Abstract
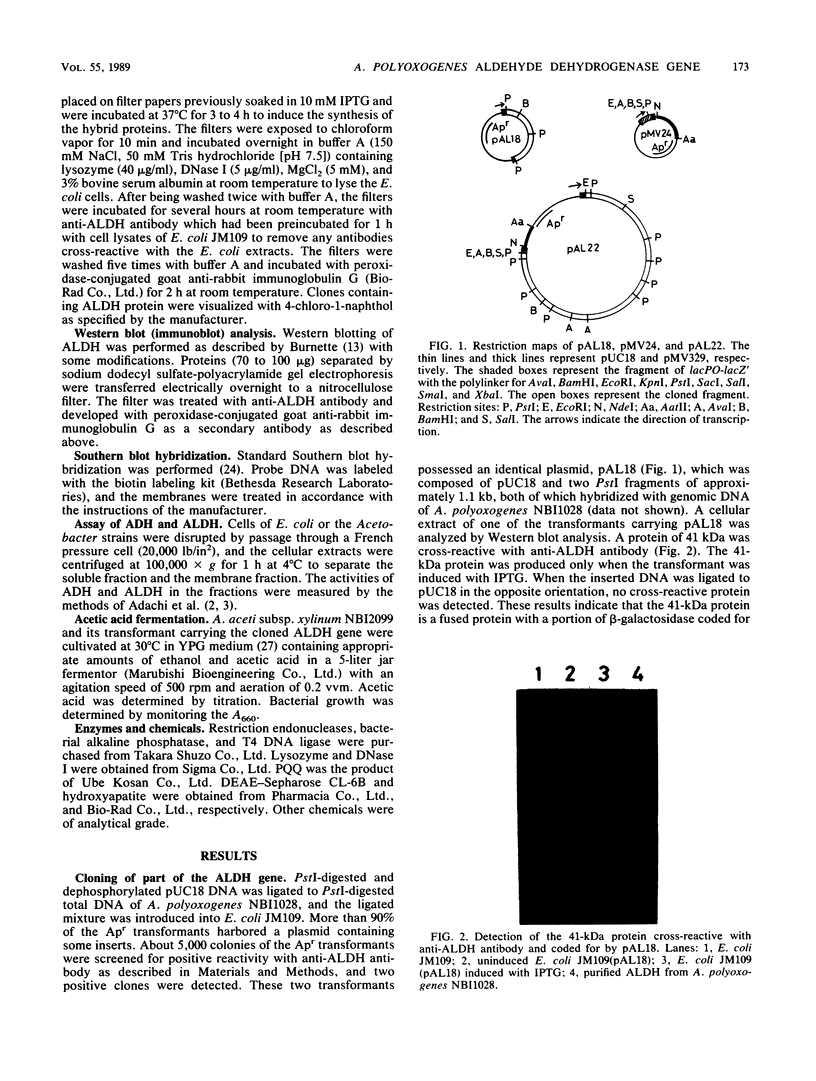
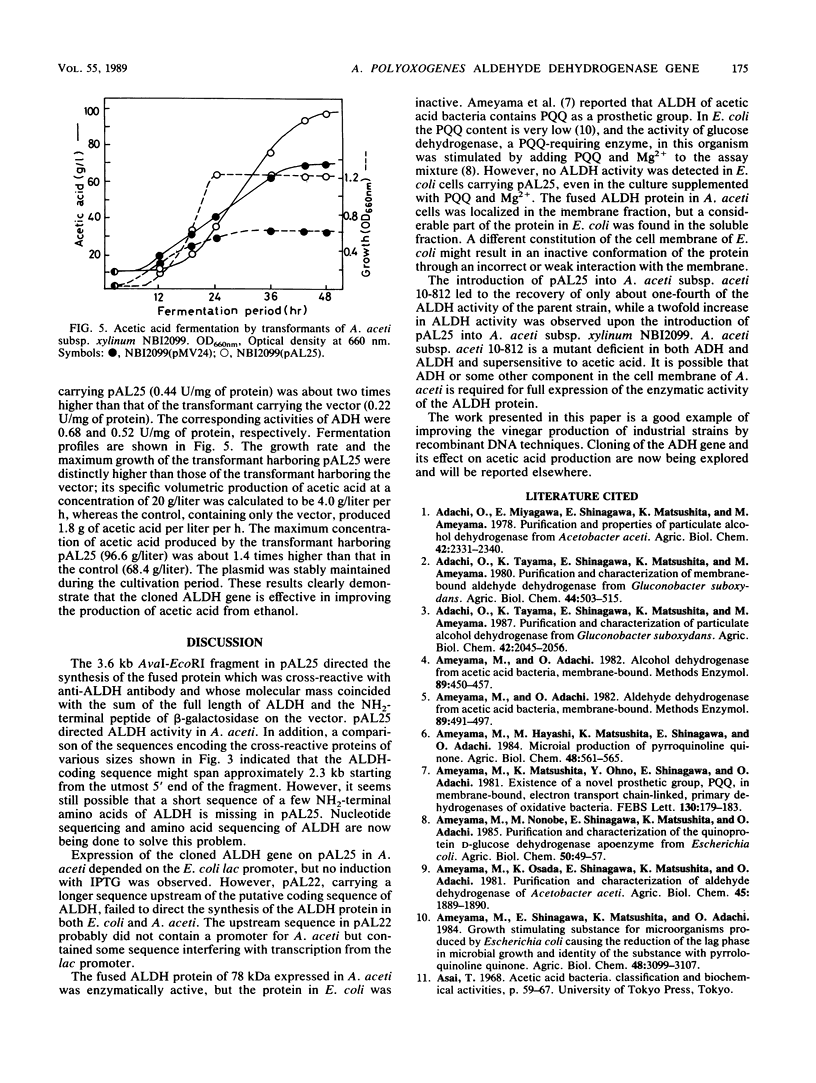
A genomic clone bank of Acetobacter polyoxogenes NBI1028 constructed in Escherichia coli by use of the expression vector pUC18 was screened with antibody raised against membrane-bound aldehyde dehydrogenase (ALDH; 75 kilodaltons [kDa]) from A. polyoxogenes NBI1028. A clone that synthesized a 41-kDa protein cross-reactive with anti-ALDH antibody was isolated. For cloning of the full-length ALDH structural gene, a cosmid gene bank was screened by Southern blot hybridization with the cloned DNA as a probe, and subcloning from the positive cosmid clone was performed with shuttle vector pMV24. Plasmid pAL25, containing the full-length ALDH structural gene, was isolated and expressed in both E. coli and Acetobacter aceti to produce a fused protein (78 kDa) with a short NH2-terminal β-galactosidase peptide. pAL25 conferred ALDH production on a mutant of A. aceti lacking the enzyme activity. Transformation of A. aceti subsp. xylinum NBI2099 with pAL25 caused 2- and 1.4-fold increases in the production rate and in the maximum concentration of acetic acid in submerged fermentation, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameyama M., Matsushita K., Ohno Y., Shinagawa E., Adachi O. Existence of a novel prosthetic group, PQQ, in membrane-bound, electron transport chain-linked, primary dehydrogenases of oxidative bacteria. FEBS Lett. 1981 Aug 3;130(2):179–183. doi: 10.1016/0014-5793(81)81114-3. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leisinger T., Jäggi W., Weber P., Ettlinger L. Spontane Mutationen bei Essigsäurebakterien. II. Die Bedeutung der Mutationen für die Systematik. Arch Mikrobiol. 1967 May 17;57(1):76–92. [PubMed] [Google Scholar]
- SCHELL J., DE LEY J. Variability of acetic acid bacteria. Antonie Van Leeuwenhoek. 1962;28:445–465. doi: 10.1007/BF02538760. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]





