Abstract
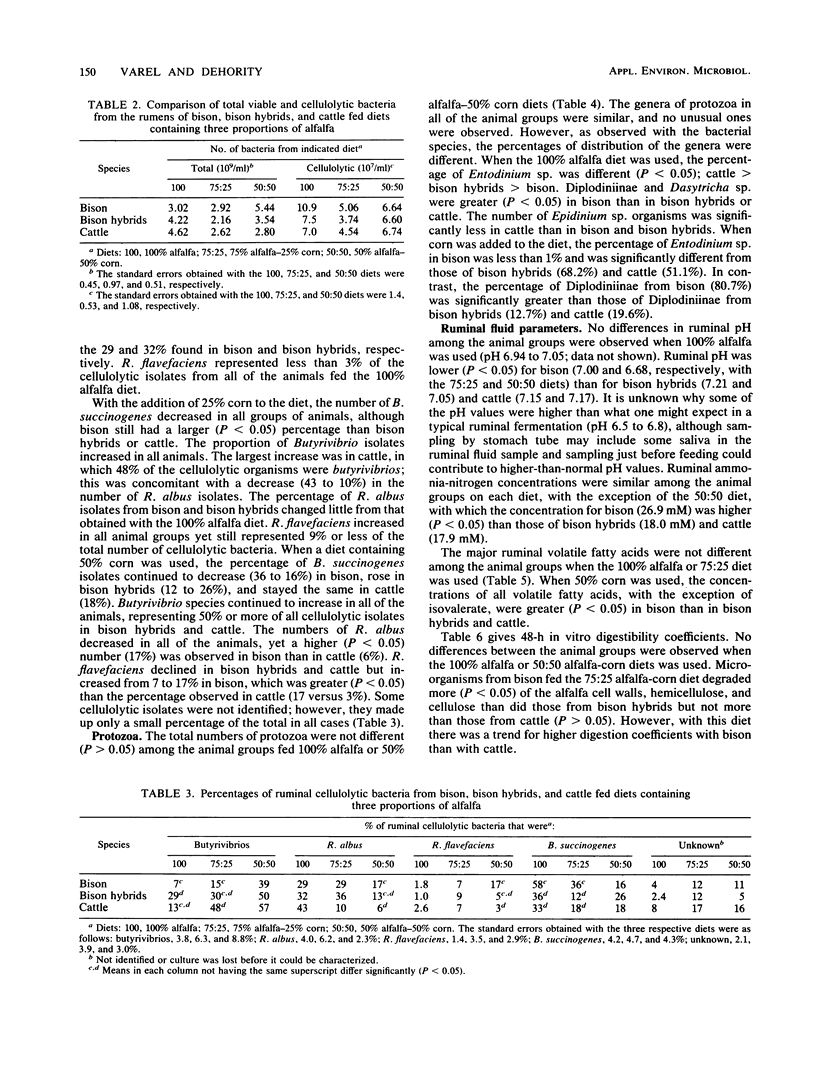
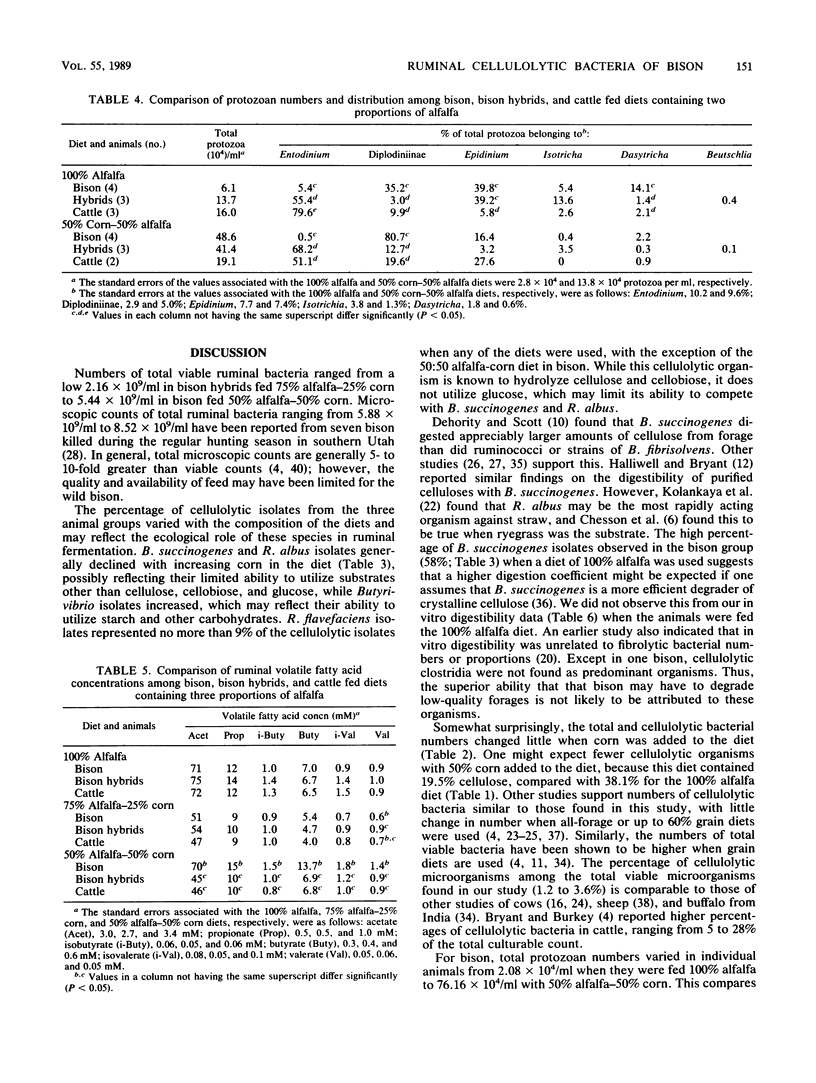
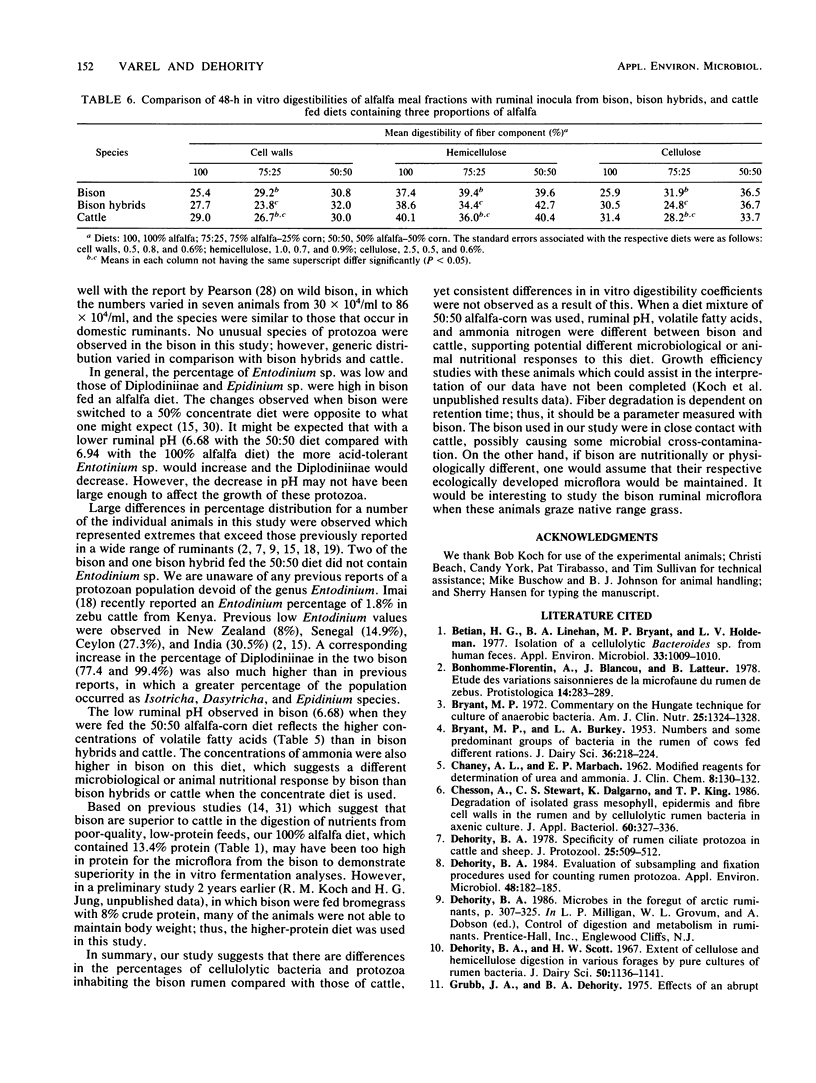
Ruminal cellulolytic bacteria and protozoa and in vitro digestibility of alfalfa fiber fractions were compared among bison, bison hybrids, and crossbed cattle (five each) when they were fed alfalfa and corn in a ratio of 100:0, 75:25, and 50:50, respectively. The total number of viable bacteria (2.16 x 10(9) to 5.44 x 10(9)/ml of ruminal fluid) and the number of cellulolytic bacteria (3.74 x 10(7) to 10.9 x 10(7)/ml) were not different among groups of animals fed each diet. The genera of protozoa in all of the animal groups were similar; however, when either the 100:0 or 50:50 diet was used the percentage of Entodinium sp. was lower and the percentage of Diplodiniinae was higher (P less than 0.05) in bison than in bison hybrids or cattle. Bacteroides succinogenes made up the largest number of cellulolytic isolates from bison (58 and 36%, respectively, on the 100:0 and 75:25 diets), which were more numerous (P less than 0.05) than those from bison hybrids (36 and 12%) and cattle (33 and 18%). This was offset by a lower number of cellulolytic Butyrivibrio isolates. The numbers of Ruminococcus albus and R. flavefaciens isolates, in general, were similar among the bovid species, although R. flavefaciens generally made up less than 10% of the cellulolytic isolates. In vitro digestibility coefficients were greater (P less than 0.05) for the bison when the 75:25 diet was used and similar for the other two diets. The concentration of ruminal volatile fatty acids was larger (P less than 0.05) in bison than in bison hybrids and cattle when the 50:50 diet was used.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betian H. G., Linehan B. A., Bryant M. P., Holdeman L. V. Isolation of a cellulotytic Bacteroides sp. from human feces. Appl Environ Microbiol. 1977 Apr;33(4):1009–1010. doi: 10.1128/aem.33.4.1009-1010.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Dehority B. A. Evaluation of subsampling and fixation procedures used for counting rumen protozoa. Appl Environ Microbiol. 1984 Jul;48(1):182–185. doi: 10.1128/aem.48.1.182-185.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb J. A., Dehority B. A. Effects of an abrupt change in ration from all roughage to high concentrate upon rumen microbial numbers in sheep. Appl Microbiol. 1975 Sep;30(3):404–412. doi: 10.1128/am.30.3.404-412.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL G., BRYANT M. P. THE CELLULOLYTIC ACTIVITY OF PURE STRAINS OF BACTERIA FROM THE RUMEN OF CATTLE. J Gen Microbiol. 1963 Sep;32:441–448. doi: 10.1099/00221287-32-3-441. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. Microorganisms in the rumen of cattle fed a constant ration. Can J Microbiol. 1957 Mar;3(2):289–311. doi: 10.1139/m57-034. [DOI] [PubMed] [Google Scholar]
- Hauser K. J., Zabransky R. J. Modification of the gas-liquid chromatography procedure and evaluation of a new column packing material for the identification of anaerobic bacteria. J Clin Microbiol. 1975 Jul;2(1):1–7. doi: 10.1128/jcm.2.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S. Ciliate protozoa in the rumen of Kenyan zebu cattle, Bos taurus indicus, with the description of four new species. J Protozool. 1988 Feb;35(1):130–136. doi: 10.1111/j.1550-7408.1988.tb04092.x. [DOI] [PubMed] [Google Scholar]
- Jung H. G., Varel V. H. Influence of forage type on ruminal bacterial populations and subsequent in vitro fiber digestion. J Dairy Sci. 1988 Jun;71(6):1526–1535. doi: 10.3168/jds.S0022-0302(88)79716-7. [DOI] [PubMed] [Google Scholar]
- Kelly W. J., Asmundson R. V., Hopcroft D. H. Isolation and characterization of a strictly anaerobic, cellulolytic spore former: Clostridium chartatabidum sp. nov. Arch Microbiol. 1987 Mar;147(2):169–173. doi: 10.1007/BF00415279. [DOI] [PubMed] [Google Scholar]
- Kolankaya N., Stewart C. S., Duncan S. H., Cheng K. J., Costerton J. W. The effect of ammonia treatment on the solubilization of straw and the growth of cellulolytic rumen bacteria. J Appl Bacteriol. 1985 Apr;58(4):371–379. doi: 10.1111/j.1365-2672.1985.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Sharpe M. E., Sutton J. D. The microbial flora of the rumen of cows fed hay and high cereal rations and its relationship to the rumen fermentation. J Appl Bacteriol. 1971 Jun;34(2):425–434. doi: 10.1111/j.1365-2672.1971.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Leedle J. A., Bryant M. P., Hespell R. B. Diurnal variations in bacterial numbers and fluid parameters in ruminal contents of animals fed low- or high-forage diets. Appl Environ Microbiol. 1982 Aug;44(2):402–412. doi: 10.1128/aem.44.2.402-412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H. A. Rumen microorganisms in buffalo from southern utah. Appl Microbiol. 1967 Nov;15(6):1450–1451. doi: 10.1128/am.15.6.1450-1451.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Fairchilds I. G., Zgornicki Y. D. Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol. 1974 Apr;27(4):678–687. doi: 10.1128/am.27.4.678-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw F. G., Eadie J. M., Mann S. O., Reid R. S. Some effects of rumen ciliate protozoa in cattle given restricted amounts of a barley diet. Br J Nutr. 1972 Mar;27(2):425–437. doi: 10.1079/bjn19720108. [DOI] [PubMed] [Google Scholar]


