Abstract
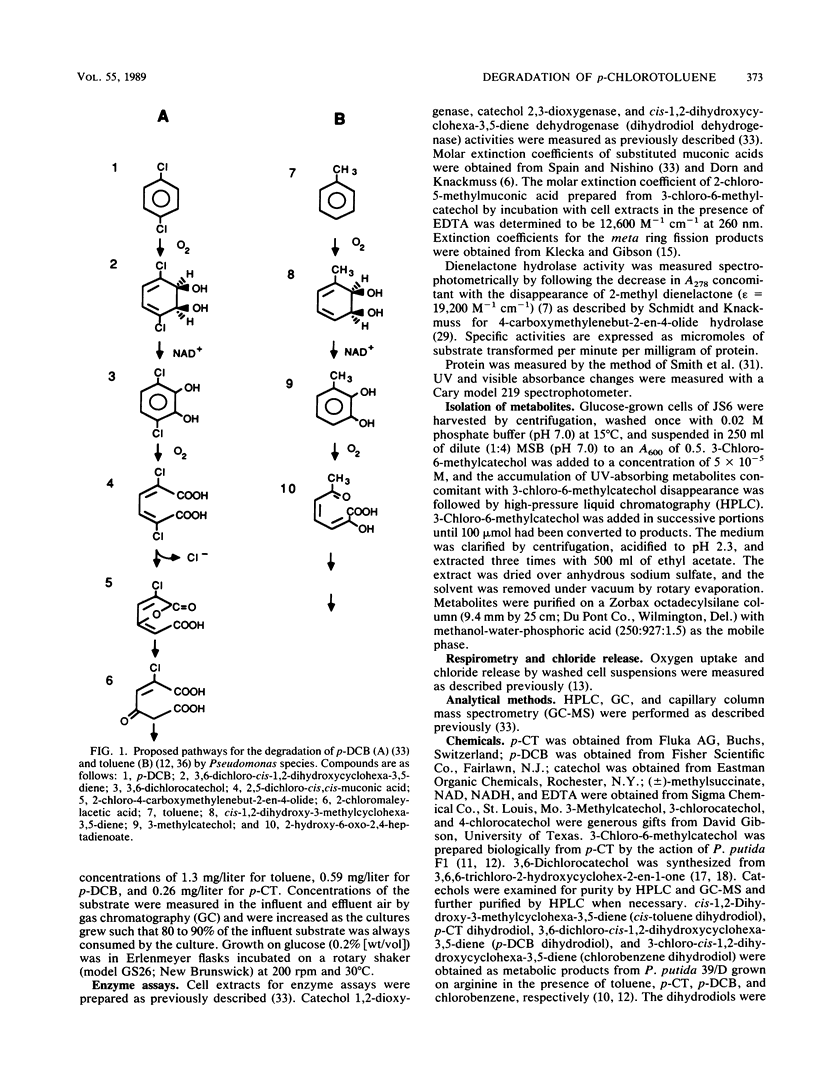
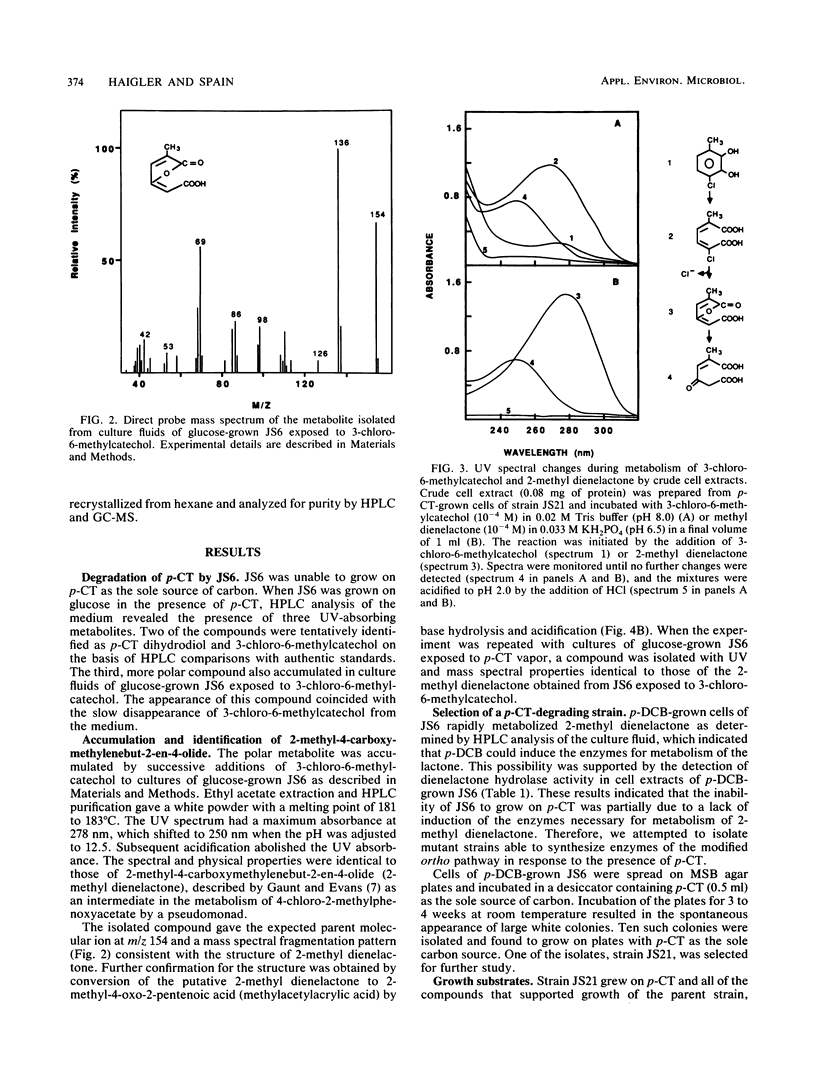
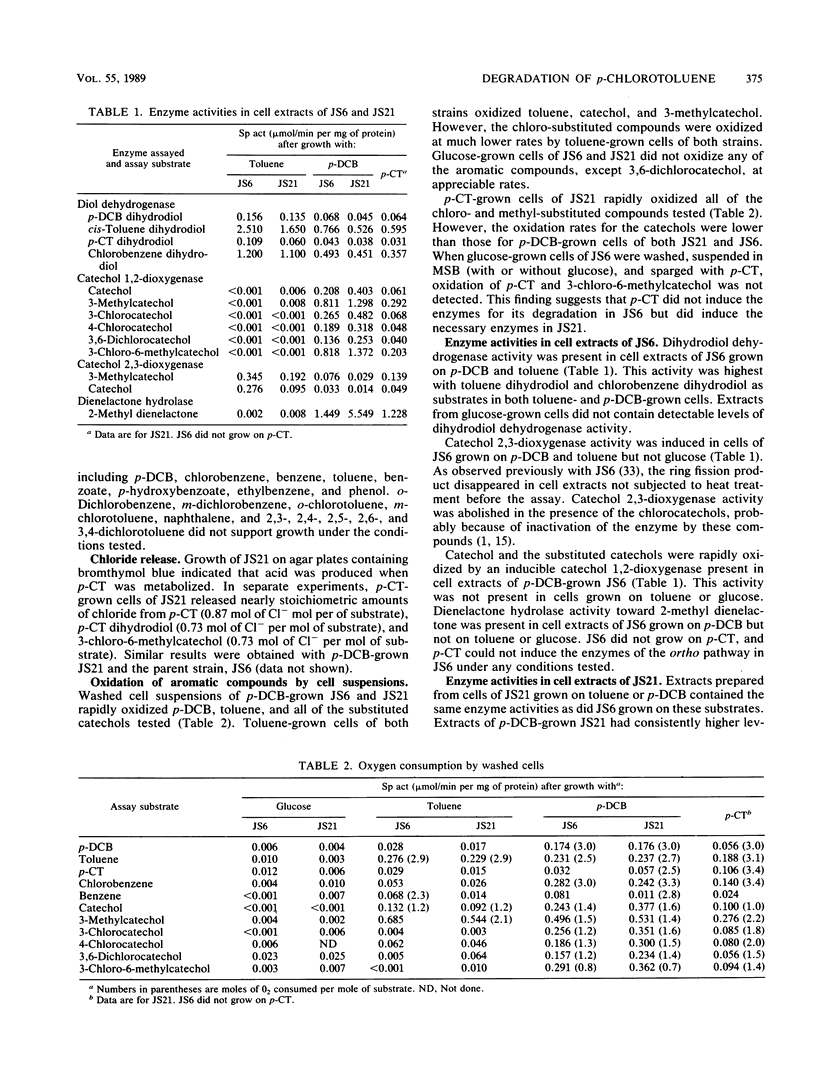
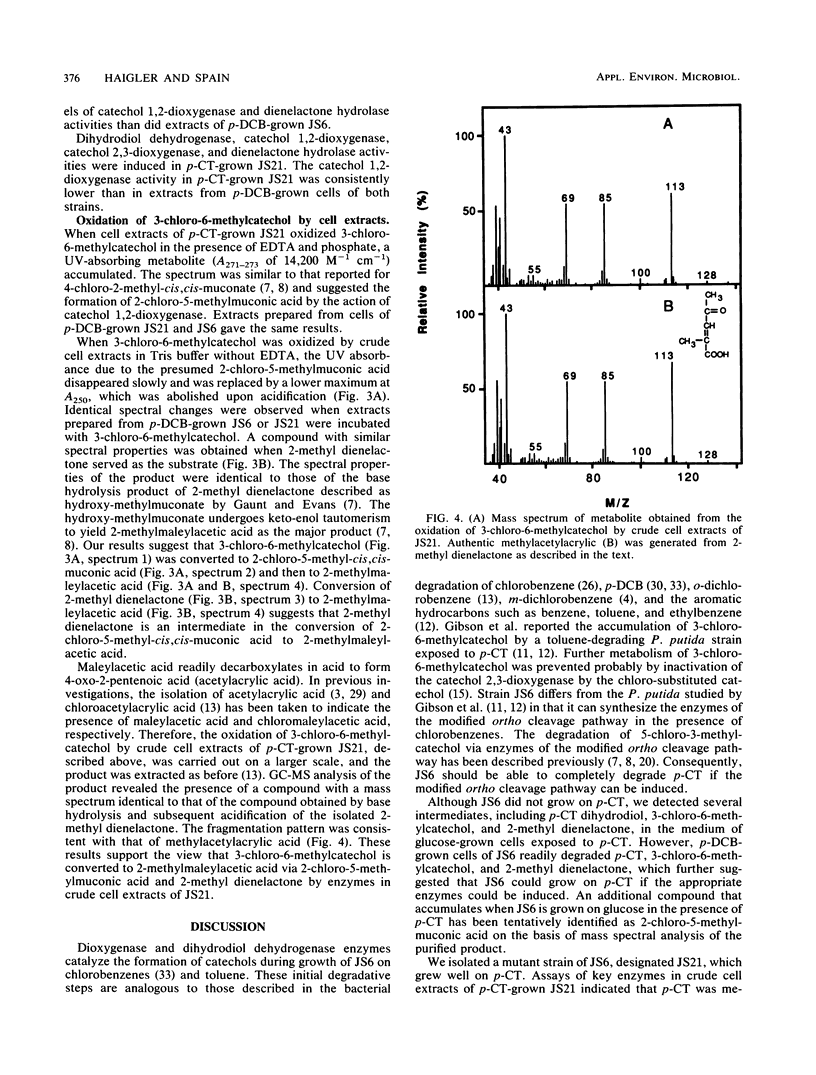
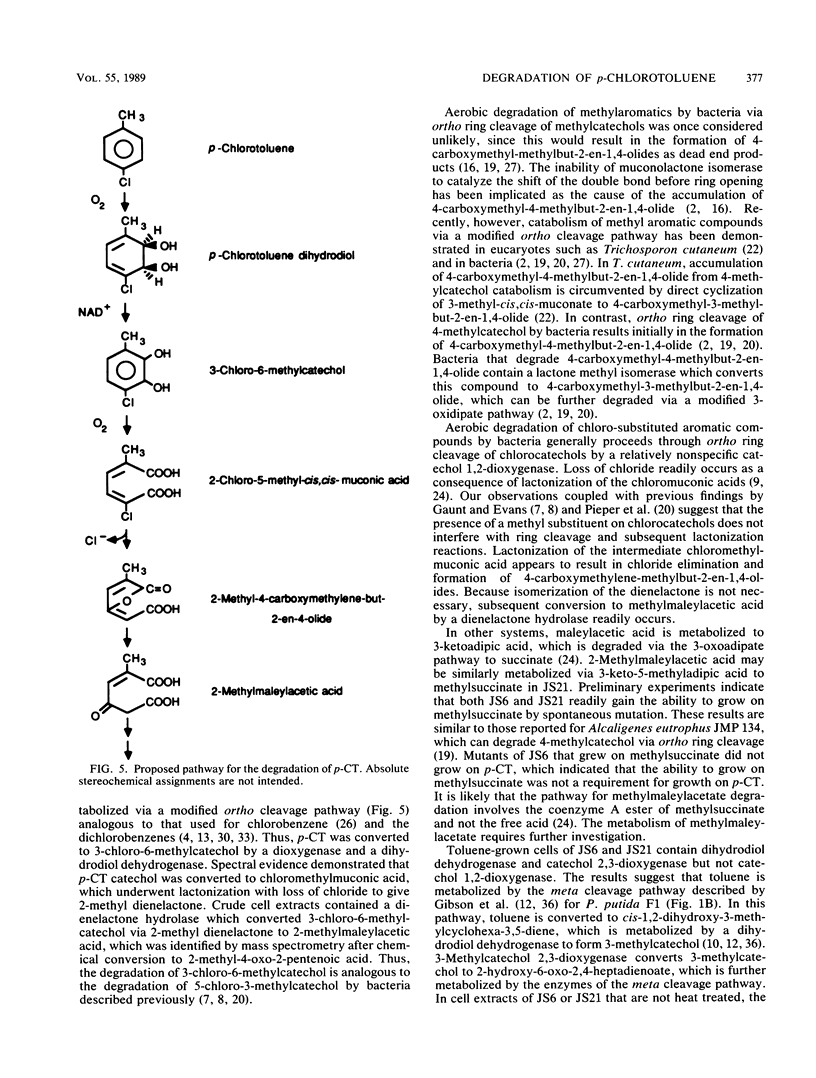
Pseudomonas sp. strain JS6 grows on chlorobenzene, p-dichlorobenzene, or toluene as a sole source of carbon and energy. It does not grow on p-chlorotoluene (p-CT). Growth on glucose in the presence of p-CT resulted in the accumulation of 4-chloro-2,3-dihydroxy-1-methylbenzene (3-chloro-6-methylcatechol), 4-chloro-2,3-dihydroxy-1-methylcyclohexa-4,6-diene (p-CT dihydrodiol), and 2-methyl-4-carboxymethylenebut-2-en-4-olide (2-methyl dienelactone). Strain JS21, a spontaneous mutant capable of growth on p-CT, was isolated from cultures of strain JS6 after extended exposure to p-CT. In addition to growing on p-CT, JS21 grew on all of the substrates that supported growth of the parent strain, including p-dichlorobenzene, chlorobenzene, benzene, toluene, benzoate, p-hydroxybenzoate, phenol, and ethylbenzene. The pathway for degradation of p-CT by JS21 was investigated by respirometry, isolation of intermediates, and assay of enzymes in cell extracts. p-CT was converted to 3-chloro-6-methylcatechol by dioxygenase and dihydrodiol dehydrogenase enzymes. 3-Chloro-6-methylcatechol underwent ortho ring cleavage catalyzed by a catechol 1,2-dioxygenase to form 2-chloro-5-methyl-cis,cis-muconate, which was converted to 2-methyl dienelactone. A dienelactone hydrolase converted 2-methyl dienelactone to 2-methylmaleylacetic acid. Preliminary results indicate that a change in wild-type induction patterns allows JS21 to grow on p-CT.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels I., Knackmuss H. J., Reineke W. Suicide Inactivation of Catechol 2,3-Dioxygenase from Pseudomonas putida mt-2 by 3-Halocatechols. Appl Environ Microbiol. 1984 Mar;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. J. Constructing microbial strains for degradation of halogenated aromatic hydrocarbons. Basic Life Sci. 1988;45:81–95. doi: 10.1007/978-1-4899-0824-7_5. [DOI] [PubMed] [Google Scholar]
- Chapman P. J., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. J Bacteriol. 1976 Mar;125(3):985–998. doi: 10.1128/jb.125.3.985-998.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt J. K., Evans W. C. Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Preliminary evidence for the metabolic pathway. Biochem J. 1971 May;122(4):519–526. doi: 10.1042/bj1220519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt J. K., Evans W. C. Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Ring-fission, lactonizing and delactonizing enzymes. Biochem J. 1971 May;122(4):533–542. doi: 10.1042/bj1220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Schuld C. L., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry. 1968 Nov;7(11):3795–3802. doi: 10.1021/bi00851a003. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Nishino S. F., Spain J. C. Degradation of 1,2-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1988 Feb;54(2):294–301. doi: 10.1128/aem.54.2.294-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Inhibition of catechol 2,3-dioxygenase from Pseudomonas putida by 3-chlorocatechol. Appl Environ Microbiol. 1981 May;41(5):1159–1165. doi: 10.1128/aem.41.5.1159-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powlowski J. B., Dagley S. beta-Ketoadipate pathway in Trichosporon cutaneum modified for methyl-substituted metabolites. J Bacteriol. 1985 Sep;163(3):1126–1135. doi: 10.1128/jb.163.3.1126-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Stolz A., Reineke W., Timmis K. N. Altered effector specificities in regulators of gene expression: TOL plasmid xylS mutants and their use to engineer expansion of the range of aromatics degraded by bacteria. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8467–8471. doi: 10.1073/pnas.83.22.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Jeenes D. J., Williams P. A., Knackmuss H. J. TOL plasmid pWW0 in constructed halobenzoate-degrading Pseudomonas strains: prevention of meta pathway. J Bacteriol. 1982 Apr;150(1):195–201. doi: 10.1128/jb.150.1.195-201.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Appl Environ Microbiol. 1984 Feb;47(2):395–402. doi: 10.1128/aem.47.2.395-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo F., Pieper D. H., Engesser K. H., Knackmuss H. J., Timmis K. N. Assemblage of ortho cleavage route for simultaneous degradation of chloro- and methylaromatics. Science. 1987 Dec 4;238(4832):1395–1398. doi: 10.1126/science.3479842. [DOI] [PubMed] [Google Scholar]
- Schmidt E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J. 1980 Oct 15;192(1):339–347. doi: 10.1042/bj1920339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraa G., Boone M. L., Jetten M. S., van Neerven A. R., Colberg P. J., Zehnder A. J. Degradation of 1,4-dichlorobenzene by Alcaligenes sp. strain A175. Appl Environ Microbiol. 1986 Dec;52(6):1374–1381. doi: 10.1128/aem.52.6.1374-1381.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Oxidation of substituted phenols by Pseudomonas putida F1 and Pseudomonas sp. strain JS6. Appl Environ Microbiol. 1988 Jun;54(6):1399–1404. doi: 10.1128/aem.54.6.1399-1404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Nishino S. F. Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1987 May;53(5):1010–1019. doi: 10.1128/aem.53.5.1010-1019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Vandenbergh P. A., Olsen R. H., Colaruotolo J. F. Isolation and genetic characterization of bacteria that degrade chloroaromatic compounds. Appl Environ Microbiol. 1981 Oct;42(4):737–739. doi: 10.1128/aem.42.4.737-739.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylstra G. J., McCombie W. R., Gibson D. T., Finette B. A. Toluene degradation by Pseudomonas putida F1: genetic organization of the tod operon. Appl Environ Microbiol. 1988 Jun;54(6):1498–1503. doi: 10.1128/aem.54.6.1498-1503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bont J. A., Vorage M. J., Hartmans S., van den Tweel W. J. Microbial degradation of 1,3-dichlorobenzene. Appl Environ Microbiol. 1986 Oct;52(4):677–680. doi: 10.1128/aem.52.4.677-680.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


