Abstract
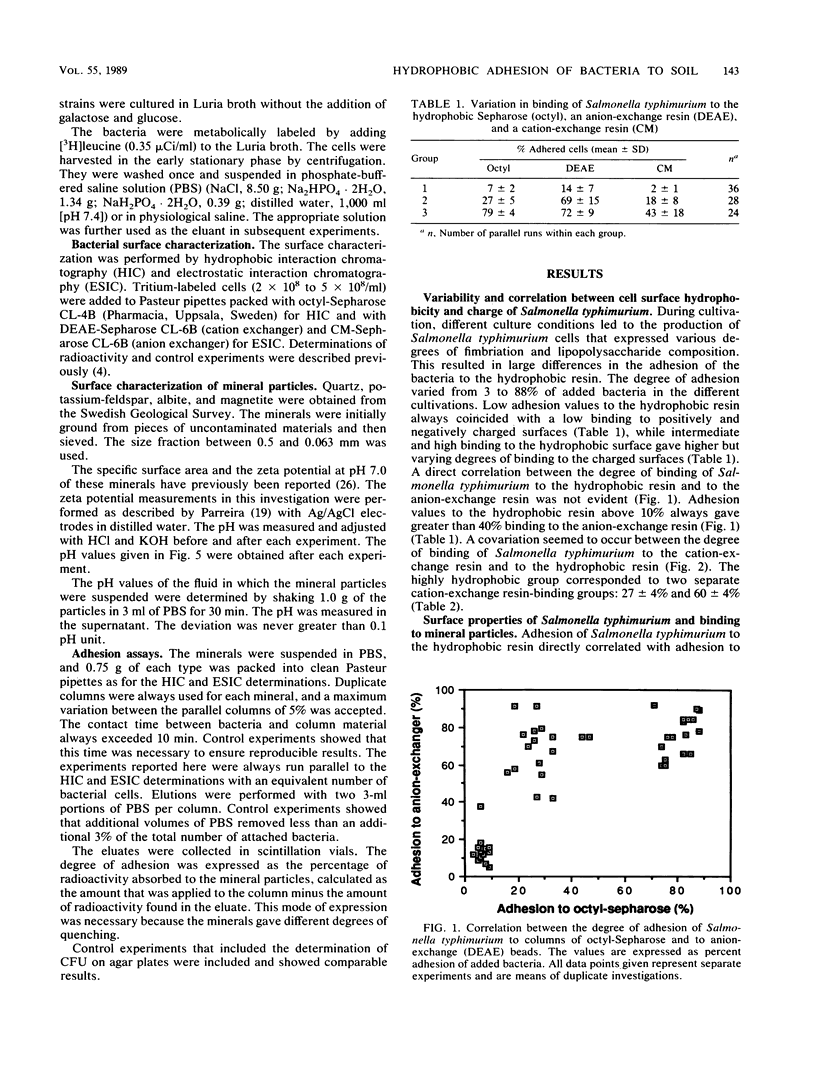
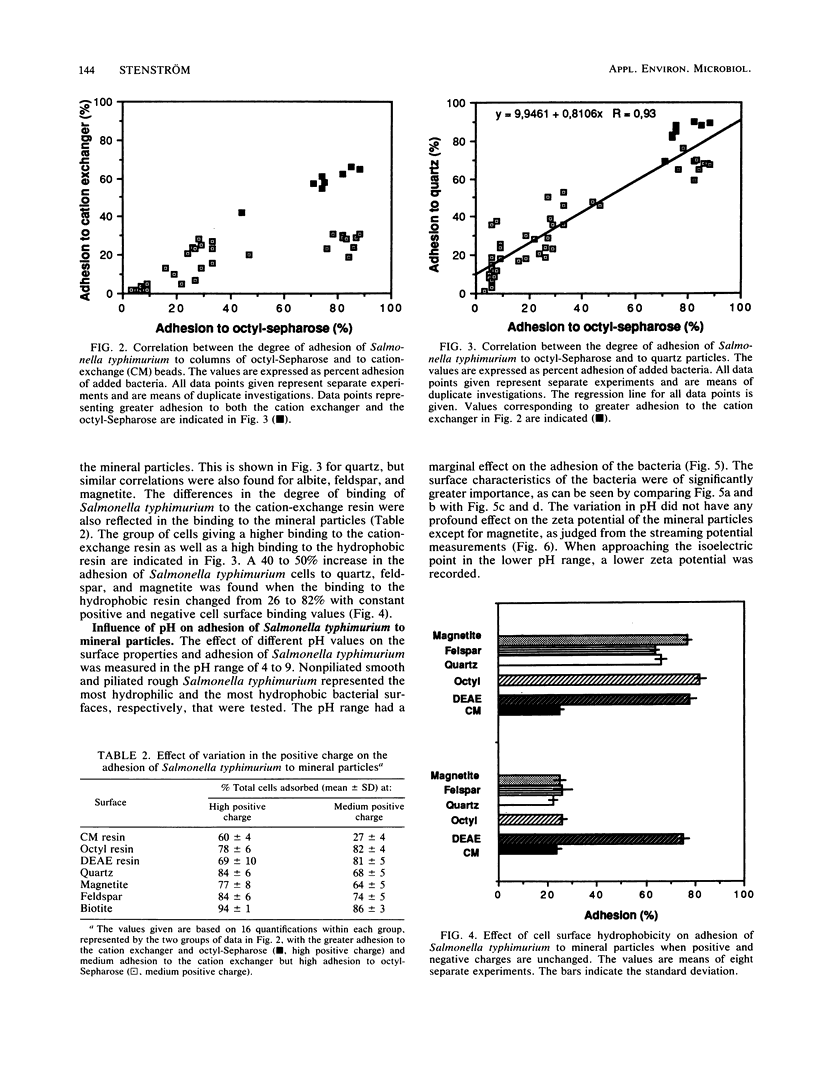
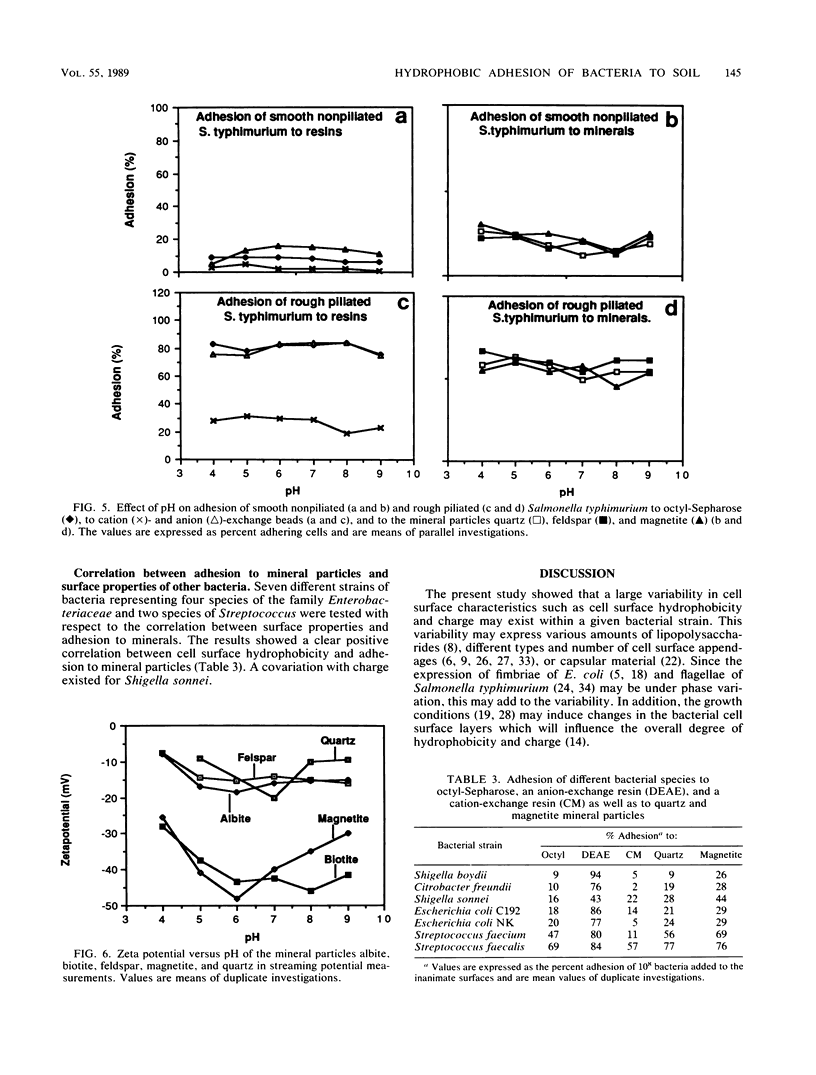
The adhesion of Salmonella typhimurium to the mineral particles quartz, albite, feldspar, and magnetite was shown to correlate with the hydrophobicity of the cell surface as measured by hydrophobic interaction chromatography. The same effects were also seen for seven other selected test strains, including Streptococcus faecalis, Streptococcus faecium, Escherichia coli, Citrobacter freundii, Shigella sonnei, and Shigella boydii. When the test strain of Salmonella typhimurium, was repeatedly cultivated in Luria broth, thus selecting for different degrees of fimbriation and roughness of the cell surface, varied cell hydrophobicity but constant negative and positive charge values were obtained. High hydrophobicity values always coincided with enhanced adhesion to the mineral particles. The negative charge of the bacterial surface as measured by electrostatic interaction chromatography appeared to play no role in the adhesion event. However, the positive charges on the cell surface contributed to the adhesion process. This was especially evident for cells exhibiting a high degree of hydrophobicity. Alteration of the pH between 4 and 9 did not significantly affect the adhesion process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahlbäck B., Hermansson M., Kjelleberg S., Norkrans B. The hydrophobicity of bacteria - an important factor in their initial adhesion at the air-water interface. Arch Microbiol. 1981 Jan;128(3):267–270. doi: 10.1007/BF00422527. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Dodd D. C. Pseudocatabolite repression of type 1 fimbriae of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1560–1567. doi: 10.1128/jb.151.3.1560-1567.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Millero F. J. Electrolyte effects on attachment of an estuarine bacterium. Appl Environ Microbiol. 1984 Mar;47(3):495–499. doi: 10.1128/aem.47.3.495-499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Lounatmaa K., Ranta H., Kuusi N. Characterization of type 1 pili of Salmonella typhimurium LT2. J Bacteriol. 1980 Nov;144(2):800–805. doi: 10.1128/jb.144.2.800-805.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounatmaa K., Mäkelä P. H., Sarvas M. Effect of polymyxin on the ultrastructure of the outer membrane of wild-type and polymyxin-resistant strain of Salmonella. J Bacteriol. 1976 Sep;127(3):1400–1407. doi: 10.1128/jb.127.3.1400-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagi S., Miyake Y., Fujioka Y., Tsuru H., Suginaka H. Cell-surface hydrophobicity of Candida species as determined by the contact-angle and hydrocarbon-adherence methods. J Gen Microbiol. 1986 Apr;132(4):1111–1115. doi: 10.1099/00221287-132-4-1111. [DOI] [PubMed] [Google Scholar]
- Neihof R. A., Echols W. H. Chemical modification of the surfaces of bacterial cell walls. Physiol Chem Phys. 1978;10(4):329–343. [PubMed] [Google Scholar]
- Pringle J. H., Fletcher M. Influence of substratum hydration and adsorbed macromolecules on bacterial attachment to surfaces. Appl Environ Microbiol. 1986 Jun;51(6):1321–1325. doi: 10.1128/aem.51.6.1321-1325.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Leibowitz M. Cyclic AMP-dependent synthesis of fimbriae in Salmonella typhimurium: effects of cya and pts mutations. J Bacteriol. 1978 Apr;134(1):356–358. doi: 10.1128/jb.134.1.356-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenström T. A., Kjelleberg S. Fimbriae mediated nonspecific adhesion of Salmonella typhimurium to mineral particles. Arch Microbiol. 1985 Oct;143(1):6–10. doi: 10.1007/BF00414759. [DOI] [PubMed] [Google Scholar]
- Zieg J., Hilmen M., Simon M. Regulation of gene expression by site-specific inversion. Cell. 1978 Sep;15(1):237–244. doi: 10.1016/0092-8674(78)90098-3. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



