Abstract
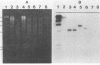
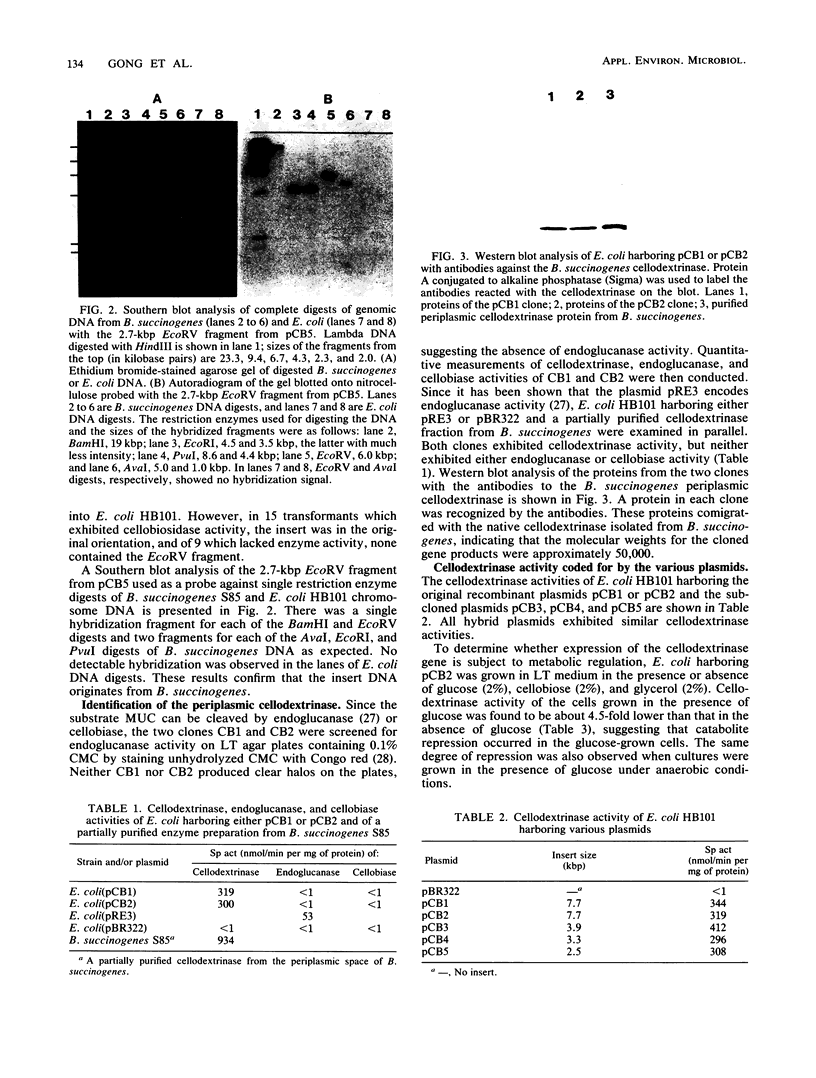
A DNA fragment coding for a cellodextrinase of Bacteroides succinogenes S85 was isolated by screening of a pBR322 gene library in Escherichia coli HB101. Of 100,000 colonies screened on a complex medium with methylumbelliferyl-beta-D-cellobioside as the indicator substrate, two cellodextrinase-positive clones (CB1 and CB2) were isolated. The DNA inserts from the two recombinant plasmids were 7.7 kilobase pairs in size and had similar restriction maps. After subcloning from pCB2, a 2.5-kilobase-pair insert which coded for cellodextrinase activity was isolated. The enzyme was located in the cytoplasm of the E. coli host. It exhibited no activity on carboxymethyl cellulose, Avicel microcrystalline cellulose, acid-swollen cellulose, or cellobiose but hydrolyzed p-nitrophenyl-beta-D-cellobioside and p-nitrophenyl-beta-D-lactoside. The Km (0.1 mM) for the hydrolysis of p-nitrophenyl-cellobioside by the enzyme expressed in E. coli was similar to that reported for the purified enzyme from B. succinogenes. Expression of the cellodextrinase gene was subjected to catabolite repression by glucose and was not induced by cellobiose. The origin of the DNA insert from B. succinogenes was confirmed by Southern blot analysis. Western blotting (immunoblotting) using antibodies raised against the purified B. succinogenes cellodextrinase revealed a protein with a molecular weight of approximately 50,000 in E. coli clones which comigrated with the native enzyme isolated from B. succinogenes. These data indicate that the cellodextrinase gene expressed in E. coli is fully functional and codes for an enzyme with properties similar to those of the native enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deshpande M. V., Eriksson K. E., Pettersson L. G. An assay for selective determination of exo-1,4,-beta-glucanases in a mixture of cellulolytic enzymes. Anal Biochem. 1984 May 1;138(2):481–487. doi: 10.1016/0003-2697(84)90843-1. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Crosby B., Thomas D. Y. Potential for manipulation of the rumen fermentation through the use of recombinant DNA techniques. J Anim Sci. 1986 Jul;63(1):310–325. doi: 10.2527/jas1986.631310x. [DOI] [PubMed] [Google Scholar]
- Greene J. R., Guarente L. Subcloning. Methods Enzymol. 1987;152:512–522. doi: 10.1016/0076-6879(87)52058-4. [DOI] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Purification and Comparison of the Periplasmic and Extracellular Forms of the Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1988 Jun;54(6):1488–1493. doi: 10.1128/aem.54.6.1488-1493.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W., Thomas D. Y. Purification and characterization of a chloride-stimulated cellobiosidase from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2923–2932. doi: 10.1128/jb.170.7.2923-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Cameron L. A. A simple immunological detection method for the direct screening of genes from clone banks. Biochem Cell Biol. 1986 Jan;64(1):73–76. doi: 10.1139/o86-012. [DOI] [PubMed] [Google Scholar]
- McGavin M., Forsberg C. W. Isolation and characterization of endoglucanases 1 and 2 from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2914–2922. doi: 10.1128/jb.170.7.2914-2922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Wilson D. B. Potential opportunities and problems for genetically altered rumen microorganisms. J Nutr. 1988 Feb;118(2):271–279. doi: 10.1093/jn/118.2.271. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Shoemaker N. B., Guthrie E. P. Recent advances in Bacteroides genetics. Crit Rev Microbiol. 1987;14(1):49–71. doi: 10.3109/10408418709104435. [DOI] [PubMed] [Google Scholar]
- Sipat A., Taylor K. A., Lo R. Y., Forsberg C. W., Krell P. J. Molecular cloning of a xylanase gene from Bacteroides succinogenes and its expression in Escherichia coli. Appl Environ Microbiol. 1987 Mar;53(3):477–481. doi: 10.1128/aem.53.3.477-481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Crosby B., McGavin M., Forsberg C. W., Thomas D. Y. Characteristics of the endoglucanase encoded by a cel gene from Bacteroides succinogenes expressed in Escherichia coli. Appl Environ Microbiol. 1987 Jan;53(1):41–46. doi: 10.1128/aem.53.1.41-46.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]