Abstract
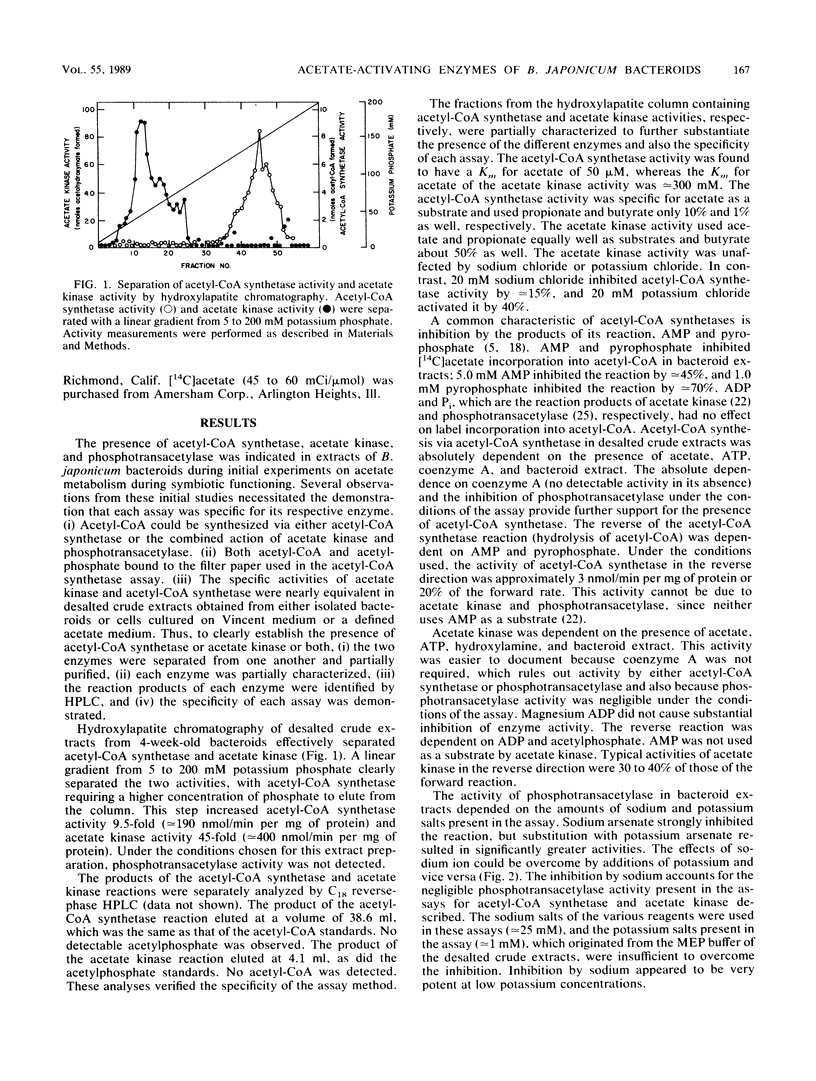
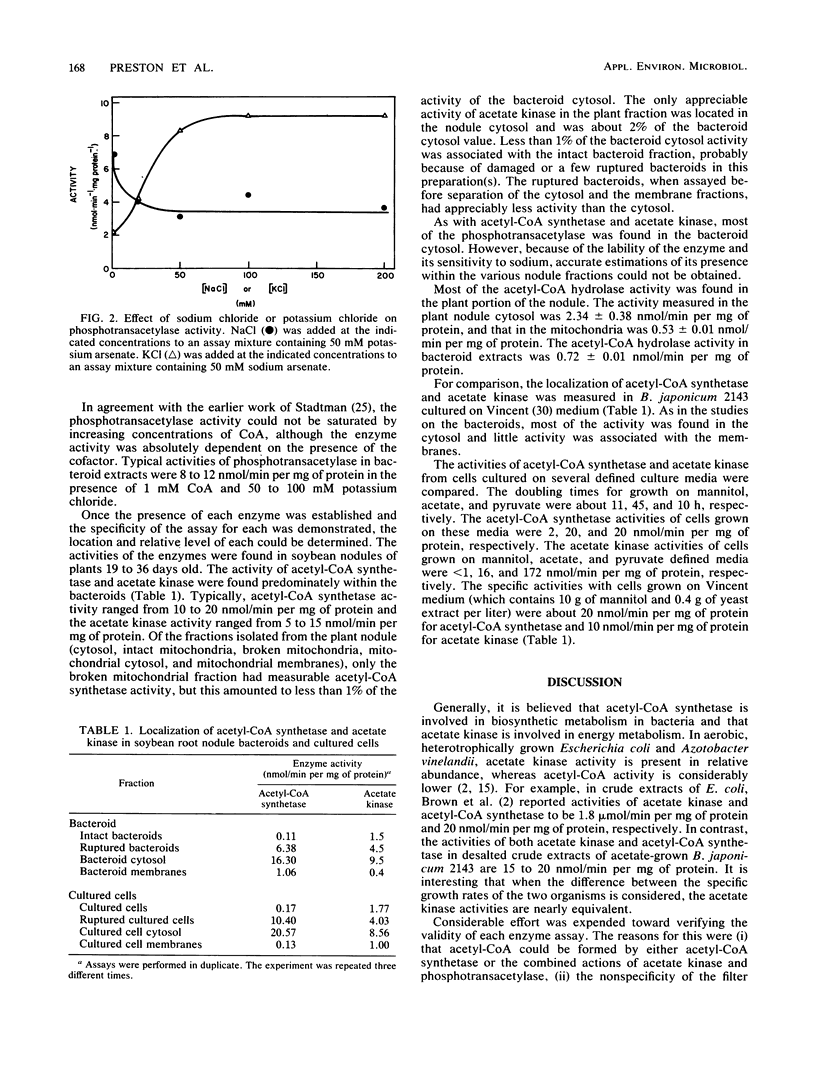
Acetyl coenzyme A (acetyl-CoA) synthetase and acetate kinase were localized within the soluble portion of Bradyrhizobium japonicum bacteroids, and no appreciable activity was found elsewhere in the nodule. The presence of each acetate-activating enzyme was confirmed by separation of the two enzyme activities on a hydroxylapatite column, by substrate dependence of each enzyme in both the forward and reverse directions, by substrate specificity, by inhibition patterns, and also by identification of the reaction products by C18 reverse-phase high-pressure liquid chromatography. Phosphotransacetylase activity, found in the soluble portion of the bacteroid, was dependent on the presence of potassium and was inhibited by added sodium. The greatest acetyl-CoA hydrolase activity was found in the root nodule cytosol, although appreciable activity also was found within the bacteroids. The combined specific activities of acetyl-CoA synthetase and acetate kinase-phosphotransacetylase were approximate to that of the pyruvate dehydrogenase complex, thus providing B. japonicum with sufficient capacity to generate acetyl-CoA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. D., Jones-Mortimer M. C., Kornberg H. L. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977 Oct;102(2):327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Fox D. K., Meadow N. D., Roseman S. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J Biol Chem. 1986 Oct 15;261(29):13498–13503. [PubMed] [Google Scholar]
- Huang K. P., Stumpf P. K. Fat metabolism in higher plants. XLI. Properties of potato acetyl coenzyme A synthetase. Arch Biochem Biophys. 1970 Sep;140(1):158–173. doi: 10.1016/0003-9861(70)90019-6. [DOI] [PubMed] [Google Scholar]
- Ingebretsen O. C., Farstad M. Direct measurement of free coenzyme A in biological extracts by reversed-phase high-performance liquid chromatography. J Chromatogr. 1980 Dec 26;202(3):439–445. doi: 10.1016/s0021-9673(00)91829-6. [DOI] [PubMed] [Google Scholar]
- Karr D. B., Waters J. K., Emerich D. W. Analysis of Poly-beta-Hydroxybutyrate in Rhizobium japonicum Bacteroids by Ion-Exclusion High-Pressure Liquid Chromatography and UV Detection. Appl Environ Microbiol. 1983 Dec;46(6):1339–1344. doi: 10.1128/aem.46.6.1339-1344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr D. B., Waters J. K., Suzuki F., Emerich D. W. Enzymes of the Poly-beta-Hydroxybutyrate and Citric Acid Cycles of Rhizobium japonicum Bacteroids. Plant Physiol. 1984 Aug;75(4):1158–1162. doi: 10.1104/pp.75.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. V. Studies on soybean nodule senescence. Plant Physiol. 1974 Oct;54(4):612–616. doi: 10.1104/pp.54.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel B., Stumpf P. K. Origin of acetate in spinach leaf cell. Plant Physiol. 1982 Apr;69(4):897–903. doi: 10.1104/pp.69.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney D., Melton T. Isolation and characterization of ack and pta mutations in Azotobacter vinelandii affecting acetate-glucose diauxie. J Bacteriol. 1986 Jan;165(1):6–12. doi: 10.1128/jb.165.1.6-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J., Ettlinger L. Characterization of the acetyl-CoA synthetase of Acetobacter aceti. Biochim Biophys Acta. 1976 Dec 20;450(3):410–417. doi: 10.1016/0005-2760(76)90014-x. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., LaRue T. A. Soluble aldehyde dehydrogenase and metabolism of aldehydes by soybean bacteroids. J Bacteriol. 1982 Sep;151(3):1473–1484. doi: 10.1128/jb.151.3.1473-1484.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. B., Larue T. A. Utilization of aldehydes and alcohols by soybean bacteroids. Plant Physiol. 1981 Aug;68(2):489–493. doi: 10.1104/pp.68.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R. The purification and properties of phosphotransacetylase. J Biol Chem. 1952 May;196(2):527–534. [PubMed] [Google Scholar]
- Skarstedt M. T., Silverstein E. Escherichia coli acetate kinase mechanism studied by net initial rate, equilibrium, and independent isotopic exchange kinetics. J Biol Chem. 1976 Nov 10;251(21):6775–6783. [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. Biosynthesis of malonate in roots of soybean seedlings. Plant Physiol. 1981 Nov;68(5):992–995. doi: 10.1104/pp.68.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S., Larue T. A. Enzymes for acetaldehyde and ethanol formation in legume nodules. Plant Physiol. 1982 Aug;70(2):388–392. doi: 10.1104/pp.70.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. P., Evans H. J. Poly-beta-hydroxybutyrate Utilization by Soybean (Glycine max Merr.) Nodules and Assessment of Its Role in Maintenance of Nitrogenase Activity. Plant Physiol. 1971 Jun;47(6):750–755. doi: 10.1104/pp.47.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]



