Abstract
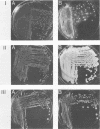
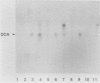
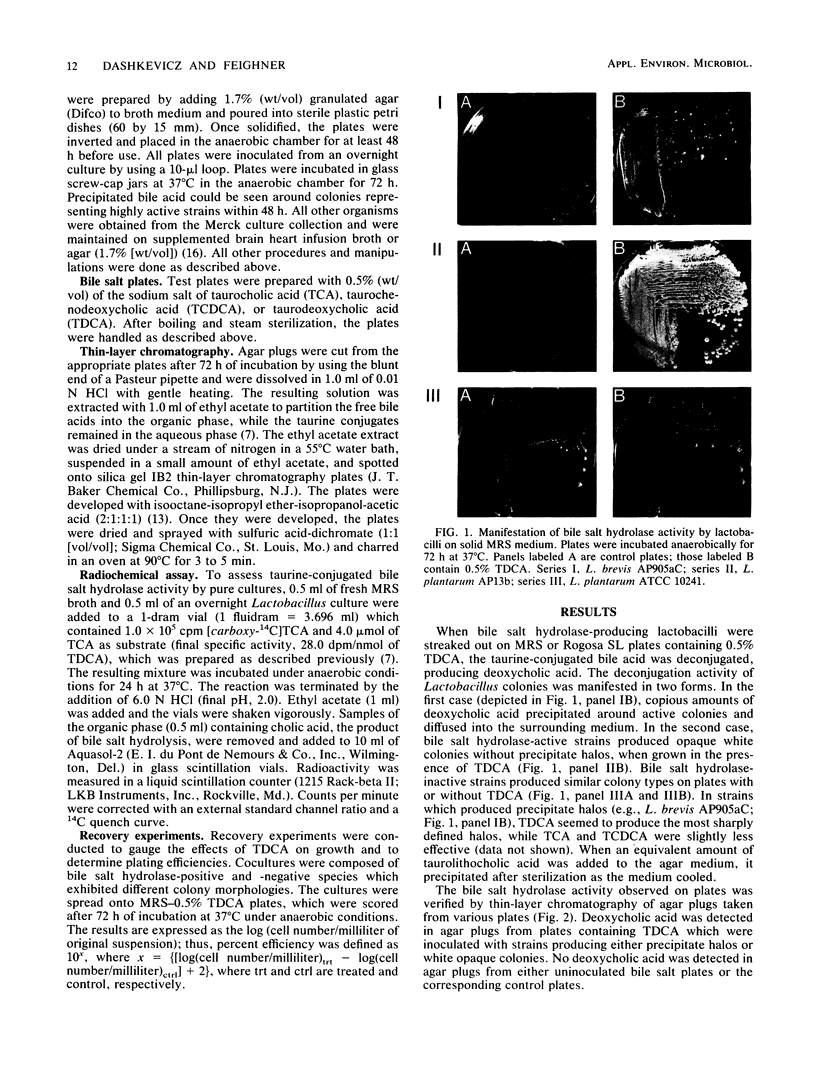
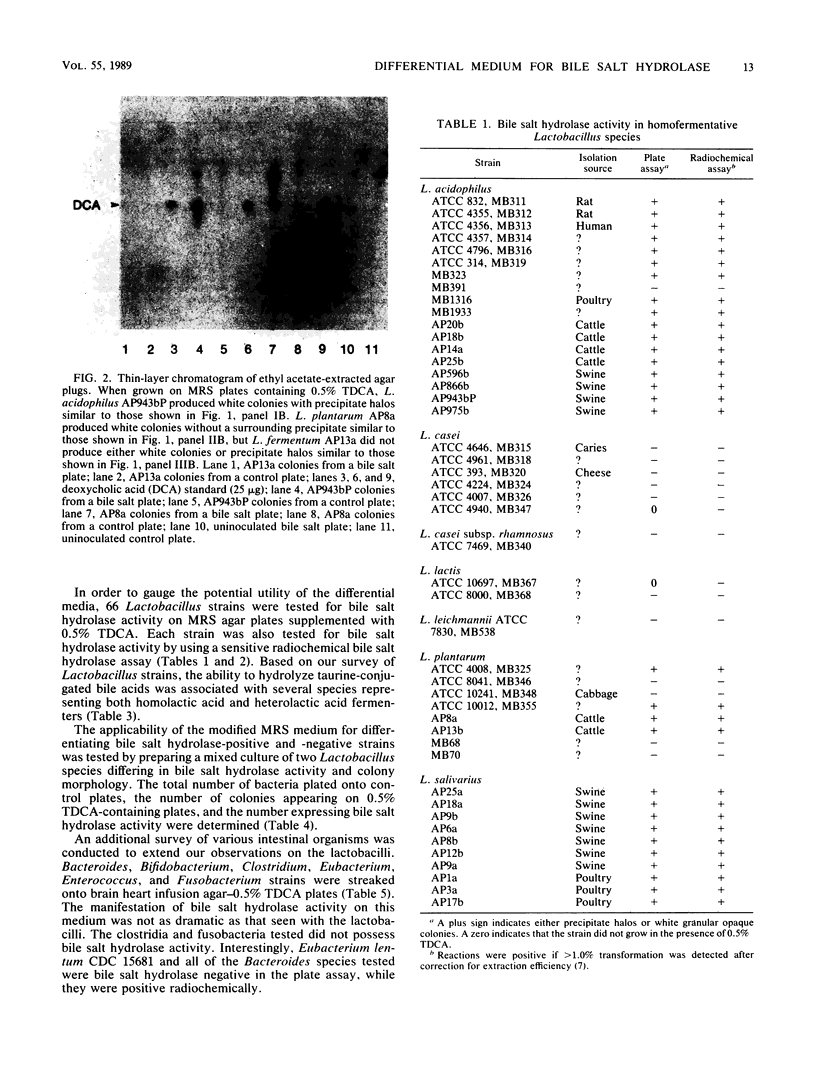
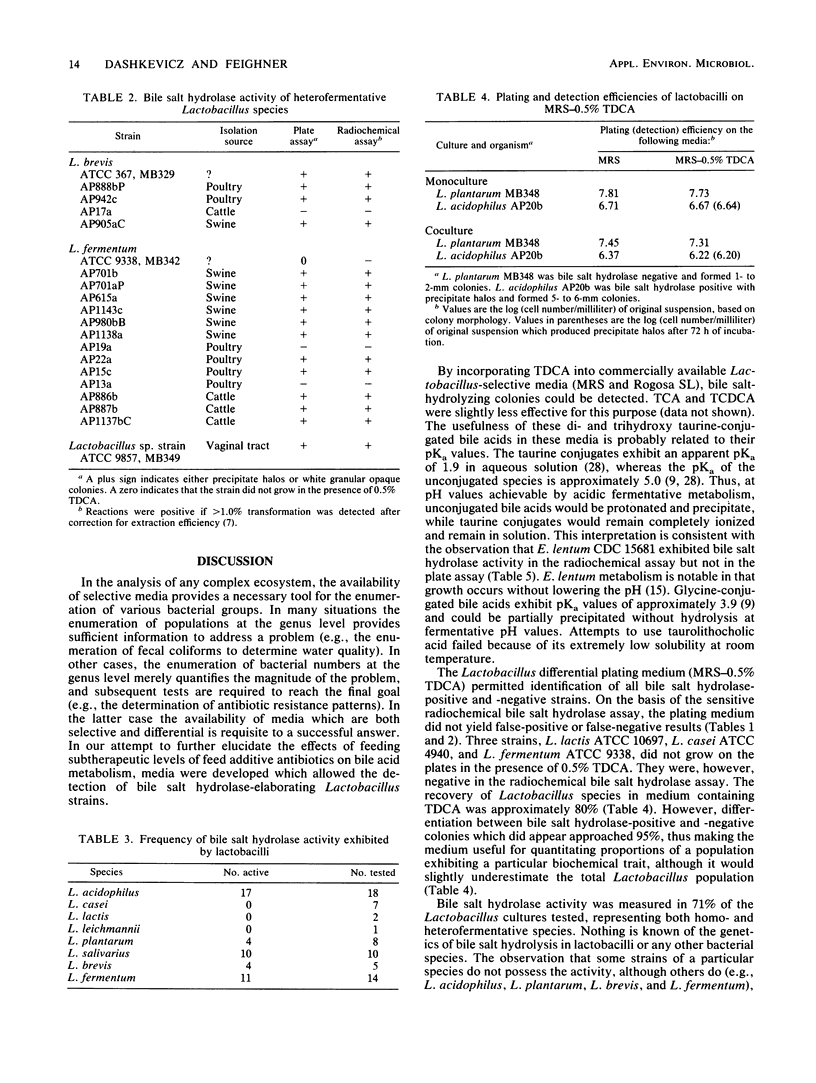
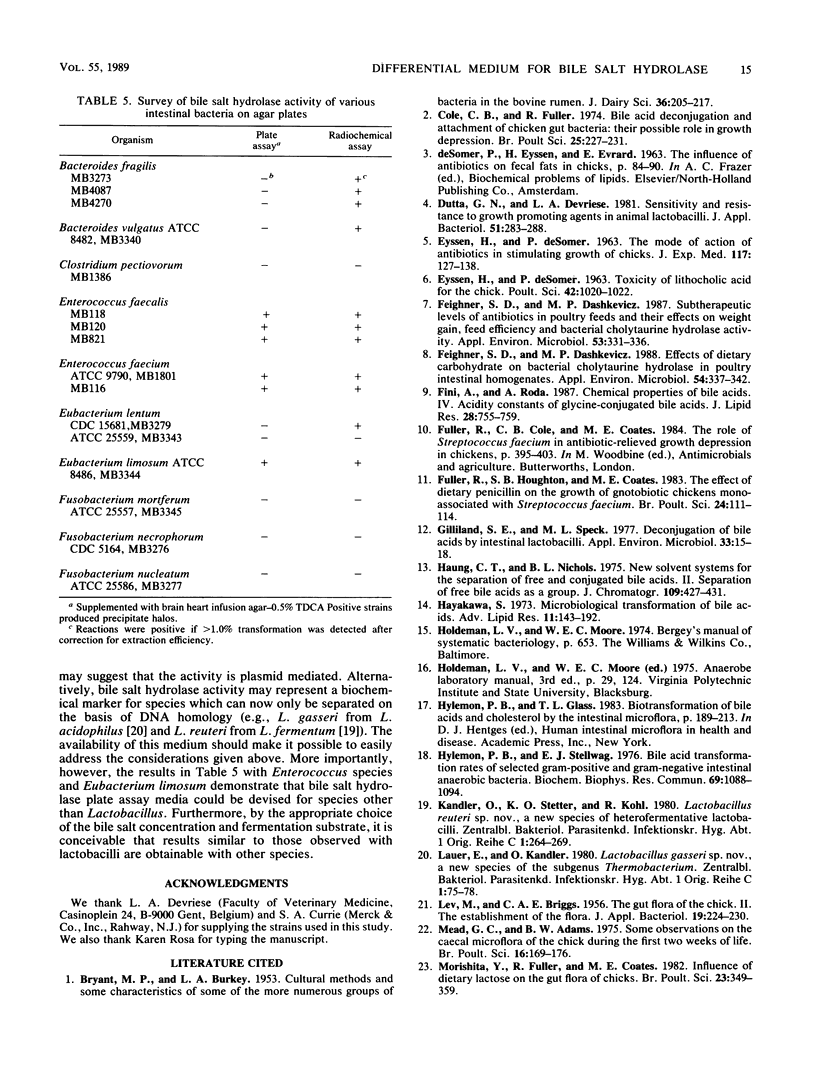
An agar plate assay was developed to detect bile salt hydrolase activity in lactobacilli. On Lactobacillus-selective MRS or Rogosa SL medium supplemented with taurodeoxycholic, taurocholic, or taurochenodeoxycholic acids, bile salt hydrolysis was manifested at two intensities: (i) the formation of precipitate halos around colonies or (ii) the formation of opaque granular white colonies. Sixty-six lactobacilli were tested for bile salt hydrolase activity by both the plate assay and a sensitive radiochemical assay. No false-positive or false-negative results were detected by the plate assay. Based on results of experiments with Eubacterium lentum and Bacteroides species, the plate assay was dependent on two factors: (i) the presence of bile salt hydrolytic activity and (ii) the ability of the organism to sufficiently acidify the medium to protonate free bile acids. The availability of a differential medium for determination of bile salt hydrolase activity will provide a rapid method for determining shifts in a specific functional activity of intestinal Lactobacillus species and provide a rapid screening capability for identifying bile salt hydrolase-deficient mutants. The latter application should allow bile salt hydrolase activity to be used as a marker enzyme in genetic experiments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole C. B., Fuller R. Bile acid deconjugation and attachment of chicken gut bacteria: their possible role in growth depression. Br Poult Sci. 1984 Apr;25(2):227–231. doi: 10.1080/00071668408454861. [DOI] [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Sensitivity and resistance to growth promoting agents in animal lactobacilli. J Appl Bacteriol. 1981 Oct;51(2):283–288. doi: 10.1111/j.1365-2672.1981.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Feighner S. D., Dashkevicz M. P. Effect of dietary carbohydrates on bacterial cholyltaurine hydrolase in poultry intestinal homogenates. Appl Environ Microbiol. 1988 Feb;54(2):337–342. doi: 10.1128/aem.54.2.337-342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner S. D., Dashkevicz M. P. Subtherapeutic levels of antibiotics in poultry feeds and their effects on weight gain, feed efficiency, and bacterial cholyltaurine hydrolase activity. Appl Environ Microbiol. 1987 Feb;53(2):331–336. doi: 10.1128/aem.53.2.331-336.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini A., Roda A. Chemical properties of bile acids. IV. Acidity constants of glycine-conjugated bile acids. J Lipid Res. 1987 Jul;28(7):755–759. [PubMed] [Google Scholar]
- Fuller R., Houghton S. B., Coates M. E. The effect of dietary penicillin on the growth of gnotobiotic chickens monoassociated with Streptococcus faecium. Br Poult Sci. 1983 Jan;24(1):111–114. doi: 10.1080/00071668308416719. [DOI] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. Deconjugation of bile acids by intestinal lactobacilli. Appl Environ Microbiol. 1977 Jan;33(1):15–18. doi: 10.1128/aem.33.1.15-18.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. T., Nichols B. L. New solvent systems for the separation of free and conjugated bile acids. II. Separation of free bile acids as a group. J Chromatogr. 1975 Jun 18;109(2):427–431. doi: 10.1016/s0021-9673(01)91822-9. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Stellwag E. J. Bile acid biotransformation rates of selected gram-positive and gram-negative intestinal anaerobic bacteria. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1088–1094. doi: 10.1016/0006-291x(76)90484-8. [DOI] [PubMed] [Google Scholar]
- Mead G. C., Adams B. W. Some observations on the caecal microflora of the chick during the first two weeks of life. Br Poult Sci. 1975 Mar;16(2):169–176. doi: 10.1080/00071667508416174. [DOI] [PubMed] [Google Scholar]
- Morishita Y., Fuller R., Coates M. E. Influence of dietary lactose on the gut flora of chicks. Br Poult Sci. 1982 Jul;23(4):349–359. doi: 10.1080/00071688208447968. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirehead P. A., Maglio M., Goodman J. R. Bacteria isolated from the duodenum, ileum, and cecum of young chicks. Appl Environ Microbiol. 1978 Apr;35(4):782–790. doi: 10.1128/aem.35.4.782-790.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka S. [Comparison of bacterial flora in the digestive tract of ducks and chickens. 1. Bacterial flora of feces]. Nihon Saikingaku Zasshi. 1970 Jun;25(5):300–304. doi: 10.3412/jsb.25.300. [DOI] [PubMed] [Google Scholar]
- Shirasaka S. [Comparison of bacterial flora in the digestive tract of ducks and chickens. 2. Bacterial flora in various parts of the digestive tract of poultry fed ad libitum and on fasting]. Nihon Saikingaku Zasshi. 1970 Jul;25(7):355–361. doi: 10.3412/jsb.25.355. [DOI] [PubMed] [Google Scholar]