Abstract
The broad clinical implementation of cancer vaccines targeting the induction of specific T cell-mediated immunity is hampered because T cell defined tumor-associated peptides are currently available for only a restricted range of tumor types. Current epitope identification strategies require a priori the generation of T “indicator” cell lines that specifically recognize the tumor antigenic epitope in in vitro assay systems. An alternative to this strategy is the use of “memory” T cells freshly isolated from the peripheral blood of patients with cancer in concert with sensitive effector cell readout assays (such as the cytokine enzyme-linked immunospot assay) and MS to identify relevant peptide epitopes. In a model system, we have evaluated the capacity of natural Epstein–Barr virus (EBV)-transformed B-lymphoblastoid cell line-extracted peptides to activate “memory” viral-specific CD4+ or CD8+ T cells freshly isolated from the blood of an EBV-seropositive individual using the IFN-γ enzyme-linked immunospot assay. After HPLC fractionation and loading onto autologous dendritic cells, multiple naturally processed HLA class I and II-associated lymphoblastoid cell line-derived peptides were isolated that were capable of inducing IFN-γ spot production by “memory” T lymphocytes. Using MS analysis on a HPLC fraction recognized by CD8+ T cells, we were able to sequence natural 9-, 10-, and 11-mer peptides naturally processed from the latent EBV antigen LMP-2 (latent membrane protein-2) and presented in the context of HLA-A2. This approach provides a useful methodology for the future identification of MHC-presented viral and tumor epitopes using freshly isolated patient materials.
Studies in animal models provide evidence that, with a few exceptions, CD4+ and CD8+ T lymphocytes play an important role in the immune response against viruses and cancers (1, 2). CD4+ T cells recognize MHC class II-presented peptides that are preferentially derived from the degradation of proteins accessing the endocytic pathway, whereas CD8+ cytotoxic T lymphocytes (CTL) recognize MHC class I-presented peptides processed primarily from endogenous proteins in the cytosol (3). In humans, numerous (mostly CD8+) T cell-defined tumor peptide antigens have been identified using either gene expression cloning techniques or immunoaffinity purification of MHC molecules followed by acid extraction of peptides and subsequent MS sequence analysis (4, 5). Despite these advances, however, recent evidence suggests that most T cell-defined cancer antigens remain unknown (6). A major obstacle in the systematic search for new tumor antigens is that the epitope identification strategies described above require a priori the generation of T “indicator” cell lines/clones that specifically recognize the tumor antigenic epitopes in vitro. Although several groups have succeeded in isolating tumor-reactive T cells from blood or tumor samples of cancer patients, the laborious and nontrivial effort required to generate and maintain antitumor T lymphocytes in vitro precludes their analysis in large numbers of patients. This is particularly true for “poorly immunogenic” tumor histologies. Further complicating matters, the use of long-term cultured T cell lines/clones as a screening tool to identify T cell-defined epitopes raises the general objection that these repeatedly stimulated bulk T cell populations may no longer reflect the full range of antitumor specificities present in the patient’s repertoire in vivo: preferential expansion of certain T cell subpopulations may occur in extended in vitro cultures (7, 8). Accordingly, approaches that allow for direct (ex vivo) analysis of tumor antigen-reactive patient T cell populations will provide an important advance for broadening the capabilities of antigen-identification strategies.
Enzyme-linked immunospot (ELISPOT) assays are able to detect and quantitate low numbers of antigen-specific T cells in freshly isolated blood lymphocytes without the need of in vitro expansion (9). With the combined use of computer-assisted video image analysis, the resulting cytokine spots can be automatically counted, providing objective and precise results (10). In particular, IFN-γ ELISPOT assays are regarded as up to an order of magnitude more sensitive for detecting low frequencies of antigen-specific T cells in lymphocyte populations versus ELISAs (11) or 51Cr-release cytotoxicity assays used in the context of limiting-dilution assays (12). Given these characteristics, we have evaluated the ability of an IFN-γ ELISPOT assay to detect in freshly isolated, peripheral blood-derived CD4+ and CD8+ T lymphocytes, those “memory” T cell responses directed against MHC-presented tumor peptides. As a model tumor, we used EBV-transformed B-LCL that present strongly immunogenic peptide epitopes derived from latent EBV antigens and recognized by HLA class I- and II-restricted “memory” T cells in healthy donors that are EBV-seropositive (13). Because dendritic cells (DC) have been shown to have a superior ability in stimulating CD4+ and CD8+ T cell immune responses (14), we applied them as antigen-presenting cells (APC) in ELISPOT assays. By using this approach, we were able to discern multiple MHC class I- and class II-presented EBV-derived epitopes and directly sequence a series of LMP-2 epitopes using MS.
Materials and Methods
Donor and Cell Lines.
The donor IP1 was a healthy individual without signs of acute EBV infection. As determined by Western blotting (kindly performed by David Rowe, Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh), donor IP1’s serum was positive for IgG antibodies (Ab) to the EBV protein EBV-associated nuclear antigen (EBNA)-1 (titer 1:100-1:250) and negative for reactivity against EBV viral capsid antigens. According to standard HLA serotyping procedures, donor IP1 was HLA-A2,32; B7,62; Cw3; DR4,15.
A CTL clone recognizing the EBV epitope LMP-2A 426–434 was kindly provided by A.B. Rickinson, University of Birmingham, United Kingdom. It was cultured as described (15). The autologous LCL was established by EBV (B95.8 strain) transformation of donor’s peripheral blood mononuclear cells (PBMC). Cells were expanded in RPMI medium 1640/10% FCS (Life Technologies, Gaithersburg, MD), washed and subsequently recultured for an additional 3 days in AIM-V medium (Life Technologies) to remove FCS proteins and to reduce the number of HLA-presented FCS-derived epitopes.
APC.
For the generation of DC, PBMC were isolated by Ficoll density centrifugation and were washed five times in Hanks’ balanced salt solution to remove platelets. Immunomagnetic CD4/CD8 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used to deplete T cells from PBMC. CD4+ or CD8+ T cells positively isolated by this technique were directly applied in ELISPOT assays or were cryopreserved until used. The remaining CD4/CD8-negative cells were resuspended at 107 cells per ml in AIM-V and were incubated for 90 min in 75-cm2 tissue culture flasks (Costar, Corning, NY) at 37°C. Nonadherent cells were gently removed by washing with Hanks’ balanced salt solution. The plastic adherent cells were cultured (37°C, 5% CO2) in 10 ml of AIM-V supplemented with 1,000 units/ml recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) and 1,000 units/ml rhIL-4 (both from Schering-Plough). At day 3, cells were fed with 5 ml of fresh AIM-V containing GM-CSF and IL-4. At day 6, nonadherent cells were harvested and cultured for 48 h in six-well plates (Costar) in 3 ml of AIM-V supplemented with GM-CSF and IL-4 (both 1,000 units/ml). DC generated in this way had an “immature” phenotype (no expression of CD83 and low to intermediate expression of CD54, CD80, CD86, and HLA class I and II) as assessed by flow cytometry. To obtain mature DC (high expression of CD54, CD80, CD83, CD86, and HLA class I and class II), day 6 cultured DC were treated with a cytokine mixture consisting of 10 ng/ml recombinant human tumor necrosis factor α (Sigma), 10 ng/ml rhIL-1β (Genzyme), 1,000 units/ml rhIL-6 (Novartis, Basel, Switzerland), and 1 μg/ml prostaglandin E2 (Sigma) for 48 h (16).
Synthetic Peptides.
All synthetic EBV peptides used in our study were derived from different viral proteins and known to be recognized by CTL. Peptides presented in association with HLA-A2.1 were BMLF-1 280–288 (17), LMP-2 329–337 (18), LMP-2A 426–434 (15), EBNA-2A 67–76 (19), EBNA-3 596–604 (20), and EBNA-6 284–293 (21), while EBNA-3A 379–387 and EBNA-3C 881–889 were peptides presented in association with HLA-B7 (22). Control peptides were the HLA-A2.1-restricted CTL epitopes influenza matrix protein (Flu MP) 58–66 (23) and HIV nef 180–189 (24). Peptides were synthesized by standard fluorenylmethoxycarbonyl chemistry and purified by HPLC.
Extraction of Naturally Processed Peptides from Viable Cells.
LCL cells (109) were incubated with 50 ml of citrate-phosphate buffer at pH 3 (25) for 1 min after centrifugation for 3 min at 880 × g. To remove remaining cell fragments, the supernatant was spun down at 1250 × g for 10 min (both at 4°C). Cell-free supernatant containing eluted peptides was concentrated on a SepPak C18 cartridge (Millipore). Bound peptides were eluted by 60% followed by 100% acetonitrile (ACN; in ddH2O), concentrated in a Speed-Vac, and stored at −70°C until use.
Extraction of Naturally Processed Peptides from Affinity-Purified HLA–DR Molecules.
Pellets from 1.5 × 109 LCL cells were lysed in 20 ml of CHAPS detergent (Sigma, 5% in ddH2O) containing protease inhibitors (Boehringer Mannheim) for 45 min on ice. After centrifugation at 880 × g for 10 min, followed by 15,000 × g for 30 min (both at 4°C), supernatant was passed through chromatography columns filled with Sepharose beads (Sigma) coupled with mAb L243 (anti-HLA–DR monomorphic). Sepharose matrices were then treated with 0.1% trifluoroacetic acid (TFA) (in ddH2O) for 15 min at room temperature. After initial centrifugation to pellet the Sepharose beads (1250 × g, 10 min), supernatant was recovered, lyophilized, and stored at −70°C until use.
Reverse-Phase HPLC (RP-HPLC).
Synthetic latent EBV peptide antigens (4 μg), naturally processed peptides extracted from 4 × 108 viable IP1-LCL cells or extracted from affinity-purified HLA–DR complexes (total of 109 IP1-LCL cells) were resuspended in 200 μl of 0.1% TFA/10% ACN (in ddH2O) and were separated on an analytical C18 column using 0.1% TFA/99.9% ddH2O (buffer A) and 99.9% ACN/0.1% TFA (buffer B) in a Rainin Instruments HPLC system at a flow rate of 0.8 ml/min. RP-HPLC runs were performed with linear gradients (synthetic peptides or natural peptides eluted from viable IP1-LCL cells: 0–5 min 10% buffer B, 5–75 min 10–75% buffer B; natural peptides eluted from HLA–DR complexes: 0-5 min 10% buffer B, 5–95 min 10-100% buffer B). Absorbance was assessed at 214 nm. Individual HPLC fractions of 0.8 ml each were collected. To screen bioactivity of fractionated synthetic or natural EBV peptides in ELISPOT or 51Cr release assays, fractions were lyophilized, resuspended in 40 μl of PBS/5% DMSO, and stored at −70°C until use.
Nanospray MS.
HPLC fractions were lyophilized to near dryness and resuspended in 5 μl of 0.1 M acetic acid. One microliter of this material was loaded onto a microcapillary C18 HPLC column (150 mm × 75 μm i.d.). Peptides were eluted with a linear gradient (0–80% buffer B, 20 min) using a buffer system consisting of 0.1 M acetic acid in ddH2O (buffer A) and 0.1 M acetic acid in 100% acetonitrile. Effective flow rates for the nanospray probe (200 nl/min) were achieved by using the Rainin HPLC system previously described equipped with an Accurate microflow processor (LC Packings, San Francisco, CA) for flow splitting. The nanospray probe was operated at a voltage differential of +3.2 keV. The source temperature was maintained at 30°C. Mass spectra were obtained by scanning from 400–1,500 every 2.6 sec and summing individual spectra on a Fisons Quattro II (Fisons, Loughborough, U.K.). Collision-induced dissociation was performed by selecting mass ions of interest and scanning at 500 atomic mass units/sec using 3 mtorr of Ar in the collision chamber.
T Cell Culture.
CD4+ T lymphocytes were positively isolated from PBMC by immunomagnetic CD4 MicroBeads (Miltenyi) and were seeded at 3 × 106 cells per well in 24-well plates (Costar). Eight-day-old autologous mature DC (105 per well, irradiated with 2,500 rad) prepulsed on day 6 of culture with a freeze/thaw lysate (≥10 kDa) of IP1-LCL were added. Culture medium was AIM-V supplemented with 5% human AB serum (Sigma) at a final volume of 2 ml per well. On day 3, 10 units/ml of rhIL-2 (Chiron, Emeryville, CA) was added. Responding T cells were restimulated on day 7 and day 14 using irradiated, antigen-pulsed DC at a responder/stimulator ratio of 30:1 in AIM-V medium containing 10 units/ml IL-2 and 5 ng/ml rhIL-7 (Genzyme).
IFN-γ ELISPOT Assay.
ELISPOT assays were performed as previously described (10) by using the anti-human IFN-γ capture (1-D1K) and detection (7-B6-1) mAbs (MABTECH, Stockholm, Sweden). Spot numbers were automatically determined with the use of a computer-assisted video image analyser [Zeiss-Kontron, Jena, Germany (10)]. To calculate the number of antigen-responsive T cells, the mean numbers of spots induced by DC alone were subtracted from mean spot numbers induced by antigen-loaded DC. For statistical evaluation, a t test for unpaired samples was used. Values of P < 0.05 were considered as significant and are indicated in relevant Figures with an asterisk (∗).
Cytotoxicity Assay.
Four-hour 51Cr-release assays were performed as described (25).
Results
Analysis of the CD8+ T Cell Response to Synthetic EBV Peptide Epitopes in a Healthy EBV Carrier.
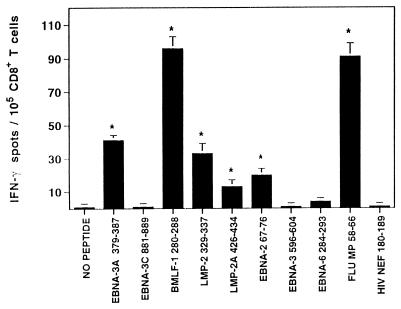
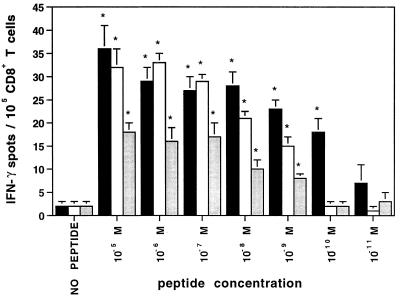
CD8+ T cells were isolated from ex vivo blood lymphocytes of the healthy anti-EBV-positive donor IP1 (HLA class I type A2, 32; B7, 62; Cw3) and were analyzed in IFN-γ ELISPOT assay for reactivity against known HLA-A2.1/-B7-binding EBV peptide epitopes. As controls, HLA-A2.1-restricted peptide antigens derived from influenza virus (positive control) and HIV (negative control) were included. Autologous monocyte-derived immature DC cultured for 8 days in IL-4- and GM-CSF-containing medium served as peptide-presenting cells. “Memory” CD8+ T cells reactivity against five peptide epitopes derived from different EBV proteins and known to be recognized in association with HLA-A2.1 (BMLF-1 280–288, LMP-2 329–337, LMP-2A 426–434, EBNA-2A 67–76) or HLA-B7 (EBNA-3A 379–387) were clearly demonstrated (Fig. 1). The frequencies of IFN-γ spot forming lymphocytes ranged between 14 and 95 per 105 CD8+ T cells. In addition, reactivity against the HLA-A2.1-restricted influenza epitope Flu MP 58-66 was also detected, while T cell responsiveness to the HLA-A2.1-binding HIV nef 180–189 epitope in this HIV-seronegative normal donor was not observed. As shown in Fig. 2, peptide-induced IFN-γ spot production was still detectable when EBV peptide concentrations were titrated down to 100 pM for peptide EBNA-3A 379–387 and to 1 nM for peptides LMP-2 329–337 and LMP-2 426–434, respectively.
Figure 1.
T cell responsiveness to synthetic EBV peptide antigens in healthy, EBV-seropositive donor IP1. CD8+ T cells were isolated from PBMC and were analyzed in IFN-γ ELISPOT assays for reactivity against EBV peptide epitopes known to bind to HLA-A2.1 (BMLF-1 280–288, LMP-2 329–337, LMP-2A 426–434, EBNA-2 67–76, EBNA-3 596–604, EBNA-6 284–293) or HLA-B7 (EBNA-3A 379–387, EBNA-3C 881–889). Autologous immature DC served as APC. Control wells contained CD8+ T cells with nonpeptide-pulsed DC or CD8+ T cells with DC loaded with the HLA-A2.1-restricted CTL epitopes Flu MP 58–66 (positive control) or HIV nef 180–189 (negative control). For these latter controls and for experimental EBV-derived epitopes, DC were loaded with 10 μg/ml concentrations of the indicated peptides. After a culture period of 20 h, IFN-γ spots were developed and counted by computer-assisted video image analysis. Each bar represents the mean spot number of triplicates ± SD per 105 CD8+ T lymphocytes initially seeded per well. The numbers of peptide-responsive T cells per 105 CD8+ T lymphocytes are calculated by subtraction of mean spot numbers induced by DC alone from mean spot numbers induced by peptide-loaded DC (∗ indicate significant results, i.e., P < 0.05). Results were confirmed in three independent experiments.
Figure 2.
Efficiency in detecting EBV-reactive ex vivo-isolated CD8+ T lymphocytes at low concentrations of synthetic peptide antigens. In IFN-γ ELISPOT assay, CD8+ T cells (105 per well) freshly isolated from PBMC of healthy donor IP1 were seeded with autologous immature DC pulsed with a series of 1:10 dilutions of latent EBV peptide antigens LMP-2 329–337 (open bar), LMP-2A 426–434 (shaded bar) or EBNA-3A 379–387 (filled bar). Spots developed after a culture period of 20 h were evaluated and presented as described in Fig. 1. The data are representative experiment of two experiments performed.
HPLC Fractionated Naturally Processed Peptides Extracted from Autologous LCLs Are Recognized by CD8+ T Cells.
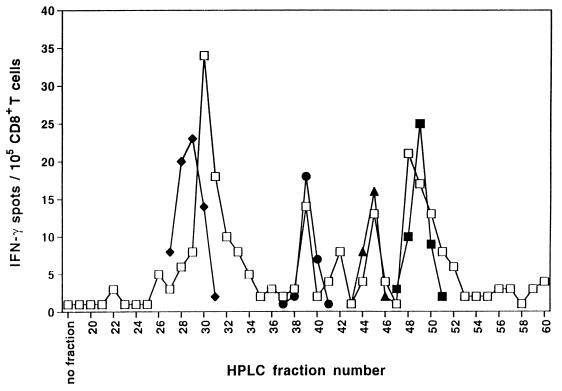
In IFN-γ ELISPOT analysis, we generally observed that autologous LCLs induced strong spot production when cultured with purified blood-derived CD8+ T cells obtained from EBV seropositive, healthy individuals. In donor IP1, for example, the frequency of LCL-reactive CD8+ T lymphocytes was 845/105 (data not shown). Accordingly, “memory” CTL specifically lysing autologous LCL cells in a standard 51Cr-release assay were readily reactivated from blood lymphocytes of donor IP1 by three weekly stimulations with the autologous LCL in vitro (data not shown). These results suggested that the autologous LCL express immunogenic HLA class I complexes containing naturally processed latent EBV peptide antigens recognized by donor IP1’s CD8+ T cells. We next extracted natural MHC class I-presented peptides from viable autologous LCL cells by mild acid treatment and fractionated them by RP-HPLC. Individual RP-HPLC fractions were screened for their capacity to induce reactivity by CD8+ “memory” T cells freshly isolated from blood of donor IP1 in IFN-γ ELISPOT analysis. As peptide-presenting cells, autologous immature DC were used since results presented in Fig. 2 had shown their ability to efficiently present latent EBV epitopes at very low peptide concentrations. In ELISPOT analysis, multiple RP-HPLC fractions induced enhanced IFN-γ spot production by ex vivo CD8+ T cells of donor IP1, consistent with the presence of several immunogenic EBV-derived epitopes in the bulk mixture of peptides (Fig. 3). In parallel, we also performed RP-HPLC fractionation of the synthetic latent EBV peptide antigens known to be recognized by donor IP1’s CD8+ lymphocytes (see Fig. 1). Many of the synthetic sequences coeluted in HPLC fractions, consistent with those observed for the naturally processed EBV peptides that were detected by the donor’s CD8+ T cells. This result suggested the potential identity of peptide species in synthetic and acid-eluted natural EBV peptide preparations.
Figure 3.
HPLC-fractionated naturally processed peptides that have been acid-eluted from viable LCL cells contain multiple epitopes recognized by freshly isolated “memory” CD8+ T cells. Natural peptides were acid-eluted from 4 × 108 LCL cells derived from donor IP1 and separated by RP-HPLC. In parallel, synthetic latent EBV peptide antigens (4 μg each) known to be recognized by donor IP1’s CD8+ lymphocytes were also HPLC-fractionated using the identical protocol. Individual HPLC fractions of natural EBV peptides (10 μl ≈ 108 LCL cell equivalents per well) or synthetic EBV peptides [10 μl per well; EBNA-3A 379–387 (♦), EBNA-2 67–76 (●), LMP-2A 426–434 (▴), LMP-2 329–337 (■)] were tested for recognition by purified CD8+ T cells freshly isolated from the blood of donor IP1 in IFN-γ ELISPOT assay. Autologous immature DC were used as APC. Spots were developed after a culture period of 40 h and were evaluated as described in Fig. 1. Each value represents the mean spot number of duplicate determinations with 105 CD8+ T lymphocytes initially seeded per well. Results were confirmed in two independent experiments.
MS Analysis.
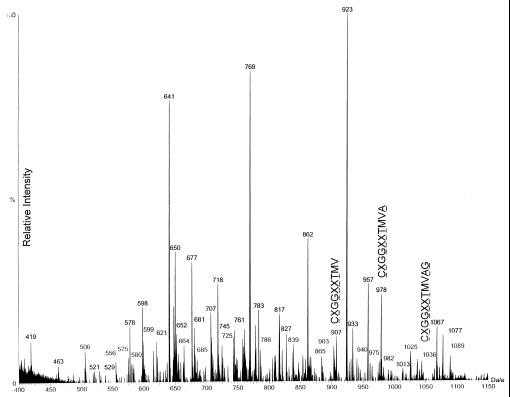
HPLC fraction 45 of the naturally processed EBV B-LCL-derived peptides was analyzed using nanospray tandem MS. One microliter of fractionated material, representing 4 × 108 EBV B-LCL cell equivalents, was loaded onto the microcapillary/nanospray system as described in Materials and Methods. The summation of mass spectra of peptides with m/z 400–1,150 is shown in Fig. 4. A peptide matching the predicted mass of the LMP-2A 9-mer epitope [residues 426–434 (m/z 907)] was detected. In addition, peptides were found that potentially conformed to one natural 10-mer [i.e., residues 426–435 (m/z 978)] and 11-mer [residues 426–436 (m/z 1,036)] containing this core 9-mer epitope. Collision-induced dissociation analyses performed on all three peptide species yielded daughter ion spectra that were interpreted as defining a common peptide core with the sequence CXGGXXT (where X indicates Leu or Ile). This result demonstrates that in addition to the expected CTL-defined 426–434 epitope (10), a 10-mer (CLGGLLTMVA) and an 11-mer (CLGGLLTMVAG) LMP-2A-derived epitope containing genomically encoded C-terminal flanking amino acids are also naturally processed and presented on the surface of this HLA-A2.1-positive EBV B-LCL.
Figure 4.
MS analysis of HPLC-fractionated crude peptides that have been acid-eluted from viable LCL cells reveals three naturally processed LMP-2A peptides. HPLC fraction 45, derived from 4 × 108 LCL cells of HLA-A2.1-positive donor IP1, was injected into the electrospray ionization source of a triple quadrupole mass spectrometer. Summation of the mass spectra obtained in the 880–1,100 m/z range is depicted. The (M+H)+ ions at m/z = 907, 978, and 1,036 were selected for collision-induced dissociation spectrum analysis based on theoretical concordance with LMP-2A-derived sequences and for fragmentation to generate sequence data. The deduced sequences of these peptides are indicated, where X represents an amino acid residue of indeterminant identity based on the fragmentation data.
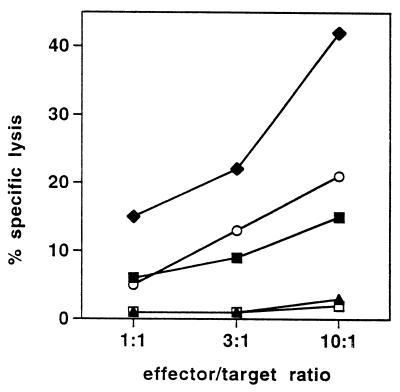
We next confirmed that the EBV B-LCL derived from donor IP1 expresses a sufficient amount of naturally processed LMP-2A peptide to trigger lysis by a CTL clone recognizing the LMP-2A 426–434 epitope (Fig. 5). Although the cytotoxicity observed against this EBV B-LCL was only moderate (i.e., 15% at an effector/target ratio of 10:1), this degree of lysis is in accordance with a previous study reporting that the cytolytic activity against HLA-A2.1-positive LCLs mediated by LMP-2A-specific, HLA-A2.1-restricted CTL ranges from 10% to 40% specific lysis at an effector/target ratio of 10:1 (15). We further demonstrated that this particular CTL clone recognized HPLC fraction 45 containing the naturally processed LMP-2A peptides prepared from the EBV B-LCL of donor IP1 when loaded onto DC. Overall, these results suggest that IFN-γ ELISPOT analysis performed on freshly isolated “memory” CD8+ lymphocytes is a suitable approach to identify single peptide species serving as CTL target antigens among HPLC fractionated peptide preparations extracted from viable cells by mild acid treatment.
Figure 5.
LMP-2A-specific CTL clone recognizes the naturally processed target epitope acid-eluted from LCL. A CTL clone recognizing the LMP-2A 426–434 epitope in association with HLA-A2.1 (15) was tested in a 51Cr-release assay at the indicated effector/target ratios for cytolytic activity against the HLA-A2.1-positive LCL derived from donor IP1 (♦) and against DC generated from IP1 and loaded with HPLC-fractionated (fraction 45, ■) or unfractionated (▴) naturally processed peptides acid-eluted from IP1’s LCL at a ratio of 108 LCL cell equivalents of peptide per well. Control targets were untreated DC (□) and DC pulsed with 10−8 M of the synthetic LMP-2A 426–434 peptide epitope (○). Results were confirmed in two independent experiments.
ELISPOT Fingerprinting of RP-HPLC Resolved Naturally Processed Peptides Extracted from LCL-Derived HLA Class II Molecules Using CD4+ T Cells.
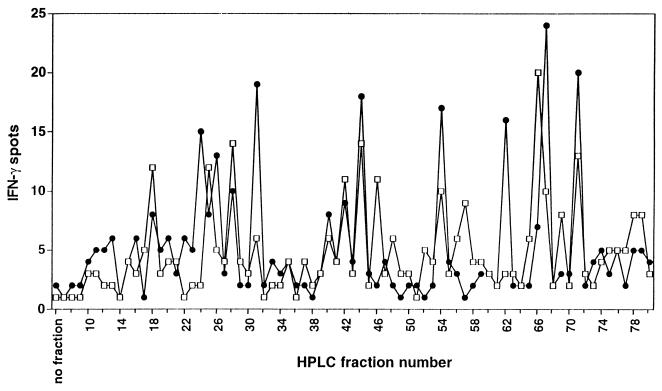
Results shown in Fig. 3 clearly demonstrated that RP-HPLC fractionated HLA class I peptides can be successfully screened by ex vivo isolated memory CD8+ T cells in IFN-γ ELISPOT analysis. Based on the observation that autologous LCLs also induced significant IFN-γ spot production when cultured with CD4+ T cells freshly isolated from the blood of healthy EBV-seropositive individuals (e.g., 203 LCL-reactive lymphocytes/105 CD4+ T cells of donor IP1; data not shown), we next extracted naturally processed peptides from immunoaffinity-purified HLA–DR complexes prepared from 109 LCL cells of donor IP1 and fractionated these by using RP-HPLC. Individual HPLC fractions were loaded onto autologous immature DC and were analyzed for reactivity in IFN-γ ELISPOT assay. Autologous CD4+ T cells used for screening were both freshly isolated from donor IP1’s blood or day 21 cultured responder lymphocytes generated by weekly stimulations of CD4+ T cells with autologous mature DC preloaded with a freeze-thaw lysate prepared from the IP1-LCL (see Materials and Methods). Compared with freshly isolated CD4+ T cells of donor IP1, day 21 cultured CD4+ T cell responder populations stimulated with DC and the IP1-LCL lysate had shown a 6-fold increase in the frequency of T cells recognizing the autologous LCL (W.H., unpublished data). This result supports the in vitro amplification of HLA-DR-restricted anti-EBV (Th1-type) CD4+ T effector cells. In IFN-γ ELISPOT fingerprinting analysis, several RP-HPLC fractions containing naturally processed HLA-DR-associated peptides extracted from IP1-LCL cells induced enhanced IFN-γ spot production by autologous, freshly isolated (day 0) CD4+ T cells or day 21 CD4+ T lymphocyte responders stimulated on a weekly basis with autologous IP1-LCL lysate-loaded DC (Fig. 6). Interestingly, although the profiles of the curves were largely overlapping (although differing in magnitude), several HPLC fractions were observed that appeared to be recognized by one or the other CD4+ T cell responder population. This may reflect the in vitro expansion of CD4+ T cells responding to “subdominant” epitopes or the failure to retain certain T cell specificities during reiterated ex vivo stimulations. This latter aspect may also suggest the potential inability of DC to yield a subset of peptide epitopes expressed by the autologous EBV B-LCL caused by differential protein processing by these APC, as has been noted by others (27, 28).
Figure 6.
ELISPOT fingerprinting of RP-HPLC-fractionated natural peptides extracted from LCL-derived HLA class II molecules. Naturally processed peptides were isolated from affinity-purified HLA–DR complexes prepared from 109 IP1-LCL cells and were fractionated by using RP-HPLC. Individual HPLC fractions were lyophilized, reconstituted in Hanks’ balanced salt solution and then tested for IFN-γ spot production by CD4+ T cells obtained from EBV-seropositive donor IP1 by ex vivo isolation from the blood (1.5 × 105 per well; □) or by three weekly stimulations with IP1-LCL freeze-thaw lysate-loaded mature DC (1.5 × 104 per well; ●). Autologous immature DC were used as APC. Each determination was done in duplicate. Spots developed after a culture period of 40 h were evaluated and presented as described in Fig. 3.
Discussion
We used an IFN-γ ELISPOT assay to characterize the CD8+ T cell response directed against known synthetic HLA-A2.1- or HLA-B7-binding peptide epitopes derived from different EBV proteins in EBV-seropositive healthy individual IP1 (Fig. 1). The frequency of EBV peptide-reactive T cells in donor IP1 and in other donors tested (data not shown) ranged from 10 to 100 per 105 CD8+ T cells. This range is consistent with reported frequencies of blood-derived T cells recognizing HLA class I-binding EBV peptides once the long-term EBV carrier-state has been established (26). We were able to generate EBV peptide-specific T cell lines in healthy EBV-seropositive individuals by repeated in vitro stimulations of CD8+ T cells with DC loaded with those EBV peptide antigens that induced enhanced IFN-γ spot production in ELISPOT assay (E.R., unpublished data). This result confirms that IFN-γ ELISPOT assay is able to reliably predict T cell responsiveness against peptide epitopes without the need for in vitro expansion of peptide-specific CD8+ T cell precursors before testing. In combination with computer-assisted video image analysis (10), ELISPOT assays provide objective data and therefore appear suitable for monitoring EBV-reactive T cells during the natural course of EBV infection, during immunotherapy trials, or during transplantation protocols where posttransplantation lymphoproliferative disorder may occur.
We also demonstrated that autologous monocyte-derived immature DC were very efficient APC in stimulating CD8+ T cell responsiveness against low concentrations of HLA class I-restricted latent EBV peptide antigens in IFN-γ ELISPOT assay. By using these immature DC, ex vivo purified CD8+ T cells of donor IP1 recognized the HLA-B7-restricted peptide EBNA-3A 379–387 at concentrations ≥100 pM (Fig. 2). This is an impressive result given the previous report that an EBNA-3A 379–387-specific CTL clone failed to recognize antigen-pulsed cells in cytotoxicity assays if the loading concentration of this particular peptide was <1 nM (22). ELISPOT assays performed on CD8+ T cells (of donor IP1) against LMP-2 peptide-loaded DC (≥1 nM, Fig. 2) provided antigen-specific frequency determinations that were comparable to those obtained using limiting-dilution assay analyses of LMP-2 329–337- and LMP-2 426–434-specific CTL cultures stimulated with low concentrations of their target epitopes (15, 18). Based on our observation that autologous immature DC are very efficient peptide-presenting cells in IFN-γ ELISPOT assays, particularly at low synthetic antigen concentrations, we applied them as APC for presenting naturally processed HLA-presented EBV peptides (isolated from IP1-LCL) to CD8+ T cells derived from EBV-seropositive donor IP1.
Mild acid treatment is a feasible approach to easily isolate the full spectrum of HLA class I-associated naturally processed peptides from the surface of viable tumor cells. Of major advantage, it does not require the immunoaffinity purification of HLA class I molecules from large starting cell numbers with subsequent acid dissociation of class I-binding peptides from purified complexes. In this study, we have demonstrated that after HPLC fractionation and loading on autologous immature DC, these natural peptides are bioactive and can be readily screened for recognition by blood-derived, freshly isolated “memory” CD8+ T cells using a very sensitive IFN-γ ELISPOT assay (Fig. 3). In addition, acid-eluted peptide material is of sufficient quantity and quality to yield sequence information on single peptide species using MS analysis. Using this approach, we were able to sequence naturally processed 9-, 10-, and 11-mer LMP-2A peptides acid-eluted from viable LCL cells (Fig. 4). We also demonstrated that IFN-γ ELISPOT analysis of ex vivo-purified CD4+ T cells can be successfully applied to screen autologous DC pulsed with RP-HPLC-resolved, naturally processed peptides extracted from affinity-purified HLA-DR complexes derived from the autologous LCL. Again, many RP-HPLC fractions contained HLA-DR-associated natural peptides that induced increased IFN-γ spot production by CD4+ T cells freshly isolated from the blood of EBV-seropositive donor IP1. The reactivity pattern observed with nonstimulated (day 0) CD4+ T cells was rather similar to the one found with day 21 cultured CD4+ responder lymphocytes obtained by weekly stimulations with IP1-LCL freeze-thaw lysate-loaded DC. However, some HPLC fractions were exclusively detected by one of the two CD4+ T cell populations tested. This observation suggests that the in vitro expansion of multispecific T lymphocytes by repeated antigenic stimulations can selectively eliminate or enrich in vivo primed T cell specificities, resulting in a distortion of the in vivo immune repertoire of specificities (7, 8).
The advantages of the described strategy for the purpose of antigen identification are obvious. The use of the very sensitive IFN-γ ELISPOT analysis to screen blood-derived, freshly isolated T cell reactivity against HPLC fractionated naturally processed peptides circumvents the need to generate and maintain antigen-specific T cell lines/clones for this purpose. This “classic” approach of using T cell lines and clones has proven typically laborious and time-consuming and is likely hampered by a drift in the T cell repertoire as a result of extended in vitro culture. Because the ELISPOT assay is able to determine the frequencies of T cells reactive against peptides present in individual HPLC fractions, this technique allows one to focus further sequence analysis on the identification of naturally processed peptides that represent the most immunogenic T cell epitopes in vivo. However, the finding that background levels of IFN-γ spot numbers by purified T cells in response to control autologous immature DC are usually very low (<5 spots per 105 T cells) lends confidence that one may identify subdominant epitopes using this approach that are recognized by rare T cells present in peripheral blood. This result is of major importance in light of recent evidence that protective immunity does not necessarily correlate with the hierarchy of T cell responses to immunodominant naturally processed peptide antigens (29). In conclusion, the screening of HPLC-fractionated naturally processed peptides with ex vivo-purified CD4+ or CD8+ T cells by IFN-γ ELISPOT assay should greatly facilitate the MS identification of HLA class I- (as shown in Fig. 3) or class II- (unpublished data) binding natural tumor or viral peptide antigens. In the cancer setting, this approach may prove particularly important for tumor histologies where it has been traditionally difficult to generate antigen-specific T cell lines and clones. The identification of such specificities allows for the rational design of peptide-based vaccines and/or for effective clinical monitoring (i.e., using ELISPOT) of patient responses to immunotherapy.
Acknowledgments
This work was supported by National Institutes of Health Grant CA 57840 (W.J.S.), a Clinical Investigator Award from the Cancer Research Institute (W.J.S.), a Consiglio Nazionale delle Ricerche–North Atlantic Treaty Organization Grant (216.1919; L.G.), a North Atlantic Treaty Organization Collaborative Research Grant CRG.CRG 973153 (L.G. and W.J.S.), and by a fellowship from the Deutsche Forschungsgemeinschaft (He 2896/1-1; W.H.).
Abbreviations
- DC
dendritic cells
- ELISPOT
enzyme-linked immunospot
- EBV
Epstein–Barr virus
- EBNA
EBV-associated nuclear antigen
- LCL
lymphoblastoid cell line
- RP
reverse-phase
- PBMC
peripheral blood mononuclear cells
- APC
antigen-presenting cells
- CTL
cytotoxic T lymphocytes
- LMP-2
latent membrane protein-2
References
- 1.Greenberg P D. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 2.Zajac A J, Murali-Krishna K, Blattman J N, Ahmed R. Curr Opin Immunol. 1998;10:444–449. doi: 10.1016/s0952-7915(98)80119-2. [DOI] [PubMed] [Google Scholar]
- 3.Rammensee H G, Friede T, Stevanovic S. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 4.Van den Eynde B J, van der Bruggen P. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 5.Slingluff C L, Hunt D F, Engelhard V H. Curr Opin Immunol. 1994;6:733–740. doi: 10.1016/0952-7915(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 6.Anichini A, Mortarini R, Maccalli C, Squarcina P, Fleischhauer K, Mascheroni L, Parmiani G. J Immunol. 1996;156:208–217. [PubMed] [Google Scholar]
- 7.Dietrich P Y, Walker P R, Schnuriger V, Saas P, Perrin G, Guillard M, Gaudin C, Caignard A. Int Immunol. 1997;9:1073–1083. doi: 10.1093/intimm/9.8.1073. [DOI] [PubMed] [Google Scholar]
- 8.Faure F, Even J, Kourilsky P. Crit Rev Immunol. 1998;18:77–86. doi: 10.1615/critrevimmunol.v18.i1-2.90. [DOI] [PubMed] [Google Scholar]
- 9.Czerkinsky C, Andersson G, Ekre H P, Nilsson L A, Klareskog L, Ouchterlony O. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 10.Herr W, Linn B, Leister N, Wandel E, Meyer zum Bueschenfelde K-H, Woelfel T. J Immunol Methods. 1997;203:141–152. doi: 10.1016/s0022-1759(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 11.Kabilan L, Andersson G, Lolli F, Ekre H P, Olsson T, Troye-Blomberg M. Eur J Immunol. 1990;20:1085–1089. doi: 10.1002/eji.1830200521. [DOI] [PubMed] [Google Scholar]
- 12.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman R. Nature (London) 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Rickinson A B, Moss D J. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 15.Lee S P, Thomas W A, Murray R J, Khanim F, Kaur S, Young L S, Rowe M, Kurilla M, Rickinson A B. J Virol. 1993;67:7428–7435. doi: 10.1128/jvi.67.12.7428-7435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonuleit H, Kuehn U, Mueller G, Steinbrink K, Paragnik L, Schmitt E, Knopp J, Enk A H. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 17.Steven N M, Annels N E, Kumar A, Leese A M, Kurilla M G, Rickinson A B. J Exp Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S P, Tierney R J, Thomas W A, Brooks J M, Rickinson A B. J Immunol. 1997;158:3325–3334. [PubMed] [Google Scholar]
- 19.Schmidt C, Burrows S R, Sculley T B, Moss D J, Misko I S. Proc Natl Acad Sci USA. 1991;88:9478–9482. doi: 10.1073/pnas.88.21.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burrows S R, Gardner J, Khanna R, Steward T, Moss D J, Rodda S, Suhrbier A. J Gen Virol. 1994;75:2489–2493. doi: 10.1099/0022-1317-75-9-2489. [DOI] [PubMed] [Google Scholar]
- 21.Kerr B M, Kienzle N, Burrows J M, Cross S, Silins S L, Buck M, Benson E M, Coupar B, Moss D J, Sculley T B. J Virol. 1996;70:8858–8864. doi: 10.1128/jvi.70.12.8858-8864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill A, Worth A, Elliott T, Rowland-Jones S, Brooks J, Rickinson A, McMichael A. Eur J Immunol. 1995;25:18–24. doi: 10.1002/eji.1830250105. [DOI] [PubMed] [Google Scholar]
- 23.Bednarek M A, Sauma S Y, Gammon M C, Porter G, Tamhankar S, Williamson A R, Zweerink H J. J Immunol. 1991;147:4047–4053. [PubMed] [Google Scholar]
- 24.Haas G, Plikat U, Debre P, Lucchiari M, Katlama C, Dudoit Y, Bonduelle O, Bauer M, Ihlenfeldt H G, Jung G, et al. J Immunol. 1996;157:4212–4221. [PubMed] [Google Scholar]
- 25.Storkus W J, Zeh H J, Salter R D, Lotze M T. J Immunother. 1993;14:94–103. [PubMed] [Google Scholar]
- 26.Tan L, Gudgeon N, Annels N E, Hansasuta P, O’Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A B, Callan M F. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 27.Vidard L, Kovacsovics-Bankowski M, Kraeft S K, Chen L B, Benacerraf B, Rock K L. J Immunol. 1996;156:2809–2818. [PubMed] [Google Scholar]
- 28.Vidard L, Rock K L, Benacerraf B. J Immunol. 1992;149:1905–11. [PubMed] [Google Scholar]
- 29.Gallimore A, Dumrese T, Hengartner H, Zinkernagel R M, Rammensee H G. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]