC were cultured in 6-well dishes, and two wells were used for each determination of cAMP. Cells were pretreated for 10 min with 0.5 mmol/liter isobutyl-1-methylxanthine in medium 199 containing 5% FBS. Agonists then were added to the cells in the same medium and cells treated for 1 hr; the incubation was stopped by rinsing in PBS containing 4 mmol/liter EDTA. The cells then were removed from the dish by scraping into the same buffer. The cell pellet after centrifugation was resuspended in boiling water containing EDTA. The suspension was sonicated, placed in boiling water for 3 min, and centrifuged at 13,000 × g for 2 min to remove coagulated macromolecules. The supernatant was used to determine cAMP levels with a cAMP kit (Amersham Pharmacia, RPA 542).
Transfection Studies.
HAEC were transfected with p(κB)4-luciferase, which contains four κB sites cloned upstream of the minimal simian virus 40 promoter (20), by using the Superfect reagent (Qiagen, Chatsworth, CA) according to the manufacturer’s instructions. pSV-β- galactosidase (Promega) was used as a control plasmid to normalize the luciferase activity. Cells were incubated for 4 h at 37°C with the indicated agents 24 h posttransfection. In establishing the conditions for this method we used a green fluorescent protein (GFP) reporter and established conditions to routinely obtain a 15% transfection efficiency. In several experiments we also included a combination of GFP plasmid and plasmid of interest to be certain that transfection of several plasmids did not affect efficiency.
Voltage Clamp Studies.
The cystic fibrosis transmembrane conductance regulator (CFTR)-containing plasmid (pACF23) obtained from J. Riordan (Mayo Clinic, Scottsdale, AZ) was linearized and transcribed in vitro into capped cRNA with SP6 RNA polymerase (Ambion, Austin, TX). The CFTR cRNA (40 ng) was injected into stage IV-V Xenopus laevis oocytes, which had been isolated by enzymatic digestion (2 mg/ml collagenase), then oocytes were incubated for 24–48 h at 19°C to allow for protein synthesis and transport of the protein to the cell membrane. Whole-oocyte currents were recorded at room temperature with the two-electrode voltage-clamp technique (21) using a Dagan Instruments (Minneapolis, MN) CA-1 oocyte clamp amplifier, a TL-1 DMA interface for data acquisition, and pclamp software (Axon Instruments, Foster City, CA). Phospholipids were dried under a stream of nitrogen and resuspended into the bath solution by vortexing before addition to clamped oocytes.
Northern Analysis.
HAEC were incubated with oxidized phospholipids and/or lipopolysaccharide (LPS) as for cell adhesion experiments, and total RNA was isolated from cells by using the guanidine thiocyanate/phenol method. Northern blot analysis using E-selectin cDNA was performed as described (7). 36B4 or 28S cDNA were used to control for RNA loading.
Nuclear Runoff Transcription Assays.
Nuclear runoff experiments were performed essentially as described (22). For these studies cells were untreated or treated for 1 h with LPS (2 ng/ml), OxPAPC (100 μg/ml), or LPS plus OxPAPC. Briefly, nuclear pellets from these cells in a 75-cm2 culture flask were resuspended in 90 μl of nuclear storage buffer (50 mmol/liter Tris⋅HCl, pH 8.3/40% glycerol/0.1 mmol/liter EDTA/0.1 mmol/liter DTT). To the nuclear preparation 100 μl of 2× reaction buffer (10 mmol/liter Tris⋅HCl, pH 7.5/5 mmol/liter MgCl2/0.3 mol/liter KCl/5 mmol/liter DTT/1 mmol/liter each of ATP, GTP, and CTP/10 μl [α-32P]UTP (3,000 Ci/mmol) were added and incubated at room temperature for 20 min. DNA was digested by 1 μl of 20,000 units/ml RNase-free DNase at room temperature for 5 min, then 10 μl of yeast tRNA (10 mg/ml) was added. The labeled RNA was isolated by using Trizol. RNA was redissolved in 500 μl of 20 mmol/liter Tris⋅HCl, pH 7.9, and 20 mmol/liter EDTA. Membranes were prepared by applying 2 μg of E-selectin cDNA or α-tubulin cDNA per slot and baking at 80°C for 2 h. Membranes were prehybridized at 65°C for 2 h and then hybridized with the isolated labeled RNA at 65°C for 48 h. Membranes were washed in 2× SSC and 0.1% SDS at room temperature for 15 min, in 2× SSC, 0.1% SDS and 10 μg/ml RNase A at 37°C for 20 min and in 0.2× SSC and 0.1% SDS at 65°C for 10 min before exposing to films.
Results
Effects of Oxidized Phospholipids on Leukocyte Adhesion and Adhesion Molecule Expression.
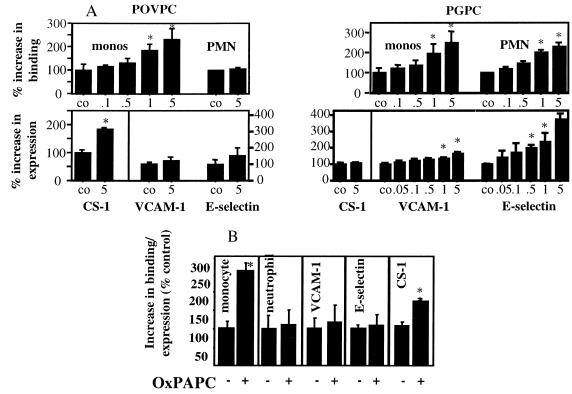
Treatment of HAEC for 4 h with POVPC dose-dependently induced monocyte, but not neutrophil, adhesion without increasing the surface expression of VCAM-1 or E-selectin (Fig. 1A). However, POVPC caused an increase in surface deposition of CS-1-containing fibronectin, an alternative monocyte ligand. In contrast, treatment of endothelial cells for 4 h with PGPC increased both monocyte and neutrophil adhesion. PGPC also dose-dependently increased expression of E-selectin and VCAM-1 but had no effect on expression of CS-1 fibronectin (Fig. 1A). OxPAPC (150 μg/ml), which contains approximately 5 μg of POVPC and 5 μg of PGPC, showed a pattern similar to POVPC, inducing monocyte-specific binding and CS-1 fibronectin surface expression (Fig. 1B). Unoxidized PAPC at the same concentration neither increased leukocyte binding nor adhesion molecule expression (not shown). Control experiments showed that OxPAPC up to a concentration of 300 μg/ml was not toxic to HAEC (data not shown). The ability of POVPC, PGPC, and OxPAPC to stimulate leukocyte endothelial interactions did not depend on the concentration of serum in the medium, because serum concentrations from 0 to 15% gave similar results (data not shown).
Figure 1.
Effects of POVPC, PGPC, and OxPAPC on leukocyte binding and adhesion molecule expression. OxPAPC, POVPC, and PGPC, which had been resuspended in chloroform, were dried under nitrogen and resuspended in tissue culture medium. (A) HAEC were treated with POVPC or PGPC at the indicated concentrations in μg/ml for 4 h at 37°C. Monocyte and polymorphonuclear neutrophil (PMN) binding then was performed in 48-well plates, and cell surface ELISAs were used for determination of VCAM-1, E-selectin, and CS-1 expression in 96-well plates. (B) HAEC were treated for 4 h with 100 μg/ml of OxPAPC, and experiments were performed as in A. Data are expressed as % of control (mean ± SD, n = 6 wells or fields/data point), where control represents cells treated with medium only. ★, P < 0.001 as calculated by one-way ANOVA. LPS (2 ng/ml) was used as positive control. Each experiment is representative of four independent studies.
The Effects of PGPC and POVPC Are Mediated by Specific Receptors.
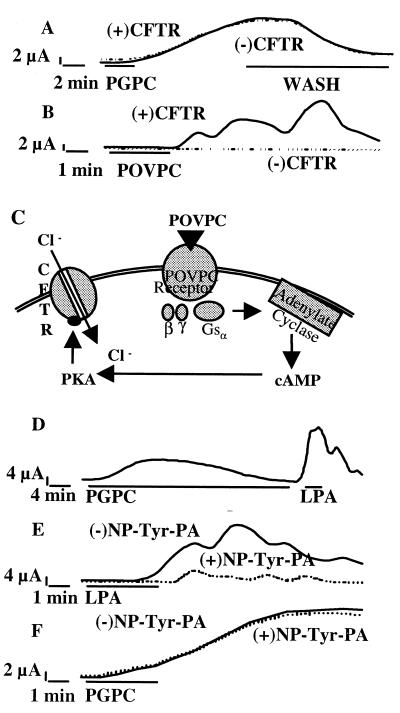
Based on the above data, we hypothesized that POVPC and PGPC acted on two different receptors. To test this hypothesis, two-electrode ramped voltage-clamp recordings were performed in X. laevis oocytes perfused with PGPC or POVPC. PGPC activated a chloride ion conductance (Fig. 2A). This effect was reversible upon washout of the ligand and could be desensitized by prolonged treatment with PGPC (Fig. 2D), both characteristic of receptor-mediated events. In contrast, POVPC did not directly activate chloride currents in Xenopus oocytes (Fig. 2B). Previous work from our laboratory indicated that the effects of MM-LDL were mediated by a G-protein-coupled receptor (7) via a cAMP-dependent pathway (6). However, Xenopus oocytes do not endogenously express cAMP-activated ion channels (23), but can be made responsive to changes in cAMP by heterologous expression of the human CFTR, a cAMP-dependent chloride channel (24, 25). Xenopus oocytes were microinjected with cRNA encoding CFTR and incubated for 24–48 h, and then the effect of POVPC was examined. Functional expression of CFTR in oocytes was confirmed by using the cAMP-elevating agent forskolin (not shown). Only in oocytes heterologously expressing CFTR, a POVPC-inducible chloride current was observed (Fig. 2 B and C). The effect of POVPC was also reversible upon washout and could be homologously desensitized after an incubation for 5 min (data not shown). Conversely, PGPC did not activate a CFTR-mediated ion current, as evidenced by the similar activation pattern independent of CFTR-expression (Fig. 2A). Furthermore, the receptor for PGPC was found to be distinct from the high affinity lysophosphatidic acid (LPA) receptor (which is also present in Xenopus oocytes), because the action of LPA was not desensitized by treatment with PGPC (Fig. 2D). In addition, LPA receptor antagonists, which specifically block the effects of LPA (Fig. 2E), could not abrogate the PGPC-induced response (Fig. 2F). In addition, we examined the levels of cAMP in HAEC treated with oxidized phospholipids (Table 1). POVPC (5 μg/ml) but not PGPC (5 μg/ml) increased levels of cAMP by approximately 70% as compared with an approximate 300% increase seen with isoproteronol (5 μmol/liter). Thus, we conclude that different receptors are stimulated by PGPC and POVPC, the latter coupling to a cAMP-mediated pathway.
Figure 2.
POVPC and PGPC act on distinct novel receptors present on Xenopus oocytes. PGPC (A) or POVPC (B) (5 μmol/liter) were added to clamped oocytes either expressing (+CFTR) or not expressing CFTR (−CFTR), and a chloride current was measured. Oocytes treated with PGPC were washed after 8–9 min to reverse the PGPC-induced chloride current (A). POVPC-induced currents were recorded for 8 min at which time they had returned to baseline (B). Binding of POVPC to its receptor results in activation of Gsα. Adenylate cyclase is activated by Gsα leading to an increase in cAMP, which activates PKA. CFTR is phosphorylated by PKA, resulting in a chloride current (C). Oocytes were treated with PGPC (5 μmol/liter) for 20 min to desensitize putative PGPC receptors, and then were treated with LPA (100 nmol/liter) (D). Oocytes were pretreated with the LPA-receptor antagonist N-palmitoyl-tyrosine (NP-Tyr-PA, 10 μmol/liter) or bath solution for 10 min, followed by treatment with LPA (100 nmol/liter) (E) or PGPC (5 μmol/liter) (F). Each condition was repeated three times with similar results.
Table 1.
cAMP levels in HAEC treated with oxidized phospholipids
| Condition | cAMP |
|---|---|
| Control | 5.1 ± 0.6 |
| POVPC | 8.6 ± 0.4* |
| PGPC | 5.0 ± 0.4 |
| IsoP | 15.1 ± 1.5* |
HAEC were preincubated for 10 min with 5 μmol/liter isobutyl-1-methylxanthine then POVPC (5 μg/ml), or isoproterenol (5 μmol/liter) were added for 1 h. Levels of cAMP were determined by using RIA. Values represent mean ± SD of quadruplicate samples. Values are given as pmol/mg protein per min. These data are representative of three experiments. *, P < 0.05. IsoP, isoproterenol.
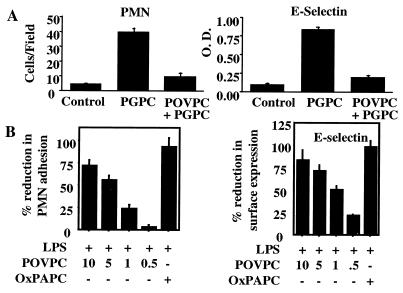
Inhibition of Neutrophil Binding and E-Selectin Expression by POVPC and OxPAPC.
It has been shown by others that elevation of cAMP inhibits E-selectin expression (26–28), and we have demonstrated that MM-LDL and other cAMP-elevating agents inhibit LPS-induced neutrophil binding to HAEC (6, 7). We hypothesized that POVPC, because of its ability to increase cAMP, would inhibit agonist-induced E-selectin expression and neutrophil binding. Therefore we tested the effect of POVPC and OxPAPC on PGPC-induced E-selectin expression and neutrophil binding. POVPC inhibited neutrophil adhesion and E-selectin expression induced by PGPC (Fig. 3A), suggesting one mechanism whereby neutrophils may be excluded from MM-LDL- or OxPAPC- stimulated endothelium. POVPC, and to a greater extent OxPAPC, also dose-dependently inhibited LPS-induced neutrophil binding and E-selectin expression (Fig. 3B). Similar results were obtained when tumor necrosis factor was used as the stimulating agent (data not shown). Evidence for the presence of additional inhibitory lipids (in addition to POVPC) was obtained by testing HPLC fractions of OxPAPC that did not contain POVPC (data not shown).
Figure 3.
Inhibitory action of POVPC and OxPAPC on neutrophil binding and E-selectin expression. (A) Effect of POVPC on PGPC action. HAEC were untreated (Control), coincubated for 4 h with PGPC alone (5 μg/ml) or PGPC (5 μg/ml) plus POVPC (5 μg/ml), and polymorphonuclear neutrophil (PMN) binding or E-selectin expression was measured. (B) Effect of POVPC and OxPAPC on LPS action. HAEC were coincubated for 4 h with LPS (2 ng/ml) and either POVPC at concentrations shown (μg/ml) or OxPAPC (100 μg/ml), and polymorphonuclear neutrophil binding and E-selectin expression was determined. Data are expressed as % reduction (mean ± SD), where 0% represents cells treated with LPS only and 100% represents cells treated with medium only. These data are representative of four experiments. ∗, P < 0.001 as calculated by one-way ANOVA, n = 6 fields or 6 wells/condition.
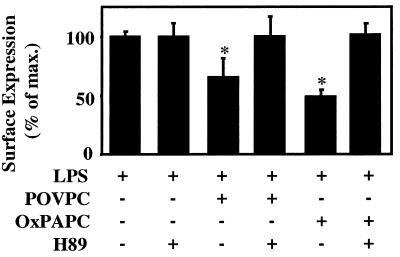
To confirm that inhibition of E-selectin expression by OxPAPC and POVPC indeed depended on a protein kinase A (PKA)-mediated pathway, we examined whether H89, a specific inhibitor of cAMP-dependent PKA, could reverse the inhibitory effect. Pretreatment of HAEC with H89 (25 μmol/liter) for 1 h blocked the inhibitory activity of both OxPAPC and POVPC on LPS-induced E-selectin expression (Fig. 4). For these studies lower concentrations of POVPC and OxPAPC were used for proven nontoxic concentrations of H89. These data indicate that POVPC exerts its inhibitory activity via a cAMP/PKA-mediated pathway.
Figure 4.
The inhibitory activity of OxPAPC and POVPC involves a PKA-mediated mechanism. HAEC were pretreated with H89 (25 μmol/liter) for 1 h at 37°C and then coincubated with LPS (2 ng/ml) and either POVPC (1 μmol/liter) or OxPAPC (5 μmol/liter) for 4 h at 37°C in the presence or absence of 25 μmol/liter H89. Surface expression of E-selectin was determined by cell surface ELISA. Data are representative of four independent experiments.
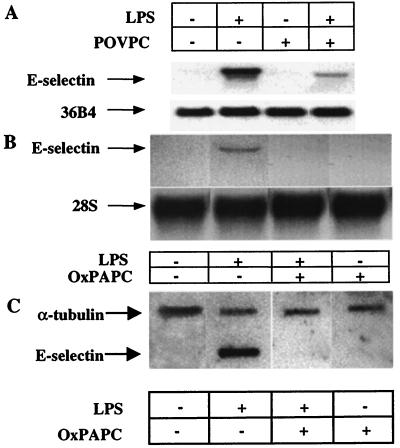
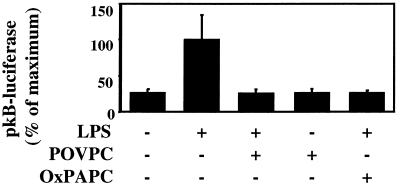
To further elucidate the mechanism of inhibition of E-selectin expression, the effects of POVPC (5 μg/ml) and OxPAPC (100 μg/ml) on LPS-induced E-selectin mRNA synthesis was examined. Northern blotting demonstrated that LPS-induced E-selectin mRNA levels were dramatically reduced by coincubation with POVPC (Fig. 5A) or OxPAPC (Fig. 5B). In addition, rates of transcription of E-selectin were examined by using nuclear runoff analysis (Fig. 5C). Transcription of E-selectin was increased by LPS and completely abrogated by coincubation with OxPAPC. NF-κB has been shown to play an important role in mediating E-selectin transcription (29–31). To determine the effects of POVPC and OxPAPC on NF-κB-mediated transcriptional activation, HAEC were transfected with p(κB)4-luciferase, and the effects of POVPC and OxPAPC on LPS-induced reporter activation were determined. Addition of POVPC or OxPAPC stongly reduced LPS-induced NF-κB promoter activity to control levels (Fig. 6).
Figure 5.
Effect of POVPC and OxPAPC on LPS-induced E-selectin mRNA. (A) HAEC were incubated for 4 h with no additives (lane 1), LPS (2 ng/ml, lane 2), 5 μg/ml POVPC (lane 3), or LPS plus POVPC (lane 4), and Northern blotting was performed. 36B4 was used to normalize for RNA loading. (B) HAEC were incubated for 4 h with no additives (lane 1), LPS (2 ng/ml, lane 2), 100 μg/ml OxPAPC (lane 3), or LPS plus OxPAPC (lane 4), and Northern blotting was performed. 28S was used to normalize for RNA loading. (C) Nuclear runoff: HAEC were untreated, treated with LPS (2 ng/ml), treated with OxPAPC (100 μg/ml), or treated with LPS plus OxPAPC for 1 h. Nuclear runoff experiments were performed as described in Materials and Methods. 32P-labeled RNA (1 × 106 cpm) was used to probe a nylon blot prefixed with 2 μg of E-selectin or α-tubulin cDNA, as indicated.
Figure 6.
POVPC and OxPAPC alter LPS-induced NF-κB activation. HAEC transiently transfected with pκB4-luciferase were coincubated with LPS (2 ng/ml) and either POVPC (5 μg/ml), OxPAPC (100 μg/ml), or POVPC alone for 4 h at 37°C. Luciferase activity then was determined and normalized to β-galactosidase activity. POVPC alone did not reduce the basal expression of luciferase. Data are representative of three experiments. Data are expressed as mean ± SD. n = 3 wells/condition, ∗, P < 0.001 as calculated by one-way ANOVA.
Discussion
The current studies have demonstrated that POVPC and PGPC, both oxidation products of PAPC that have five carbon-containing fragments at the sn-2 position, differentially regulate endothelial binding of monocytes and neutrophils. We demonstrate that POVPC can act as a powerful mediator of monocyte-specific adhesion to endothelial cells by increasing expression of the monocyte ligand CS-1 fibronectin (Fig. 1) and inhibiting neutrophil binding induced by PGPC and the potent activators LPS (Fig. 4) and tumor necrosis factor (data not shown). In contrast, PGPC induced both monocyte and neutrophil binding by stimulating the expression of E-selectin and VCAM-1. VCAM-1 has been shown to be strongly expressed in the luminal endothelium of rabbits (32) and mice (33) in the areas of monocyte entry. In human atherosclerotic lesions both CS-1 (5) and VCAM-1 (34) are expressed, though VCAM-1 is expressed mainly on adventitial microvessels. Thus previous studies suggest that both VCAM-1 and CS-1 are important monocyte binding molecules in atherosclerotic lesions.
The differences in the actions of POVPC and PGPC indicate that they might act by two different receptors. Our studies on Xenopus oocytes suggest that POVPC and PGPC recognize different novel receptors and that POVPC, but not PGPC, acts through a G-protein coupled receptor, inducing a cAMP/PKA-mediated pathway (Fig. 2 A–C). Furthermore, exposure of Xenopus oocytes to PGPC did not desensitize the oocyte to the effect of POVPC (data not shown), supporting the hypothesis that the binding sites for the two molecules are different. We previously have reported that MM-LDL increased the levels of cAMP in human aortic endothelial cells by activation of a Gs-coupled receptor and decreasing the endogenous activation of a Gi-coupled receptor (7). The present studies demonstrate that POVPC, but not PGPC, activates a Gs-coupled pathway in Xenopus oocytes (Fig. 2) and increased cAMP in endothelial cells (Table 1). The receptors for POVPC and PGPC are distinct from Edg-1 (35) and Edg-3 (36, 37) and the platelet-activating factor receptor (38), because Xenopus oocytes do not endogenously express these receptors. In addition, we have evidence that Xenopus oocytes do not express Edg-2 or Edg-4 (G.T., unpublished observation). Our studies also excluded the LPA receptor as a candidate (Fig. 2 D–F). Thus, the simplest interpretation of our data is that the different effects of POVPC and PGPC on endothelial cells are likely caused by activation of distinct, novel phospholipid receptors by these lipids.
The present studies demonstrate that the effect of POVPC is dominant in a mixture of oxidized phospholipids. At equimolar concentrations, POVPC inhibited the effect of PGPC to induce neutrophil binding. An important molecule in the regulation of neutrophil adhesion is E-selectin, whose induction also was inhibited by POVPC. Furthermore, we have shown that MM-LDL (6) and OxPAPC (Fig. 1B), which contain a mixture of oxidized lipids, reflect the inhibitory activity of POVPC. Fractionation of OxPAPC by HPLC revealed that POVPC was the most active inhibitory lipid (data not shown). However, at least one other inhibitory lipid was present. Studies on Xenopus oocytes demonstrated that POVPC did not inhibit activation of a chloride channel by PGPC (data not shown). Therefore, the inhibitory effect did not seem to be mediated at the level of the receptor. We present evidence that the inhibitory effect of POVPC and OxPAPC on LPS-induced E-selectin expression was at the level of transcription (Fig. 5 A and B).
The present studies have provided some evidence for the mechanism by which POVPC and OxPAPC inhibit E-selectin transcription. It has been shown previously that elevation of cAMP inhibits LPS- and tumor necrosis factor-induced E-selectin expression (26–28). The present studies demonstrate that a PKA inhibitor (H89) could reverse the inhibitory effect of POVPC and OxPAPC on LPS-induced E-selectin expression (Fig. 4). Transcriptional regulation of E-selectin was shown to be mediated in part by NF-κB. The E-selectin promoter contains three NF-κB binding sites, each of which is important for its up-regulation in response to LPS or tumor necrosis factor α (29, 30). Previous studies have demonstrated that after cytokine treatment of endothelial cells, elevation of intracellular cAMP can block NF-κB-mediated transactivation (20). On the other hand, the catalytic subunit of PKA has been shown to stimulate transcriptional activity of NF-κB by phosphorylating p65 (39). This effect was inhibited by H89; however, it was independent of cAMP (40). Elevation of cAMP levels within cells leads to the opposite effect, that is down-regulation of NF-κB transcriptional activity. A suggested mechanism for this effect is competition of PKA-phosphorylated cAMP response element-binding protein with p65 for limiting amounts of the coactivator CREB-binding protein (41). In our studies, the complete inhibition of LPS-induced p(κB)4-luciferase activity by POVPC and OxPAPC (Fig. 6) suggests that the transcriptional activity of nuclear NF-κB is markedly reduced. Taken together, our studies provide evidence that the inhibitory effect of POVPC and OxPAPC on LPS-induced E-selectin transcription is mediated by cAMP/PKA, resulting in down-regulation of LPS-induced NF-κB-mediated transcription. It is also possible that the inhibitory effect of POVPC and OxPAPC may be partly mediated by other elements on the E-selectin promoter, which previously have been shown to have key regulatory roles (27, 29).
In summary, these studies have shown an important role for POVPC and PGPC in regulating adhesion molecule expression in HAEC. We originally identified these lipids as being present in MM-LDL and atherosclerotic lesions. These two phospholipid oxidation products represent two of the three most active agents present in MM-LDL (12). However, it is very likely that at sites of inflammation, where increased oxidant stress has been reported, oxidized phospholipids are released from cells and contribute to the inflammatory process (42). The presence of antibodies to oxidized phospholipids in a number of chronic diseases (9–11) is further evidence for their more general importance. Although our studies have focused on two phospholipid oxidation products (active at 1–5 μmol/liter), it is possible that other lipid oxidation products also may play a role in regulating adhesion molecule expression. For example, others have shown that lyso-phosphatidylcholine, at higher concentrations of 30–50 μmol/liter, can induce VCAM-1 expression in other endothelial cell types (43). We hypothesize that the relative abundance of various biologically active oxidized phospholipids in atherosclerotic lesions, as well as at other sites of inflammation, play an important role in determining which adhesion molecules are expressed on the endothelial surface and therefore influence the type of inflammatory cells present in the lesion. Leukocyte activators, whose levels also have been shown to be increased by phospholipid oxidation products, may further influence the inflammatory cell migration.
Acknowledgments
This research was supported by U.S. Public Health Service Grant HL30568 and the Laubisch Fund.
Abbreviations
- PAPC
1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine
- OxPAPC
oxidized PAPC
- MM-LDL
minimally modified low density lipoprotein
- POVPC
1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine
- PGPC
1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine
- CS-1
connecting segment 1
- VCAM-1
vascular cell adhesion molecule 1
- HAEC
human aortic endothelial cells
- CFTR
cystic fibrosis transmembrane conductance regulator
- LPS
lipopolysaccharide
- LPA
lysophosphatidic acid
- PKA
protein kinase A
References
- 1.Ross R. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 2.Gerrity R G. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 3.Berliner J A, Heinecke J W. Free Radical Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 4.Berliner J A, Territo M C, Sevanian A, Ramin S, Kim J A, Bamshad B, Esterson M, Fogelman A M. J Clin Invest. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih P T, Elices M J, Fang Z T, Ugarova T P, Strahl D, Territo M C, Frank J S, Kovach N L, Cabanas C, Berliner J A, Vora D K. J Clin Invest. 1999;103:613–625. doi: 10.1172/JCI5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parhami F, Fang Z T, Fogelman A M, Andalibi A, Territo M C, Berliner J A. J Clin Invest. 1993;92:471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parhami F, Fang Z T, Yang B, Fogelman A M, Berliner J A. Arterioscler Thromb Vasc Biol. 1995;15:2019–2024. doi: 10.1161/01.atv.15.11.2019. [DOI] [PubMed] [Google Scholar]
- 8.Watson A D, Navab M, Hama S Y, Sevanian A, Prescott S M, Stafforini D M, McIntyre T M, Du B N, Fogelman A M, Berliner J A. J Clin Invest. 1995;95:774–782. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horkko S, Bird D A, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner J A, Friedman P, Dennis E A, Curtiss L K, et al. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horkko S, Miller E, Branch D W, Palinski W, Witztum J L. Proc Natl Acad Sci USA. 1997;94:10356–10361. doi: 10.1073/pnas.94.19.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horkko S, Miller E, Dudl E, Reaven P, Curtiss L K, Zvaifler N J, Terkeltaub R, Pierangeli S S, Branch D W, Palinski W, Witztum J L. J Clin Invest. 1996;98:815–825. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson A D, Leitinger N, Navab M, Faull K F, Horkko S, Witztum J L, Palinski W, Schwenke D, Salomon R G, Sha W, et al. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 13.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Thromb Haemostasis. 1997;78:195–199. [PubMed] [Google Scholar]
- 14.Leitinger N, Watson A D, Hama S Y, Ivandic B, Qiao J H, Huber J, Faull K F, Grass D S, Navab M, Fogelman A M, et al. Arterioscler Thromb Vasc Biol. 1999;19:1291–1298. doi: 10.1161/01.atv.19.5.1291. [DOI] [PubMed] [Google Scholar]
- 15.Ravandi A, Kuksis A, Myher J J, Marai L. J Biochem Biophys Methods. 1995;30:271–285. doi: 10.1016/0165-022x(95)00015-7. [DOI] [PubMed] [Google Scholar]
- 16.Han X, Gubitosi K R, Collins B J, Gross R W. Biochemistry. 1996;35:5822–5832. doi: 10.1021/bi952927v. [DOI] [PubMed] [Google Scholar]
- 17.Fogelman A M, Elahi F, Sykes K, Van L B, Territo M C, Berliner J A. J Lipid Res. 1988;29:1243–1247. [PubMed] [Google Scholar]
- 18.Boyum A. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 19.Sarafian T A, Vartavarian L, Kane D J, Bredesen D E, Verity M A. Toxicol Lett. 1994;74:149–155. doi: 10.1016/0378-4274(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 20.Ollivier V, Parry G N, Cobb R R, de Prost D, Mackman N. J Biol Chem. 1996;271:20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 21.Stuhmer W. Methods Enzymol. 1992;207:319–339. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- 22.Fei H, Drake T A. Biotechniques. 1993;15:838. [PubMed] [Google Scholar]
- 23.Miledi R, Woodward R M. J Physiol London. 1989;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boie Y, Rushmore T H, Darmon G A, Grygorczyk R, Slipetz D M, Metters K M, Abramovitz M. J Biol Chem. 1994;269:12173–12178. [PubMed] [Google Scholar]
- 25.Luebke A E, Dahl G P, Roos B A, Dickerson I M. Proc Natl Acad Sci USA. 1996;93:3455–3460. doi: 10.1073/pnas.93.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pober J S, Slowik M R, De L L, Ritchie A J. J Immunol. 1993;150:5114–5123. [PubMed] [Google Scholar]
- 27.De L L, Johnson D R, Whitley M Z, Collins T, Pober J S. J Biol Chem. 1994;269:19193–19196. [PubMed] [Google Scholar]
- 28.Morandini R, Ghanem G, Portier L A, Robaye B, Renaud A, Boeynaems J M. Am J Physiol. 1996;270:H807–H816. doi: 10.1152/ajpheart.1996.270.3.H807. [DOI] [PubMed] [Google Scholar]
- 29.Brostjan C, Anrather J, Csizmadia V, Natarajan G, Winkler H. J Immunol. 1997;158:3836–3844. [PubMed] [Google Scholar]
- 30.Lewis H, Kaszubska W, DeLamarter J F, Whelan J. Mol Cell Biol. 1994;14:5701–5709. doi: 10.1128/mcb.14.9.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meacock S, Pescini G R, DeLamarter J F, Hooft-van H R. J Biol Chem. 1994;269:31756–31762. [PubMed] [Google Scholar]
- 32.Cybulsky M I, Gimbrone M A J. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 33.Qiao J H, Welch C L, Xie P Z, Fishbein M C, Lusis A J. J Clin Invest. 1993;92:2386–2393. doi: 10.1172/JCI116844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien K D, McDonald T O, Chait A, Allen M D, Alpers C E. Circulation. 1996;93:672–682. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 35.Lee M J, Van B J, Thangada S, Liu C H, Hand A R, Menzeleev R, Spiegel S, Hla T. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 36.Liliom K, Bittman R, Swords B, Tigyi G. Mol Pharmacol. 1996;50:616–623. [PubMed] [Google Scholar]
- 37.An S, Dickens M A, Bleu T, Hallmark O G, Goetzl E J. Biochem Biophys Res Commun. 1997;231:619–622. doi: 10.1006/bbrc.1997.6150. [DOI] [PubMed] [Google Scholar]
- 38.Honda Z, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, Miyamoto T, et al. Nature (London) 1991;349:342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- 39.Zhong H, Voll R E, Ghosh S. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhong H, SuYang H, Erdjument B H, Tempst P, Ghosh S. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 41.Parry G C, Mackman N. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 42.Prescott S M, Patel K D, Melchior E P, Stafforini D, Lorant D E, Zimmerman G A, McIntyre T M. Atheroscler Rev. 1993;25:59–68. [Google Scholar]
- 43.Kume N, Cybulsky M I, Gimbrone M A J. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]