Abstract
Thymocyte development is reported to be inhibited by pregnancy, although the impact of this effect on fertility is unknown. We demonstrate, using progesterone receptor null mutant mice, that the inhibitory effects of pregnancy hormones on T cell development require the presence of functional progesterone receptor (PR). A combination of hysterectomy, thymic immunohistochemistry, and transplant studies reveals that local expression of PR in thymic stromal cells is specifically required for thymic involution to occur. These cells, under the influence of progesterone, block T cell development at the early pre-T cell (CD3−CD44+ CD25+) stage of development via a paracrine mechanism. In addition, age-related thymic involution is shown to occur by a separate PR-independent mechanism. Finally, pregnancy studies with thymic transplants from progesterone receptor null mutant mice to wild-type female recipients demonstrate that thymic stromal PR is required for normal fertility. Together, these observations provide evidence for a PR-dependent paracrine mechanism that blocks very early T cell lymphopoiesis during pregnancy and is essential for normal fertility.
One of the long-standing enigmas of reproductive biology is why the fetus is not rejected by the mother’s immune system (1–3). Many explanations for this phenomenon have been proposed and tested, including the idea of paternal antigen sequestration and reduced MHC expression (4, 5), the possibility of local immune changes in the uterus (6, 7), and a potential maternal shift from Th1 to Th2 immune responses (8–10). Although each of these mechanisms appears to play some role in protection of the fetus from the maternal immune system, none has been directly shown to be required. It now appears that pregnancy may in fact have many redundant mechanisms acting both systemically and at the maternal–fetal interface to protect the fetus from the mother’s immune system.
One particularly interesting effect of pregnancy on the maternal immune system is a general blockade of lymphocyte development. This blockade manifests itself grossly in the form of thymic involution and bone marrow involution. Involution of the thymus, the site of T cell differentiation, has long been recognized to occur during pregnancy in a number of species including rat, mouse, and human (11–13) and may be mimicked by the administration of the female sex hormones estrogen (E) and progesterone (P), although the relative contributions of the two hormones are controversial (14, 15). Although the functional importance of pregnancy-induced thymic involution remains unknown, the fact that it has been observed in all mammals that have been examined has led to the speculation that reduced or altered output of mature T cells by the thymus could be an important component of maternal immune regulation. It also has been reported that bone marrow, the site of B lymphocyte development, undergoes an involution process similar to that occurring in the thymus during pregnancy. The result is a specific block to B cell development that can be replicated by E + P treatment (16–18).
As the major hormone of pregnancy, P is an obvious candidate mediator for pregnancy-associated phenomena. The central importance of P to pregnancy has been amply demonstrated by the progesterone receptor knockout (PRKO) mouse. Analysis of the PRKO mouse revealed that the progesterone receptor (PR) regulates virtually all aspects of female reproduction, including ovulation, uterine decidualization, sexual lordosis behavior, and mammary gland development (19, 20). Because regulation of the maternal immune system is also an important aspect of pregnancy, we questioned whether PR might be involved in that aspect of pregnancy as well.
In the present study, we demonstrate that PR in thymic epithelial cells is required for pregnancy-related thymic involution. We show that PR causes pregnancy-induced thymic involution by causing a loss of T cell precursors at a specific early stage of T cell development. Finally, we show that loss of thymic involution during pregnancy results in decreased fertility and increased fetal loss.
Materials and Methods
Reagents.
Hamster anti-mouse CD3ɛ antibody (clone 145–2C11), rat anti-mouse CD4 (clone RM5–5), rat anti-mouse CD8α (clone 53–6.7), rat anti-mouse CD25 (clone 7D4), and rat anti-mouse CD44 (clone IM7) were obtained from PharMingen. Rabbit anti-PR antibody (catalogue no. A0098) was purchased from Dako. Biotinylated goat anti-rabbit IgG (catalogue no. BA-1000) was purchased from Vector Laboratories. Goat serum was purchased from GIBCO/BRL and heat inactivated at 56°C for 30 min before use. Progesterone, estrogen (17β-estradiol), sesame oil, DAB peroxidase substrate (Sigma Fast DAB Tablet Sets), and 2′deoxyguanosine were purchased from Sigma. E was dissolved in ethanol, then diluted to 20 ng/μl in sesame oil. P was dissolved to 20 μg/μl in sesame oil at 55°C for 1 hr. Transwell plates (catalogue no. 3412) were purchased from Corning Costar.
Fertility Studies.
Thymectomy was performed on neonatal wild-type 129/C57 hybrid mice (a wild-type strain derived from our PRKO colony) as described by Sjodin et al. (21). Surgical mortality was 5%, and deaths caused by cannibalism were approximately 5%. Donor thymuses were collected from neonatal wild-type or PRKO mice and placed in complete DMEM +1.35 mM 2′deoxyguanosine in transwell plates for 4 days, as described previously (22, 23). Donor thymuses were then transplanted beneath the kidney capsule of 4-wk-old thymectomized recipient mice and allowed to repopulate.
Six weeks later, transplanted mice were mated to vasectomized males followed by embryo transfer of eight FVB embryos into one uterine horn. FVB embryos were chosen to maximize antigenic differences between the mother and the embryo, and embryo transfers were performed to control for variations in ovulation and fertilization. Mice were sacrificed on pregnancy day 17, inspected to ensure the absence of residual thymic tissue in the chest, and scored for the number of live and resorbing embryos present in the uterus. Embryos were then fixed in Bouins’ fixative and prepared for hematoxylin/eosin (H&E) histology.
Animals.
All mice were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. PRKO mice were generated in our laboratory as described (19). Wild-type and PRKO mice were housed in the animal facility at Baylor College of Medicine (Houston, TX). Ovariectomies and hysterectomies were performed under ketamine, xylazine, and acepromazine anesthesia. Hysterectomized mice had both ovaries and both uterine horns removed starting at the level of the cervix via a midline incision. PR immunohistochemistry was performed as previously described (24).
Thymocyte Preparation.
Mice were sacrificed by CO2 inhalation and thymuses were removed and weighed. Thymocytes from wild-type and PRKO mice were depleted of erythrocytes and enriched for viable cells as described (25). The cells were resuspended in Hanks’ balanced salt solution +1% FCS for subsequent cell counting and flow cytometry.
Flow Cytometry.
All staining steps were carried out on ice in Hanks’ balanced salt solution +1% FCS. All cells were resuspended at a concentration of 5 × 106/ml. Two hundred microliters of this suspension was stained with 1 μg of the appropriate fluorochrome-conjugated antibody(ies), an isotype control, or no antibody for 20 min. Cells were then fixed in 1% paraformaldehyde in PBS. Analysis was performed on an EPICS Profile instrument (Coulter), and results are expressed as the mean percentage of cells staining above control intensity for at least three different animals or as fluorescence histograms on a log scale.
Statistics.
P values were calculated by using paired or unpaired one-tailed Student’s t tests, assuming equal variance or a χ2 test as appropriate. Statistical calculations were performed by using Excel ’97 (Microsoft).
Results
Progesterone Receptor Is Required for Hormone-Induced Thymic Involution but Not for Age-Induced Thymic Involution or for Bone Marrow Involution.
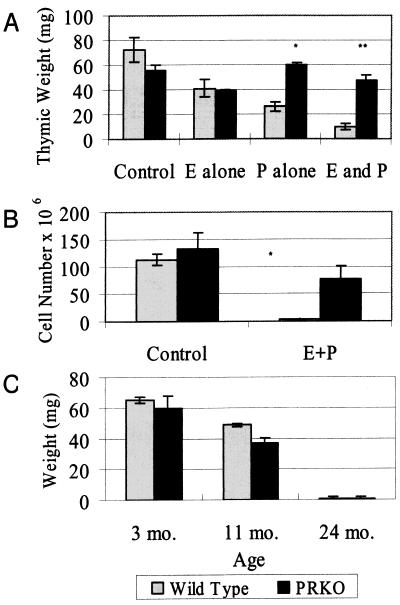
Because PRKO mice are infertile, we initially decided to examine the role of PR in pregnancy-induced thymic and bone marrow involution by treating wild-type and PRKO mice with exogenous sex steroids. To assess the importance of PR in mediating sex hormone-induced thymic involution, ovariectomized wild-type and PRKO mice were treated daily for 12 days with vehicle alone, E, or E + P (Fig. 1). After treatment, thymuses were assessed for involution based on weight and cell counts (Fig. 2, A and B). Untreated wild-type and PRKO mice showed no significant difference in thymic size, weight, or cell number. After E treatment, wild-type and PRKO mice showed a similar 30% decrease in thymic weight (Fig. 2A). Progesterone treatment alone resulted in a 30% decrease in thymic weight in the wild-type animals but showed no effect in the PRKO mice. When given together, E and P resulted in a dramatic 80% decrease in wild-type thymic size and weight (Fig. 1 and Fig. 2A) and a 99% decrease in cell number (Fig. 2B). E + P-treated PRKO mice, on the other hand, appeared similar to those treated with E alone, thus demonstrating that E and P work synergistically to cause thymic involution, and that functional PR is required for the majority of thymic involution to occur. Interestingly, wild-type and PRKO mice treated for 3 wk with E + P showed no significant difference in CD3+ T cell numbers in the spleen (27.6 ± 3.7 × 106 vs. 23.0 ± 6.0 × 106). Further, E + P-mediated suppression of preB cell numbers showed no PR dependence with E + P-treated wild-type and PRKO mice showing similar numbers of preB cells in the bone marrow.
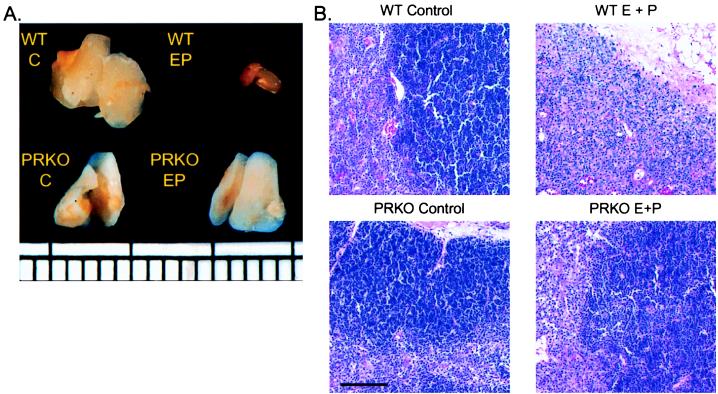
Figure 1.
Gross morphology and H&E histology of E + P treated wild-type and PRKO thymuses. (A) Photographs of wild-type and PRKO thymuses after 12 days of E + P treatment demonstrating lack of involution in PRKO mice. Ruler demarcated in millimeters. (B) Five micrometers H&E-stained sections of thymuses taken from wild-type and PRKO mice treated for 12 days with E, E + P, or vehicle alone. Notice the complete loss of cortical lymphocytes in the wild-type E + P-treated mouse, whereas the PRKO thymus appears intact. Bar = 100 μm.
Figure 2.
Hormone- and age-induced thymic and bone marrow involution. (A and B) Graph of thymic weight (A) and total thymocyte number (B) in ovariectomized wild-type and PRKO mice after 12 days of E + P treatment. Wild-type mice show significantly smaller thymuses than PRKO mice after P and E + P treatment. *, P < 0.01; **, P < 0.001 (C) Graph of thymic weights for wild-type and PRKO mice at 3, 11, and 24 mo of age. Thymic involution occurred equally with age in both wild-type and PRKO mice, demonstrating that age-related thymic involution is not PR dependent.
Histologically, 12 days of E + P treatment in wild-type mice results in an almost complete loss of cortical thymocytes (Fig. 1B). E treatment alone produces no noticeable histologic changes in the thymus of wild-type or PRKO animals, nor does E + P treatment of PRKO mice.
The thymus is also known to normally involute with age. To test whether this phenomenon is also PR dependent, we measured thymic weight in wild-type and PRKO mice at 3, 11, and 24 mo of age. Male and female wild-type and PRKO mice showed similar involution of their thymus with age, resulting in almost complete loss of this organ by 24 mo (Fig. 2C). Thus, age-related thymic involution is not PR dependent and is mechanistically distinct from that which occurs in pregnancy.
Thymic Stromal PR Is Required for Complete Hormone- and Pregnancy-Induced Thymic Involution.
PR is classically thought of as being expressed mainly in reproductive organs. To determine whether these organs play a role in thymic involution, the ovaries, oviducts, and uteri from eight wild-type female mice were removed at 8 wk of age. A sham operation in which only the ovaries were removed was performed on control mice. Two weeks later, the mice were treated with E + P or with vehicle for 12 days. Both groups displayed identical involution after E + P treatment (data not shown), demonstrating that PR in the uterus, ovaries, and oviducts is not required for thymic involution.
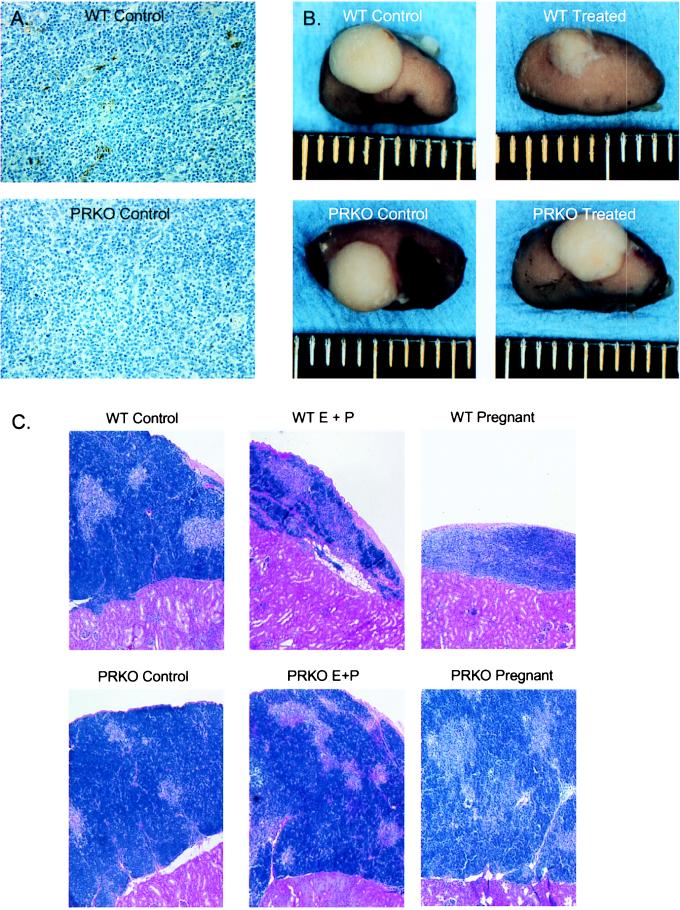
Several groups have reported the presence of PR in the thymus of rats and chickens, with expression apparently confined to the nonlymphocyte cell populations (26–29). To examine the presence of PR in the murine thymus, thymuses collected from 10-wk-old wild-type and PRKO female mice were stained with antibodies against PR (Fig. 3A). In the wild-type thymus, positively staining cells were localized to the medullary region, specifically to large cells that morphologically appear to be epitheliocytes or macrophages. No staining was seen in the smaller T cell progenitors. Staining was completely absent in the PRKO thymus, confirming that the immunostaining observed with this antibody was specific for PR. Thus, our results in the mouse agree with the reported localization of PR in the rat thymus (29) and predict that PR acts via thymic epitheliocytes to regulate thymocyte development.
Figure 3.
Thymic stromal PR is required for thymic involution. (A) Immunoperoxidase staining for PR in untreated wild-type and PRKO mouse thymus. Staining was found almost exclusively in large cells of the medullary region. (B) Photographs of wild-type- and PRKO-transplanted thymuses after 12 days of E + P treatment or pregnancy demonstrate a requirement for stromal PR in thymic involution. Ruler demarcated in millimeters. (C) Transplant thymic histology after E + P or pregnancy. Five micrometers H&E-stained sections of transplanted wild-type and PRKO thymuses taken from wild-type mice after pregnancy. Notice the relative loss of cortical lymphocytes in the wild-type pregnant thymus, whereas the thymus containing PRKO stroma appears intact. Bar = 100 μm.
To determine the role of thymic stromal PR in thymic involution, chimeric thymuses composed of PRKO stroma and wild-type lymphoid cells were produced by thymocyte-depleted PRKO thymic transplant into thymectomized wild-type recipients. After repopulating for 1 mo, recipient mice either were treated with E + P or were allowed to become pregnant. After treatment or pregnancy, thymic involution was assessed via gross morphology and histology (Fig. 3 B and C). Wild-type stromal controls displayed normal thymic involution with the accompanying loss of size and cortical lymphocytes after E + P treatment or pregnancy. Mice that received PRKO stroma, however, showed no thymic involution after E + P treatment or pregnancy and had a relative preservation of cortical tissue. Thus, thymic stromal PR is necessary for normal thymic involution after pregnancy or exogenous E + P administration, and PR in other tissues is not able to compensate for loss of PR in the thymic stroma.
PR Does Not Affect Late Thymocyte Development.
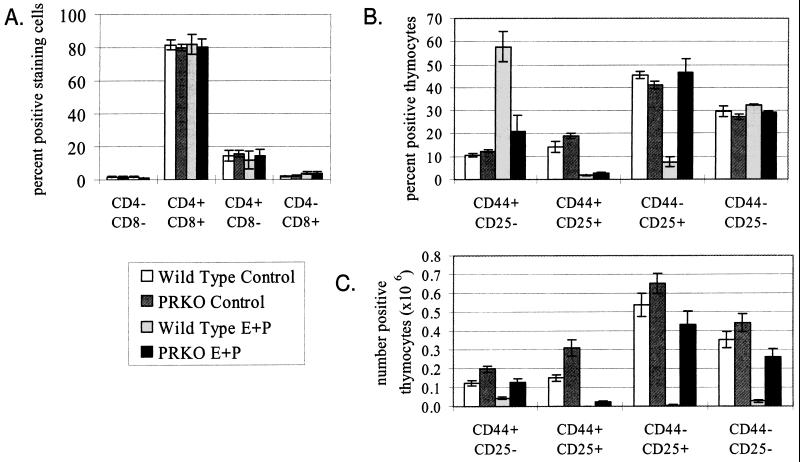
To determine the effect of thymic involution on T cell development, flow cytometry was performed on cells taken from wild-type and PRKO thymuses after 12 days of E + P treatment. All mice showed the same relative percentages of CD4+, CD8+, and CD4+CD8+ thymocytes (Fig. 4A), in spite of the large differences in total T cell numbers between the groups. Thus, long-term E + P treatment does not affect the steady-state distribution of thymocytes among the different late-differentiation compartments.
Figure 4.
Thymocyte numbers after E + P treatment. (A) Distribution of the relative numbers of thymocytes separated by CD4 and CD8 expression after 12 days of E + P treatment. Both wild-type- and PRKO-treated and untreated thymuses showed essentially identical distributions of cells among the four compartments, in spite of the significant loss of cells that had occurred in the wild-type-treated thymus because of thymic involution. (B and C) Relative (B) and absolute (C) numbers of CD25 and/or CD44 expressing triple negative thymocytes after 12 days of E + P treatment. PR appears to exert its effect at the transition from the CD44+CD25+ stage to the CD44−CD25+ stage.
PR Impairs the Transition from Pro-T Cell to Early Pre-T Cell.
On the basis of recent publications by Rijhsinghani et al., in which the effects of E + P treatment on the thymus were attributed to ER activity at a very early stage of thymocyte development (14, 30), we examined very early T cell differentiation for a PR-mediated loss of thymocytes. We separated the CD3−CD4−CD8− triple-negative population on the basis of their expression of CD25 and CD44 (Fig. 4 B and C). In wild-type mice, E + P treatment resulted in a dramatic shift in the relative number of cells into the earliest CD44+CD25− population and of the two CD25+ populations. This was reflected in terms of absolute cell numbers as a partial loss of the earliest CD44+ CD25− cell population, and an almost complete loss of all later populations. In PRKO animals, this shift occurred to a much lesser extent, with only the CD44+ CD25+ population showing some reduction. Later populations showed no difference in relative cell numbers. In terms of absolute cell numbers, PRKO mice showed a decrease similar to that of wild types in the CD44+ compartment, but in contrast to wild type showed little loss in the CD44− compartment. Thus, it appears that E causes a specific loss of cells in the CD44+ CD25+ population, whereas PR is required to prevent recovery of cell numbers in subsequent populations.
Lack of Thymic Involution Impairs Fertility.
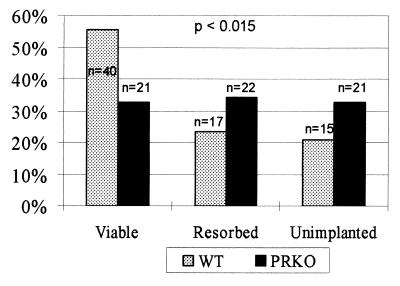
To determine the importance of thymic involution to fertility, wild-type thymectomized mice were transplanted with either PRKO or wild-type thymuses, and then implanted with eight FVB embryos via embryo transfer. Mice were sacrificed on day 17 of pregnancy and scored for the number of live and resorbing embryos present in the uterus (Fig. 5). There was a significant difference (P < 0.015) between mothers receiving wild-type and PRKO thymuses, with mice that received PRKO thymuses having smaller litters and higher resorption rates than mice that received wild-type thymuses. On histological comparison, viable embryos and placentas were indistinguishable in the wild-type and PRKO-transplanted mice, with no gross inflammation present (data not shown). Thus, mice with progesterone-insensitive thymuses show decreased fertility, demonstrating that thymic involution is important to the maintenance of pregnancy.
Figure 5.
Lack of thymic stromal PR impairs fertility. Number of viable, resorbed, and unimplanted embryos in wild-type thymectomized mice receiving wild-type and PRKO thymic transplants expressed as a percentage of the total numbers of embryos transferred. A total of nine wild-type mice received 72 embryos, and eight PRKO mice received 64 embryos. P value is calculated on the basis of a χ2 distribution with three possible categorical outcomes.
Discussion
T cell lymphopoiesis has been previously reported to be specifically inhibited by pregnancy (14, 30). We demonstrate that the observed inhibitory effects of pregnancy hormones on T cell development require the presence of a functional PR. In addition, age-related thymic involution is shown to occur by a separate PR-independent mechanism. A combination of hysterectomy, thymic immunohistochemistry, and transplant studies reveals that thymic stromal PR is specifically required for thymic involution to occur. In addition, the stages of T cell development at which E and P exert their negative effects are defined. Finally, pregnancy studies demonstrate that thymic stromal PR is required for normal fertility. Together, these observations provide evidence for a PR-dependent mechanism that blocks T cell lymphopoiesis during pregnancy and is essential to normal fertility.
Other groups have published previously that full thymic involution can be induced by E alone (14), whereas our studies show only a partial involution at best. Studies that report complete involution after E treatment, however, have used E doses several-fold higher than that used in the present work. More importantly, PRKO-transplanted thymuses showed almost no involution after pregnancy. Thus, although both E and P are capable of causing thymic involution, the PR-mediated mechanism dominates at hormone levels achieved during pregnancy.
Not only are E and P both able to induce thymic involution, but also their effects are separable in terms of the stage of T cell development they block. In E + P-treated PRKO thymuses, cell numbers in the CD44+ CD25+ compartment drop to a similar extent as in wild types, an effect that appears to be caused by estrogen activity. This is the same stage of development that is reported as blocked by Rijhsinghani et al. (14). Unlike findings in the previous report, however, PRKO animals show a rebound in cell numbers in subsequent populations, demonstrating the importance of PR in blocking proliferation at later stages of development. Thus, it appears that ER and PR act to block T cell development at specific and distinct stages, and that lack of PR allows the effects of E to be overcome by proliferation in later stages.
The effect of P on developing T cells is mediated indirectly by PR in thymic stromal cells. This is in agreement with the localization of PR to this cell population both in this work and in other published reports (27, 29). A paracrine effect of PR is not unprecedented, because PR is known to exert a paracrine antiproliferative effect on the uterine epithelium as well (31).
It has also been reported that treatment of mice with E and P results in a change in the relative numbers of CD4- and CD8-expressing thymocytes (14). This effect was not seen in our studies. The reason for this discrepancy is unknown, although significantly higher hormone dosages were used in the previous study.
It is interesting to note that 3 wk of E + P treatment did not significantly change peripheral populations of T or B lymphocytes, in spite of the profound decrease in B cell and T cell output. This result is in agreement with reports in the literature after hormone treatments (16) and pregnancy (18). Presumably, the long-lived nature of peripheral lymphocytes prevents a transient drop in their production from having a major effect on their overall numbers.
The mechanism by which PR regulation of T cell development protects the fetus is not clear, although some speculation is possible. It has been shown (32) that T cells capable of recognizing paternal antigens in the female mouse undergo clonal deletion or anergy during pregnancy. Thus, it appears that potentially harmful T cells are removed from the maternal repertoire during pregnancy. If the goal of the immune system is to remove potentially harmful T cells from the peripheral circulation, however, a logical adjunct to this goal would be to block the production of new T cells that could replace those undergoing peripheral deletion. In fact, because the peripheral deletion studies were performed in mice that were also undergoing pregnancy-induced thymic involution, it is possible that some of the reported decrease in paternally reactive T cells was caused by decreased replenishment of these cells by the thymus. In addition, it is possible that blockade of T cell development is not the only important effect of PR on the thymus. It is well known that the thymus produces a number of secreted factors that are found in the peripheral circulation, and that production of these factors can be affected by sex hormones (33). It is thus possible that PR could also be regulating the production of an unknown thymic hormone, resulting in changes in peripheral immunity that are independent of the block to T cell development. Clearly, this problem is complicated, and more studies will be required to define the specific mechanisms by which PR-induced thymic involution exerts its effect on pregnancy.
Acknowledgments
This work was supported by the National Institutes of Health (HD07857) and Organon Pharmaceuticals.
Abbreviations
- E
estrogen
- P
progesterone
- PR
progesterone receptor
- ER
estrogen receptor
- PRKO
progesterone receptor knockout
- H&E
hematoxylin/eosin
References
- 1.Gill T. Crit Rev Immunol. 1985;5:201–227. [PubMed] [Google Scholar]
- 2.Sargent I. Exp Clin Immunogenet. 1993;10:85–102. [PubMed] [Google Scholar]
- 3.Olsen N, Kovacs W. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 4.Beer A, Sio J. Biol Reprod. 1982;62:271. doi: 10.1095/biolreprod26.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Hunt J, Orr H. FASEB J. 1992;6:2344–2348. doi: 10.1096/fasebj.6.6.1544544. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan A, Johnson G I, Lewis G. J Anim Sci. 1997;75:1621–1632. doi: 10.2527/1997.7561621x. [DOI] [PubMed] [Google Scholar]
- 7.Moriyama I, Sugawa T. Nature (London) 1972;236:150–155. doi: 10.1038/newbio236150a0. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan L, Guilbert L, Russell A, Wegmann T, Mosmann T, Belosevic M. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 9.Krishnan L, Guilbert L, Wegmann T, Belosevic M, Mosmann T. J Immunol. 1996;156:653–662. [PubMed] [Google Scholar]
- 10.Wegmann T, Lin H, Guilbert L, Mosmann T. Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 11.Chambers S, Clarke A. J Reprod Fertil. 1979;55:309–315. doi: 10.1530/jrf.0.0550309. [DOI] [PubMed] [Google Scholar]
- 12.Shinomiya N, Tsuru S, Tsugita M, Katsura Y, Takemura T, Rokutanda M, Nomoto K. J Clin Lab Immunol. 1991;34:11–22. [PubMed] [Google Scholar]
- 13.Persike E. Proc Soc Exp Biol Med. 1940;45:315–317. [Google Scholar]
- 14.Rijhsinghani A, Thompson K, Bhatia S, Waldschmidt T. Am J Reprod Immunol. 1996;36:269–277. doi: 10.1111/j.1600-0897.1996.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 15.Screpanti I, Morrone S, Meco D, Santoni A, Gulino A, Paolini R, Crisanti A, Mathieson B, Frati L. J Immunol. 1989;142:3378–3383. [PubMed] [Google Scholar]
- 16.Medina K, Kincade P. Proc Natl Acad Sci USA. 1994;91:5382–5386. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kincade P, Medina K, Smithson G. Immunol Rev. 1994;137:119–134. doi: 10.1111/j.1600-065x.1994.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 18.Medina K, Smithson G, Kincade P. J Exp Med. 1993;178:1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lydon J P, DeMayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A J, Shyamala G, Conneely O M, O’Malley B W. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 20.Lydon J P, DeMayo F J, Conneely O M, O’Malley B. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 21.Sjodin K, Dalmasso A, Smith J, Martinez C. Transplantation. 1963;1:521–525. doi: 10.1097/00007890-196301040-00011. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson E, Franchi L, Kingston R, Owen J J. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 23.Ready A, Jenkinson E, Kingston R, Owen J. Nature (London) 1984;310:231–233. doi: 10.1038/310231a0. [DOI] [PubMed] [Google Scholar]
- 24.Tibbetts T, Mendoza-Meneses M, O’Malley B, Conneely O. Biol Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- 25.von Boehmer H, Shortman K. J Immunol Methods. 1973;2:293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]
- 26.Naray A. Biochem Biophys Res Commun. 1981;98:866–874. doi: 10.1016/0006-291x(81)91191-8. [DOI] [PubMed] [Google Scholar]
- 27.Pearce P, Khalid B, Funder J. Endocrinology. 1983;113:1287–1291. doi: 10.1210/endo-113-4-1287. [DOI] [PubMed] [Google Scholar]
- 28.Fujii-Hanamoto H, Grossman C, Roselle G, Mendenhall C, Seiki K. Thymus. 1990;15:31–45. [PubMed] [Google Scholar]
- 29.Kawashima I, Sakave K, Seiki K, Fujii-hanamoto H, Akatsuka A, Tsukamoto H. Thymus. 1991;18:79–93. [PubMed] [Google Scholar]
- 30.Rijhsinghani A, Bhatia S K, Kantamneni L, Schlueter A, Waldschmidt T J. Am J Reprod Immunol. 1997;37:384–390. doi: 10.1111/j.1600-0897.1997.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 31.Kurita T, Young P, Brody J R, Lydon J P, O’Malley B W, Cunha G R. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 32.Tafuri A, Alferink J, Möller P, Hämmerling G, Arnold B. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 33.Seiki K, Sakabe K. Arch Histol Cytol. 1997;60:29–38. doi: 10.1679/aohc.60.29. [DOI] [PubMed] [Google Scholar]