Abstract
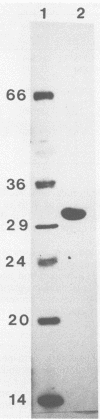
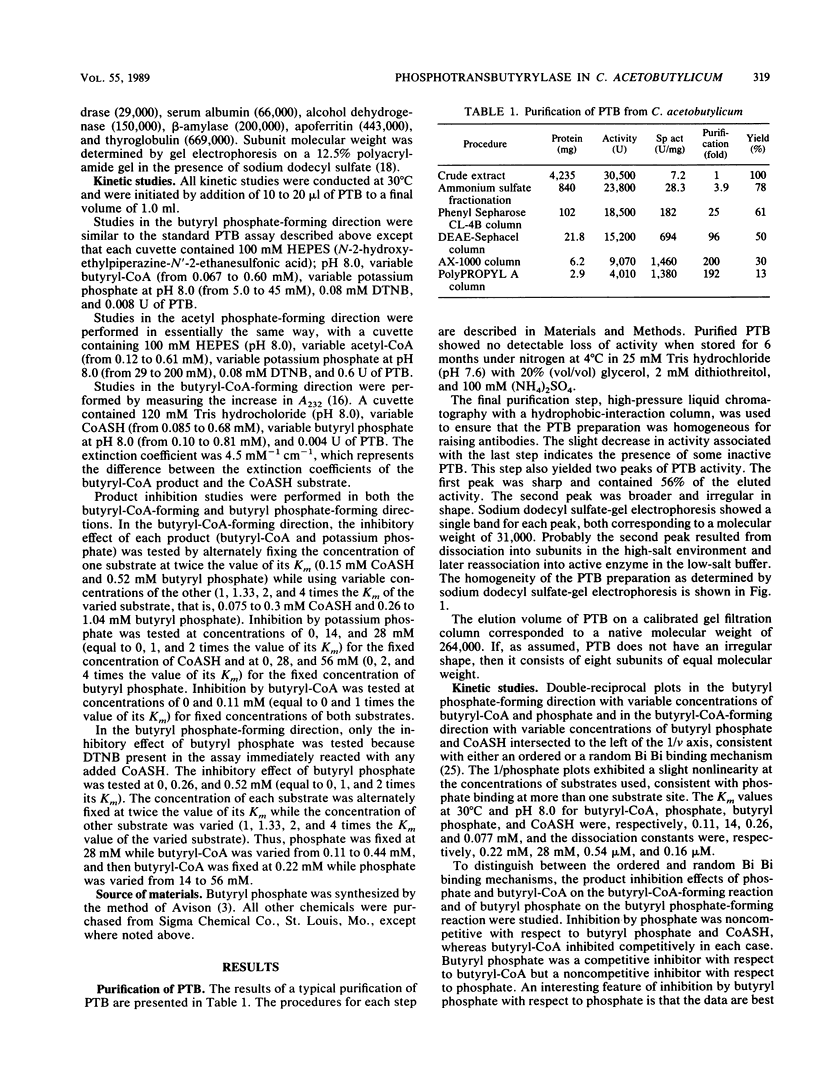
Phosphotransbutyrylase (phosphate butyryltransferase [EC 2.3.1.19]) from Clostridium acetobutylicum ATCC 824 was purified approximately 200-fold to homogeneity with a yield of 13%. Steps used in the purification procedure were fractional precipitation with (NH4)2SO4, Phenyl Sepharose CL-4B chromatography, DEAE-Sephacel chromatography, high-pressure liquid chromatography with an anion-exchange column, and high-pressure liquid chromatography with a hydrophobic-interaction column. Gel filtration and denaturing gel electrophoresis data were consistent with a native enzyme having eight 31,000-molecular-weight subunits. Within the physiological range of pH 5.5 to 7, the enzyme was very sensitive to pH change in the butyryl phosphate-forming direction and showed virtually no activity below pH 6. This finding indicates that a change in internal pH may be one important factor in the regulation of the enzyme. The enzyme was less sensitive to pH change in the reverse direction. The enzyme could use a number of substrates in addition to butyryl coenzyme A (butyryl-CoA) but had the highest relative activity with butyryl-CoA, isovaleryl-CoA, and valeryl-CoA. The Km values at 30 degrees C and pH 8.0 for butyryl-CoA, phosphate, butyryl phosphate, and CoASH (reduced form of CoA) were 0.11, 14, 0.26, and 0.077 mM, respectively. Results of product inhibition studies were consistent with a random Bi Bi binding mechanism in which phosphate binds at more than one site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GAVARD R., HAUTECOEUR B., DESCOURTIEUX H. Phosphotransbutyrylase de Clostridium acetobutylicum. C R Hebd Seances Acad Sci. 1957 Apr 29;244(18):2323–2326. [PubMed] [Google Scholar]
- Gilbert H. F., Lennox B. J., Mossman C. D., Carle W. C. The relation of acyl transfer to the overall reaction of thiolase I from porcine heart. J Biol Chem. 1981 Jul 25;256(14):7371–7377. [PubMed] [Google Scholar]
- Hartmanis M. G. Butyrate kinase from Clostridium acetobutylicum. J Biol Chem. 1987 Jan 15;262(2):617–621. [PubMed] [Google Scholar]
- Hartmanis M. G., Gatenbeck S. Intermediary Metabolism in Clostridium acetobutylicum: Levels of Enzymes Involved in the Formation of Acetate and Butyrate. Appl Environ Microbiol. 1984 Jun;47(6):1277–1283. doi: 10.1128/aem.47.6.1277-1283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Gibbins L. N., Forsberg C. W. Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol. 1985 Oct;50(4):1043–1047. doi: 10.1128/aem.50.4.1043-1047.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrtopoulos S. A., Satchell D. P. Kinetic studies with phosphotransacetylase. IV. Inhibition by products. Biochim Biophys Acta. 1972 Aug 28;276(2):383–391. doi: 10.1016/0005-2744(72)90998-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J., Rudolph F. B. The hexokinases: kinetic, physical, and regulatory properties. Adv Enzymol Relat Areas Mol Biol. 1973;39:249–326. doi: 10.1002/9780470122846.ch4. [DOI] [PubMed] [Google Scholar]
- Robinson J. R., Sagers R. D. Phosphotransacetylase from Clostridium acidiurici. J Bacteriol. 1972 Oct;112(1):465–473. doi: 10.1128/jb.112.1.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph F. B. Product inhibition and abortive complex formation. Methods Enzymol. 1979;63:411–436. doi: 10.1016/0076-6879(79)63018-5. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Suzuki T., Kameda K. Y., Abiko Y. Phosphotransacetylase of Escherichia coli B, purification and properties. Biochim Biophys Acta. 1969;191(3):550–558. doi: 10.1016/0005-2744(69)90348-9. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Purification and role of phosphotransbutyrylase. J Biol Chem. 1960 Jul;235:1948–1952. [PubMed] [Google Scholar]