Abstract
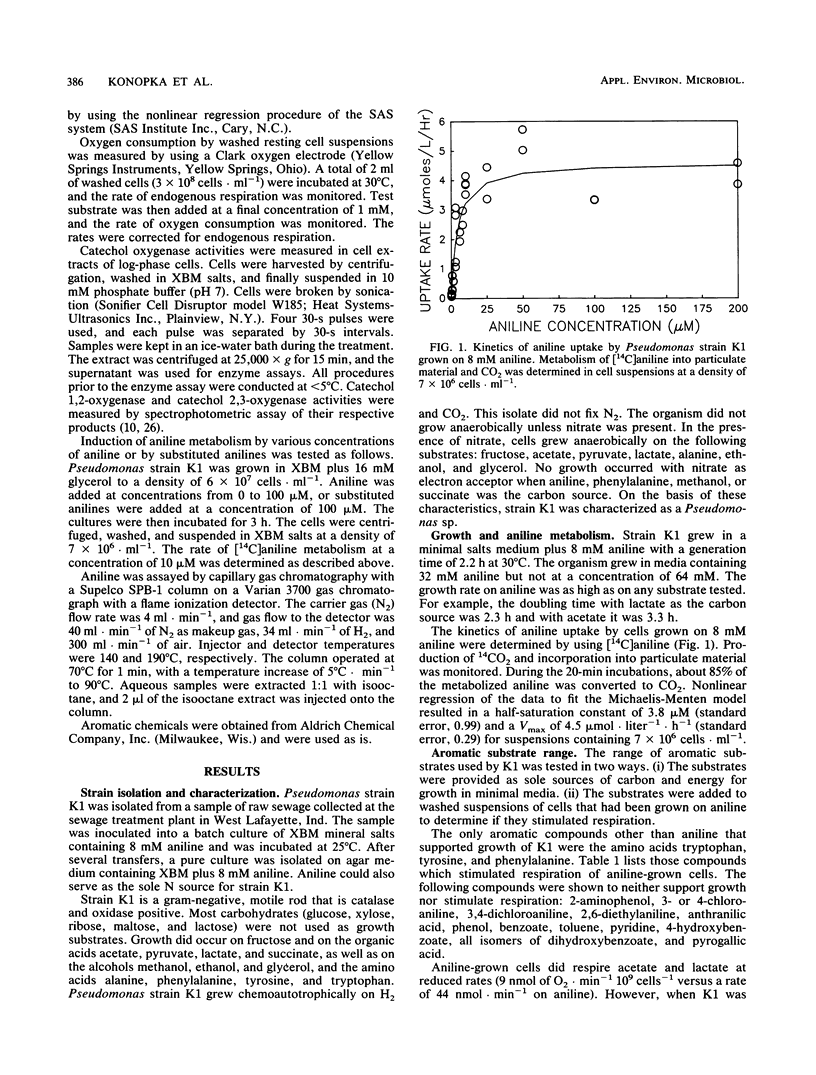
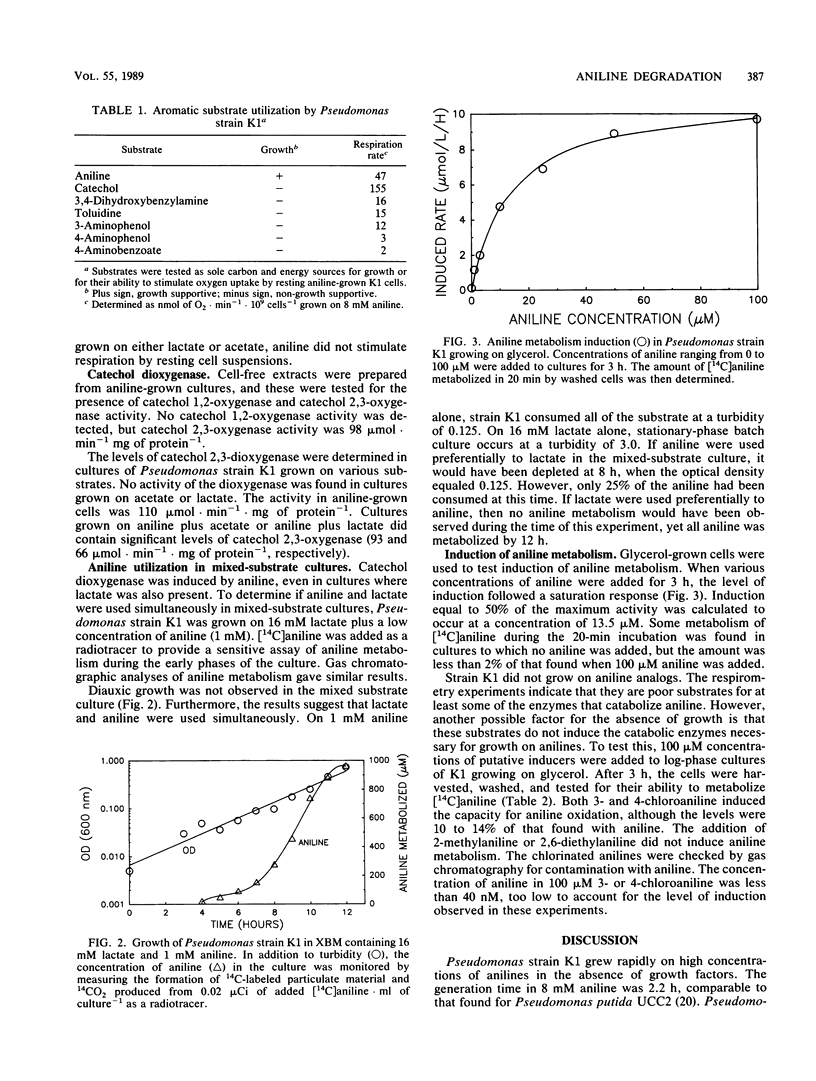
Pseudomonas strain K1 is a gram-negative rod which grows aerobically on minimal media containing aniline with a doubling time of 2 h at 30°C. The half-saturation parameter for aniline metabolism by aniline-grown cells was 3.8 μmol · liter−1. Concentrations of aniline as low as 50 nM were metabolized. Neither substituted anilines nor other aromatic compounds (other than aromatic amino acids) supported growth. Cells grew as fast on aniline as on nonaromatic substrates such as lactate. The aromatic ring was cleaved via the meta pathway. Catechol 2,3-oxygenase activity was induced by aniline, even in cultures containing alternative carbon sources such as lactate. Cultures grown on a mixture of aniline and lactate mineralized aniline in the presence of the second substrate. Lactate-grown cultures lacked catechol oxygenase activity, and resting cells from these cultures did not respire aniline. Resting cells from aniline-grown cultures exhibited high respiratory activity upon the addition of aniline or catechol, some activity with toluidine, and no activity after addition of a wide variety of other aromatic compounds, including dihydroxybenzylamine, chloroanilines, ethylanilines, aminophenols, aminobenzoates, and dihydroxybenzoates. Although substituted anilines were not metabolized, 3-or 4-chloroaniline did induce the enzymes for aniline oxidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson J. G., Mackinnon G. Novel pseudomonas plasmid involved in aniline degradation. Appl Environ Microbiol. 1984 Oct;48(4):868–869. doi: 10.1128/aem.48.4.868-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachofer R., Lingens F., Schäfer W. Conversion of aniline into pyrocatechol by a Nocardia sp.; incorporation of oxygen-18. FEBS Lett. 1975 Feb 1;50(2):288–290. doi: 10.1016/0014-5793(75)80510-2. [DOI] [PubMed] [Google Scholar]
- Button D. K. Kinetics of nutrient-limited transport and microbial growth. Microbiol Rev. 1985 Sep;49(3):270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYAISHI O., KATAGIRI M., ROTHBERG S. Studies on oxygenases; pyrocatechase. J Biol Chem. 1957 Dec;229(2):905–920. [PubMed] [Google Scholar]
- Hallas L. E., Alexander M. Microbial transformation of nitroaromatic compounds in sewage effluent. Appl Environ Microbiol. 1983 Apr;45(4):1234–1241. doi: 10.1128/aem.45.4.1234-1241.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Strategies of mixed substrate utilization in microorganisms. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):459–480. doi: 10.1098/rstb.1982.0055. [DOI] [PubMed] [Google Scholar]
- Heiman A. S., Cooper W. T. Solid-state 13C nuclear magnetic resonance spectroscopy of simultaneously metabolized acetate and phenol in a soil Pseudomonas sp. Appl Environ Microbiol. 1987 Jan;53(1):156–162. doi: 10.1128/aem.53.1.156-162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski U., Janke D., Prauser H., Fritsche W. Degradation of aniline and monochloroanilines by Rhodococcus sp. An 117 and a pseudomonad: a comparative study. Z Allg Mikrobiol. 1983;23(4):235–246. doi: 10.1002/jobm.3630230405. [DOI] [PubMed] [Google Scholar]
- Kinouchi T., Ohnishi Y. Purification and characterization of 1-nitropyrene nitroreductases from Bacteroides fragilis. Appl Environ Microbiol. 1983 Sep;46(3):596–604. doi: 10.1128/aem.46.3.596-604.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A., Szentes M. Autotrophic Growth of Gas Vacuolate Strains of Microcyclus aquaticus on Methanol and Hydrogen. Appl Environ Microbiol. 1984 Apr;47(4):870–872. doi: 10.1128/aem.47.4.870-872.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons C. D., Katz S., Bartha R. Mechanisms and pathways of aniline elimination from aquatic environments. Appl Environ Microbiol. 1984 Sep;48(3):491–496. doi: 10.1128/aem.48.3.491-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure N. C., Venables W. A. Adaptation of Pseudomonas putida mt-2 to growth on aromatic amines. J Gen Microbiol. 1986 Aug;132(8):2209–2218. doi: 10.1099/00221287-132-8-2209. [DOI] [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard R. D., Russel S., Bollag J. M. Chemical transformation of 4-chloroaniline to a triazene in a bacterial culture medium. J Agric Food Chem. 1977 Jul-Aug;25(4):841–844. doi: 10.1021/jf60212a011. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris G. E. Environmental and metabolic transformations of primary aromatic amines and related compounds. Residue Rev. 1980;76:1–30. doi: 10.1007/978-1-4612-6107-0_1. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Schmidt S. K., Alexander M. Effects of dissolved organic carbon and second substrates on the biodegradation of organic compounds at low concentrations. Appl Environ Microbiol. 1985 Apr;49(4):822–827. doi: 10.1128/aem.49.4.822-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Franklin F. C., Williams P. A. Regulation of the degradative pathway enzymes coded for by the TOL plasmid (pWWO) from Pseudomonas putida mt-2. J Bacteriol. 1978 Jun;134(3):757–764. doi: 10.1128/jb.134.3.757-764.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndham R. C. Evolved aniline catabolism in Acinetobacter calcoaceticus during continuous culture of river water. Appl Environ Microbiol. 1986 Apr;51(4):781–789. doi: 10.1128/aem.51.4.781-789.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Wasserfallen A., Timmis K. N. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol. 1985 Aug;50(2):447–453. doi: 10.1128/aem.50.2.447-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



