Abstract
Ca2+-mobilizing compounds such as the Ca2+ ionophore A23187 or the endoplasmic reticulum Ca2+ ATPase inhibitor thapsigargin can suppress or induce apoptosis in the same cells. The use of different calcineurin inhibitors has shown that both suppression and induction of apoptosis by the Ca2+-mobilizing compounds were mediated by calcineurin activation. Ca2+-mobilizing compounds activated p38 and p44/42 mitogen-activated protein kinases (MAPKs). Induction of apoptosis by the Ca2+-mobilizing compounds was suppressed by an inhibitor of p38 MAPK but not by an inhibitor of p44/42 MAPK. These MAPK inhibitors did not suppress apoptosis induction by wild-type p53 or by withdrawal of IL-6 from IL-6-dependent cells that are mediated by calcineurin-independent pathways. These MAPK inhibitors also did not affect the ability of Ca2+-mobilizing compounds to suppress apoptosis. The results indicate that (i) Ca2+- mobilizing compounds activate different and opposing pathways that diverge downstream from calcineurin activation that can either suppress or induce apoptosis in the same cells; (ii) p38 MAPK but not p44/42 MAPK is involved in induction of apoptosis but not in its suppression by the Ca2+-mobilizing compounds; and (iii) neither p38 nor p44/42 MAPKs mediate induction of apoptosis by some calcineurin-independent pathways.
Overexpression of wild-type p53 in M1 myeloid leukemic cells induces apoptosis (1), and wild-type p53 is required for induction of apoptosis by DNA damaging agents in normal myeloid precursors (2) and thymocytes (2–4). Induction of apotosis in M1 leukemic cells by wild-type p53 can be suppressed by some cytokines (1, 5–11) and Ca2+-mobilizing compounds such as the Ca2+ ionophore A23187 or the endoplasmic reticulum Ca2+ ATPase inhibitor thapsigargin (TG) (11). Suppression of wild-type p53-induced apoptosis by cytokines or Ca2+-mobilizing compounds was not the result of changes in the ability of wild-type p53 to transcriptionally activate waf-1, mdm-2, or FAS (6, 11). Cytokines and Ca2+-mobilizing compounds also can suppress apoptosis induced by cytotoxic compounds through p53-independent pathways (11). However, unlike cytokines, suppression of apoptosis by A23187 or TG required extracellular Ca2+ and was blocked by the calcineurin inhibitor cyclosporin A (CsA) (11). Furthermore, A23187 and TG could not suppress all pathways of apoptosis that were suppressed by cytokines (11). Although A23187 and TG can promote cell viability by suppressing apoptosis in some cell types (11–14), these compounds also can induce apoptosis in other cell types (reviewed in ref. 15). These different effects of Ca2+-mobilizing compounds in different cells raises the possibility that these compounds may activate opposing apoptosis-regulating signals and that this may occur even in the same cells.
The present experiments have shown that A23187 and TG activate calcineurin-dependent opposing pathways that can either induce or suppress apoptosis in the same cells. The results also indicate the involvement of the p38 mitogen-activated protein kinase (p38 MAPK) pathway in induction of apoptosis but not in suppression of apoptosis by the Ca2+-mobilizing compounds.
Materials and Methods
Cells and Cell Culture.
The cells used were a cell line, mouse M1 myeloid leukemic cells, which do not express p53, that were transfected with plasmids containing the neomycin-resistance gene (M1-neo) or both the neomycin-resistance gene and a temperature-sensitive mutant p53 gene (M1-t-p53) (1). In M1-t-p53 cells the temperature-sensitive p53 gene encodes a protein, [Val-135]p53, that behaves like a tumor-suppressing wild-type p53 at 32°C and like a mutant p53 at 37°C (16). The cells were cultured in DMEM (GIBCO/BRL) with 10% heat-inactivated (56°C, 30 min) horse serum (GIBCO/BRL) in a 10% CO2/90% air atmosphere at 37°C or 32°C.
Compounds.
The compounds used were: the Ca2+ mobilizers A23187 and TG, the calcineurin inhibitors CsA and ascomycin (FK506 analog), the FK506 analog rapamycin, the MAPK kinase inhibitor PD98059, the p38 MAPK inhibitor SB203580, the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmanin, the cyclooxygenase inhibitor indomethacin, and prostaglandins E1 and E2. PD98059 and SB203580 were obtained from Calbiochem, CsA from Sandoz Pharmaceutical, and all other compounds from Sigma. Recombinant human IL-6 was kindly provided by Steve Clark (Genetics Institute, Cambridge, MA). Except for IL-6, stock solutions of all other compounds were made in absolute ethanol and stored at −20°C.
Assay for Apoptosis and Cell Viability.
Apoptosis was induced in M1-t-p53 cells seeded at 3 × 105 cells per ml and cultured at 32°C to activate wild-type p53 (1, 5, 9–11). M1-t-p53 and M1-neo cells cultured at 37°C, which do not express wild-type p53, were tested for induction of apoptosis by addition of A23187 or TG. The percent apoptotic cells was determined on May-Grünwald Giemsa-stained cytospin preparations by counting 400 cells. Apoptotic cells were scored by their smaller size, condensed chromatin, and fragmented nuclei compared with nonapoptotic cells (1, 5, 9–11). The percent cell viability was determined from the ratio of the number of viable cells (trypan blue excluding and nonapoptotic) divided by the total number of cells (including viable, trypan blue-stained, and apoptotic cells) as described (9). As previously shown for wild-type p53-induced apoptosis that results in loss of cell viability (11, 17), TG and A23187-induced loss of cell viability was associated with appearance of typical apoptotic cells and caspase activation resulting in cleavage of poly (ADP ribose) polymerase and pro-caspase 2, determined as described (11, 17).
Determination of Phosphorylated Forms of p44/42 and p38 MAPK by Western Blotting.
Activated forms of p44/42 MAPK (ERK1/2) and p38 MAPK were detected by Western blotting using antibody to the phosphorylated forms of these kinases. Cell extracts from untreated cells and cells cultured with TG or A23187 were made by sonication in ice-cold RIPA buffer (150 mM NaCl/0.1 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS/5 mM EDTA) containing a mixture of protease inhibitors and the phosphatase inhibitors sodium orthovanadate (1 mM) and β glycerophosphate (50 mM). For Western blotting, 100 μg cell extract proteins were electrophoresed in a SDS/12% polyacrylamide gel and electroblotted to a nitrocellulose filter. Blots were blocked for 1 hr at room temperature in buffer containing 0.2% Tween 20 and 5% low fat milk and then incubated overnight at 4°C with rabbit polyclonal antibody to the unphosphorylated forms of p44/42 MAPK or p38 MAPK, or the doubly phosphorylated (Thr-202/Tyr-204) p44/42 MAPK or (Thr-180/Tyr-182) p38 MAPK (New England Biolabs). Blots then were washed five times and incubated for 1 hr at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology), washed, and then developed with the Enhanced Chemiluminescence detection kit (Amersham Pharmacia). Bands of p44/42 MAPK or p38 MAPK were visualized after exposing the blots to Fuji Super RX film.
Results
Calcineurin-Mediated Suppression of Wild-Type p53 Induced Apoptosis by Calcium-Mobilizing Compounds.
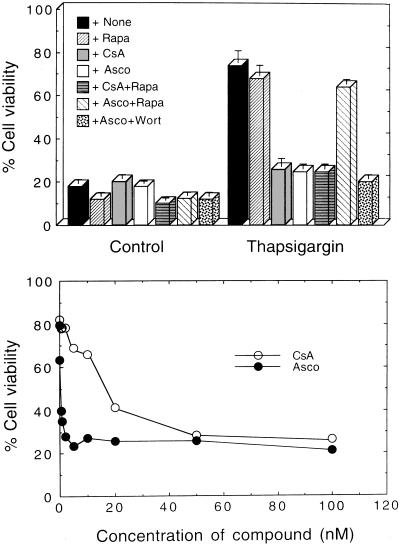
We previously have shown that A23187 and TG suppressed wild-type p53-induced apoptosis in M1 myeloid leukemic cells and that this suppression was blocked by CsA, an inhibitor of the serine/threonine protein phosphatase 2B (calcineurin) (11). The antiapoptotic effect of A23187 and TG was not blocked by okadaic acid, an inhibitor of the serine/threonine protein phosphatases 1 and 2A (PP1 and PP2A), or by several Ca2+ channel blockers that like CsA can inhibit p-glycoprotein multidrug resistance activity (11). These results indicated that the antiapoptotic effect of A23187 and TG was mediated by calcineurin. The mechanism of action of CsA involves binding to cyclophilin A, and the complex then associates with calcineurin and inhibits its activity (reviewed in ref. 18). To further establish the involvement of calcineurin in the antiapoptotic effect of A23187 and TG, we now have used a different calcineurin inhibitor, ascomycin, an analog of FK506 that binds to the FK506 binding protein 12 (FKBP12) (19), and this complex then associates with calcineurin and inhibits its activity (18). Like CsA, ascomycin completely blocked the ability of A23187 and TG to suppress wild-type p53-induced apoptosis (Fig. 1). Ascomycin was about 50-fold more effective than CsA in its ability to block the antiapoptotic effect of A23187 or TG (Fig. 1 Lower). These results provide further evidence that suppression of wild-type p53-induced apoptosis by Ca2+-mobilizing compounds is mediated by calcineurin.
Figure 1.
Calcineurin-dependent suppression of wild-type p53-mediated apoptosis. (Upper) M1-t-p53 cells were cultured for 20 hr at 32°C with no additions (Control) or with 10 nM TG in the absence (+None) or presence of: 100 nM rapamycin (+Rapa); 50 nM CsA (+CsA); 2 nM ascomycin (+Asco); CsA and rapamycin (+CsA+Rapa); ascomycin and rapamycin (+Asco+Rapa); or ascomycin and 1 μM wortmanin (+Asco+Wort). (Lower) Cells were cultured for 20 hr with 10 nM TG and different concentrations of CsA or ascomycin (Asco).
FKBP12 also can bind rapamycin, another FK506 analog, but the FKBP12-rapamycin complex does not inhibit calcineurin activity (18). Rapamycin, unlike CsA or ascomycin, did not block the antiapoptotic effect of TG (Fig. 1 Upper) or A23187. Furthermore, by competing for binding to FKBP12, rapamycin can interfere with the ability of FK506 to inhibit calcineurin activity (18). Addition of rapamycin antagonized the effect of ascomycin on suppression of apoptosis by TG (Fig. 1 Upper) or A23187, but did not antagonize CsA, which acts through cyclophilin A and not through FKBP12 (Fig. 1 Upper). The rapamycin-FKBP12 complex can inhibit activation of ribosomal p70S6kinase (20, 21). p70S6kinase can be activated in cells via the PI3K pathway, and this can be blocked by wortmanin (reviewed in ref. 22). Wortmanin did not block the antagonistic effect of ascomycin on suppression of apoptosis by TG (Fig. 1 Upper). Wortmanin also had no effect on induction of apoptosis by wild-type p53 or on its suppression by TG or A23187. Suppression of wild-type p53-induced apoptosis in M1 cells by TG or A23187 thus does not appear to be mediated by activation of PI3K or p70S6kinase.
Calcineurin-Mediated Induction of Apoptosis by Calcium-Mobilizing Compounds.
Suppression of wild-type p53-induced apoptosis by TG or A23187 was almost as effective as by the cytokine IL-6 when assayed 20 hr after culture of M1-t-p53 cells at 32°C (75 ± 5% versus 90 ± 6% cell viability, respectively) (11). However, when assayed 30 hr after culture of M1-t-p53 cells at 32°C, TG showed a much lower antiapoptotic effect than IL-6 (16 ± 4% versus 80 ± 3% cell viability, respectively). Similar results were obtained with A23187. These results indicate that the suppressive effect of TG or A23187 against wild-type p53-induced apoptosis is rapidly lost. Addition of TG together with IL-6 for 30 hr at 32°C also reduced the frequency of viable cells to 43 ± 2% compared with 80 ± 3% with IL-6 alone. These results raised the possibility that in addition to their calcineurin-mediated antiapoptotic effect, TG and A23187 may activate an opposing pathway that induces apoptosis in the same cells. To examine this possibility, we cultured M1-t-p53 cells with TG or A23187 at 37°C, a temperature at which the p53 protein behaves like a mutant p53 and does not induce apoptosis (1). There was no detectable loss of cell viability (92 ± 3% cell viability) up to 20 hr after addition of the same concentration of TG (10 nM) used to suppress induction of wild-type p53-mediated apoptosis at 32°C. However, there was a loss of cell viability at 30 hr after addition of TG (60 ± 5% cell viability) and this loss was even stronger (22 ± 2% cell viability) after 40 hr. Similar results were obtained with M1-neo cells, which do not express wild-type p53, cultured at 37°C for 40 hr with 10 nM TG or 0.5 μM A23187.
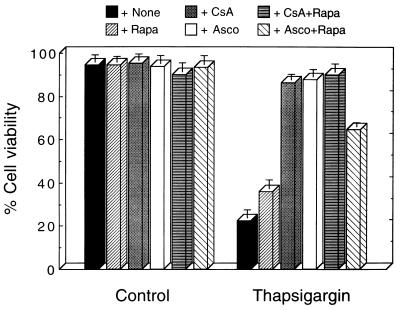
Induction of apoptosis in M1 cells at 37°C by TG was suppressed by CsA and ascomycin (Fig. 2). Rapamycin showed a small increase in viability of these TG-treated cells and antagonized the ability of ascomycin, but not of CsA, to suppress TG-induced apoptosis (Fig. 2). Similar results were obtained with A23187-treated cells. The results thus indicate that in addition to their ability to suppress apoptosis in M1 myeloid leukemic cells, Ca2+-mobilizing compounds can induce a calcineurin-mediated pathway that leads to apoptosis in the same cells. The opposing pathways for suppression or induction of apoptosis diverge downstream from activation of calcineurin.
Figure 2.
Induction of calcineurin-dependent apoptosis by TG. M1-t-p53 cells were cultured for 40 hr at 37°C without (Control) or with 10 nM TG in the abscence (+None) or presence of: 100 nM rapamycin (+Rapa); 50 nM CsA (+CsA); 1 nM ascomycin (+Asco); CsA and rapamycin (+CsA+Rapa); or ascomycin and rapamycin (+Asco+Rapa).
Involvement of p38 MAPK in Induction of Apoptosis but Not in Suppression of Apoptosis by Ca2+-Mobilizing Compounds.
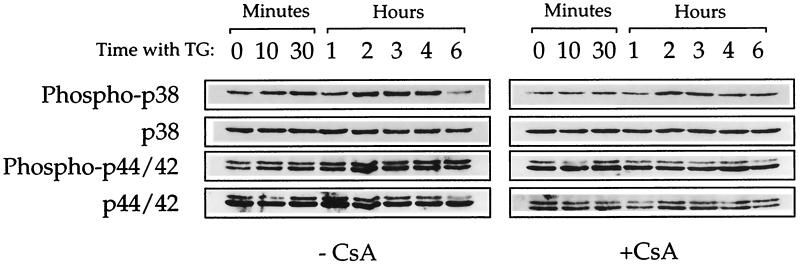
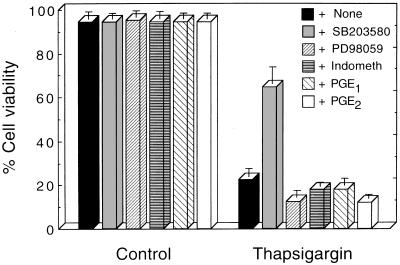
The divergence of two opposing pathways for suppression or induction of apoptosis downstream from calcineurin activation by Ca2+-mobilizing compounds presumably is caused by divergence of different signaling cascades, which could include MAPKs that form cascades of different serine/threonine kinases (reviewed in ref. 23). Induction of apoptosis may be regulated by the balance between different MAPKs (24), and p38 MAPK activation has been implicated in apoptosis induction (24–29). We therefore determined the involvement of p38 MAPK and p44/42 MAPK in induction or suppression of apoptosis in M1 cells by the Ca2+-mobilizing compounds. Using antibody to the doubly phosphorylated p38 MAPK we detected an increase in phosphorylation of p38 MAPK in M1 cells without wild-type p53, which was strongest at 2–4 hr after addition of TG, and this increase was partially blocked by the calcineurin inhibitor CsA (Fig. 3). TG also caused an increase in the phosphorylation of p44/42 MAPK, which was blocked by CsA (Fig. 3). Similar results were obtained with A23187. Addition of 1–5 μM SB203580, which inhibits the activity of p38 MAPK but not other MAPKs (30, 31), partially suppressed induction of apoptosis by TG in cells without wild-type p53 (Fig. 4). But up to 50 μM PD98054, that inhibits MAPK kinase and thus prevents activation of downstream p44/42 MAPKs (ERK1/2) (32), did not suppress TG-induced apoptosis in these cells (Fig. 4, Table 1). Neither SB203580 nor PD98059 had any suppressive effect on induction of apoptosis by wild-type p53 or by withdrawal of IL-6 from IL-6-dependent cells (Table 1) that are not mediated by calcineurin. SB203580 and PD98059 also did not have any effect on suppression of apoptosis by TG or A23187 that are calcineurin mediated, or on suppression of apoptosis by IL-6 that is not mediated by calcineurin (Table 1).
Figure 3.
Calcineurin-dependent activation of p38 and p44/42 MAPKs. M1-t-p53 cells were cultured at 37°C with 10 nM TG for various times or without TG (0 min with TG) in the absence (−) or presence (+) of 100 nM CsA. Western blotting was carried out with antibody to p38 or p44/42 MAPKs or to the doubly phosphorylated (Phospho-p38 and Phospho-p44/42) MAPKs.
Figure 4.
Suppression of TG-induced apoptosis by a p38 MAPK inhibitor. M1-t-p53 cells were cultured for 40 hr at 37°C without (Control) or with 10 nM TG in the absence (+None) or presence of: 5 μM SB203580 or PD98059; 10 μM indomethacin (+Indometh); 10 μM prostaglandins E1 or E2 (+PGE1 or PGE2).
Table 1.
Specificity of the suppressive effect of SB203580 on apoptosis induction by TG
| Induction of apoptosis by* | Suppression of apoptosis by† | % Cell viability‡
|
||
|---|---|---|---|---|
| Addition of
| ||||
| None | SB203580 | PD98059 | ||
| TG | None | 23.3 ± 5.1 | 61.0 ± 7.1§ | 16.3 ± 3.1§ |
| Wild-type p53 | None | 22.5 ± 3.1 | 19.0 ± 2.5 | 10.3 ± 3.6 |
| TG | 75.4 ± 6.1 | 77.3 ± 5.0 | 70.3 ± 6.6 | |
| A23187 | 76.2 ± 4.8 | 80.3 ± 3.1 | 68.9 ± 3.7 | |
| IL-6 | 90.6 ± 3.7 | 91.3 ± 5.3 | 86.1 ± 6.6 | |
| IL-6 withdrawal | None | 35.5 ± 5.2 | 33.5 ± 4.1 | 30.5 ± 4.0 |
| IL-6 | 84.4 ± 6.2 | 87.5 ± 4.8 | 81.3 ± 5.6 | |
M1-t-p53 cells were induced to undergo apoptosis by culture at 37°C with 10 nM TG for 40 hr, by culture at 32°C, to activate wild-type p53, for 20 hr, or by pretreatment with 20 ng/ml IL-6 at 32°C for 20 hr and then subculturing the cells at 37°C for 24 hr without IL-6 as described (11).
Apoptosis was suppressed by 10 nM TG, 0.5 μM A23187, or 20 ng/ml IL-6.
% Cell viability was determined without (None) or with 5 μM SB203580 or PD98059.
Even 1 μM SB203580 suppressed induction of apoptosis by TG (43.6 ± 5.3% cell viability) whereas there was no suppression of TG-induced apoptosis even at 50 μM PD98059 (11.6 ± 3.2% cell viability).
SB203580 and PD98054 block the activity of p38 MAPK and activation of p44/42 MAPK by MAPK kinase, respectively (30–32). But both compounds also were reported to inhibit the activity of cyclooxygenases 1 and 2 that control prostaglandin synthesis (33). The differential ability of SB203580 but not PD98054 to suppress induction of apoptosis by TG or A23187 in our experiments makes it unlikely that cyclooxygenase inhibition by SB203580 is responsible for this suppression of apoptosis. In addition, neither the cyclooxygenase inhibitor indomethacin nor prostaglandins E1 or E2 had any effect on the ability of TG to induce apoptosis (Fig. 4).
The results indicate that p38 MAPK, but not p44/42 MAPK, is involved in the pathway that leads to induction of apoptosis in M1 cells by Ca2+-mobilizing compounds downstream from calcineurin activation. However, neither p38 nor p44/42 MAPKs mediate suppression of apoptosis by Ca2+-mobilizing compounds, and p38 MAPK does not mediate induction of apoptosis by some calcineurin-independent pathways.
Discussion
Induction of apoptosis in different normal and cancer cell types by DNA damaging agents can depend on expression of wild-type p53, and there are also p53-independent pathways that can lead to apoptosis (reviewed in refs. 5, 8, and 10). Using M1 myeloid leukemic cells that express a temperature-sensitive p53 protein we previously have shown that overexpression of p53 in its wild-type form is sufficient to induce apoptosis (1, 5–11). We also have shown that different cytokines (1, 2, 5–10) as well as Ca2+-mobilizing compounds such as A23187 and TG (11) can effectively suppress this apoptosis. However, suppression of wild-type p53 induced apoptosis by Ca2+-mobilizing compounds required extracellular Ca2+ and was blocked by the calcineurin inhibitor CsA, whereas apoptosis suppression by cytokines did not show these properties (11). Cytokines also can suppress pathways that lead to apoptosis, which were not suppressed by Ca2+-mobilizing compounds (11). These different properties indicate that suppression of apoptosis by cytokines and Ca2+-mobilizing compounds is mediated by activating different viability promoting signals.
The ability of CsA to block the antiapoptotic effect of Ca2+-mobilizing compounds indicated that these compounds mediate their antiapoptotic effect through calcineurin activation (11). We now have shown that ascomycin (19), an ethyl analog of the calcineurin inhibitor FK506 that differs from CsA by binding to FKBP12 rather than to cyclophilin A (18), was even more effective than CsA in blocking the antiapoptotic effect of Ca2+-mobilizing compounds. In contrast, rapamycin, which also binds to FKBP12 but does not block calcineurin activity (18), did not block the antiapoptotic effect of Ca2+-mobilizing compounds. Rapamycin also antagonized the ability of ascomycin but not of CsA to block the antiapoptotic effect of Ca2+-mobilizing compounds, as expected from the ability of rapamycin to compete with FK506 for binding to FKBP12 (18). This effect of rapamycin does not appear to be mediated by its ability to inhibit p70S6kinase that is activated through the PI3K pathway, because the PI3K inhibitor wortmanin did not mimic the ability of rapamycin to antagonize ascomycin. The use of wortmanin also has indicated that p70S6kinase and PI3K did not mediate the suppression of apoptosis by the Ca2+-mobilizing compounds. The results provide further evidence that Ca2+-mobilizing compounds signal suppression of wild-type p53-induced apoptosis by activating calcineurin (Fig. 5).
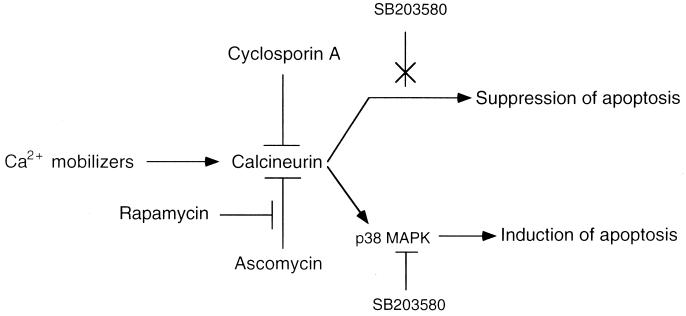
Figure 5.
Model of calcineurin-dependent pathways for suppression or induction of apoptosis. Arrow indicates pathway; ⊥, suppression of pathway; and X, pathway not suppressed.
Unlike IL-6, the ability of Ca2+-mobilizing compounds to suppress wild-type p53-induced apoptosis was rapidly lost, and on more prolonged incubation these compounds even reduced the antiapoptotic effect of IL-6. The same concentration of Ca2+-mobilizing compounds that showed an antiapoptotic effect on apoptosis induced by wild-type p53 could induce apoptosis in the absence of wild-type p53. As with the antiapoptotic effect, the proapoptotic effect of the Ca2+-mobilizing compounds was blocked by CsA and ascomycin, and the blocking effect of ascomycin was antagonized by rapamycin. Ca2+-mobilizing compounds therefore appear to induce two opposing pathways in the same cells downstream from calcineurin activation, which can either suppress or induce apoptosis (Fig. 5). However, calcineurin activation does not appear to mediate apoptosis induction by wild-type p53 or by withdrawal of a viability factor such as IL-6, or apoptosis suppression by cytokines. Calcineurin is a serine/threonine phosphatase that can bind to different substrates directly or through adaptor proteins such as FKBP12 or AKAP79 and dephosphorylate these substrates (reviewed in ref. 34). Calcineurin may suppress or induce apoptosis by dephosphorylating and thus modulating the activity of different phospho-proteins that are involved in the apoptotic process such as Bcl-2 (35, 36) or Bad (37).
We now have shown that calcineurin activation can lead to activation of p38 MAPK and that the p38 MAPK inhibitor SB203580 blocked this pathway for the induction of apoptosis (Fig. 5). The ability of SB203580 to only partially block the proapoptotic effect of Ca2+-mobilizing compounds suggests that calcineurin also may activate p38 MAPK-independent pathways that lead to induction of apoptosis. Although p44/42 MAPK was activated by Ca2+-mobilizing compounds, an inhibitor of this pathway did not block induction of apoptosis. Neither p38 nor p44/42 MAPKs appear to be involved in suppression of apoptosis by the Ca2+-mobilizing compounds, or in induction of apoptosis that is not mediated by calcineurin. The results indicate that calcineurin activation can activate opposing pathways that either suppress or induce apoptosis in the same cells and that induction of apoptosis can be mediated by the p38 MAPK pathway.
Acknowledgments
This work was supported by the National Foundation for Cancer Research (Bethesda, MD), the Esther Mazor Family (Washington, DC), and the Ebner Family Biomedical Research Foundation at the Weizmann Institute of Science in memory of Alfred and Dolfi Ebner and Lola Beer-Ebner.
Abbreviations
- CsA
cyclosporin A
- FKBP
FK506 binding protein
- MAPK
mitogen-activated protein kinase
- PI3K
phosphatidylinositol 3-kinase
- TG
thapsigargin
References
- 1.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Nature (London) 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 2.Lotem J, Sachs L. Blood. 1993;82:1092–1096. [PubMed] [Google Scholar]
- 3.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. Nature (London) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 4.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Nature (London) 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 5.Sachs L, Lotem J. Blood. 1993;82:15–21. [PubMed] [Google Scholar]
- 6.Lotem J, Sachs L. Leukemia. 1995;9:685–692. [PubMed] [Google Scholar]
- 7.Yonish-Rouach E, Grunwald D, Wilder S, Kimchi A, May E, Lawrence J, May P, Oren M. Mol Cell Biol. 1993;13:1415–1423. doi: 10.1128/mcb.13.3.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotem J, Sachs L. Leukemia. 1996;10:925–931. [PubMed] [Google Scholar]
- 9.Lotem J, Peled-Kamar M, Groner Y, Sachs L. Proc Natl Acad Sci USA. 1996;93:9166–9171. doi: 10.1073/pnas.93.17.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotem J, Sachs L. Apoptosis. 1999;4:187–196. doi: 10.1023/a:1009614723237. [DOI] [PubMed] [Google Scholar]
- 11.Lotem J, Sachs L. Proc Natl Acad Sci USA. 1998;95:4601–4606. doi: 10.1073/pnas.95.8.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues-Tarduchy G, Collins M, López-Rivas A. EMBO J. 1990;9:2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin J L, Johnson E M., Jr Trends Neurosci. 1992;15:501–508. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Tozawa Y, Iseki R, Mukai M, Iwata M. J Immunol. 1995;154:6346–6354. [PubMed] [Google Scholar]
- 15.McConkey D J, Orrenius S. J Leukocyte Biol. 1996;59:775–783. [PubMed] [Google Scholar]
- 16.Michalovitz D, Halevy O, Oren M. Cell. 1990;62:671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- 17.Lotem J, Sachs L. Proc Natl Acad Sci USA. 1997;94:9349–9353. doi: 10.1073/pnas.94.17.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber S L, Crabtree G R. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 19.Kawai M, Lane B C, Hsieh G C, Mollison K W, Carter G W, Luly J R. FEBS Lett. 1993;316:107–113. doi: 10.1016/0014-5793(93)81196-7. [DOI] [PubMed] [Google Scholar]
- 20.Kuo C J, Chung J, Fiorentino D F, Flanagan W M, Blenis J, Crabtree G R. Nature (London) 1992;358:70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- 21.Price D J, Grove J R, Calvo V, Avruch J, Bierer B E. Science. 1992;257:973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- 22.Ui M, Okada T, Hazeki K, Hazeki O. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 23.Schaeffer H J, Weber M J. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 25.Graves J D, Draves K E, Craxton A, Saklatvala J, Krebs E G, Clark E A. Proc Natl Acad Sci USA. 1996;93:13814–13818. doi: 10.1073/pnas.93.24.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 27.Kummer J L, Rao P K, Heidenreich K A. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- 28.Yue T, Ni J, Romanic A M, Gu J, Keller P, Wang C, Kumar S, Yu G, Hart T K, Wang X, et al. J Biol Chem. 1999;274:1479–1486. doi: 10.1074/jbc.274.3.1479. [DOI] [PubMed] [Google Scholar]
- 29.Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. J Immunol. 1999;162:1692–1700. [PubMed] [Google Scholar]
- 30.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 31.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 32.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Börsch-Haubold A G, Pasquet S, Watson S P. J Biol Chem. 1998;273:28766–28772. doi: 10.1074/jbc.273.44.28766. [DOI] [PubMed] [Google Scholar]
- 34.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 35.Shibasaki F, Kondo E, Akagi T, McKeon F. Nature (London) 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 36.Haldar S, Jena N, Croce C M. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Pathan N, Ethell I M, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke T F, Reed J C. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]