Abstract
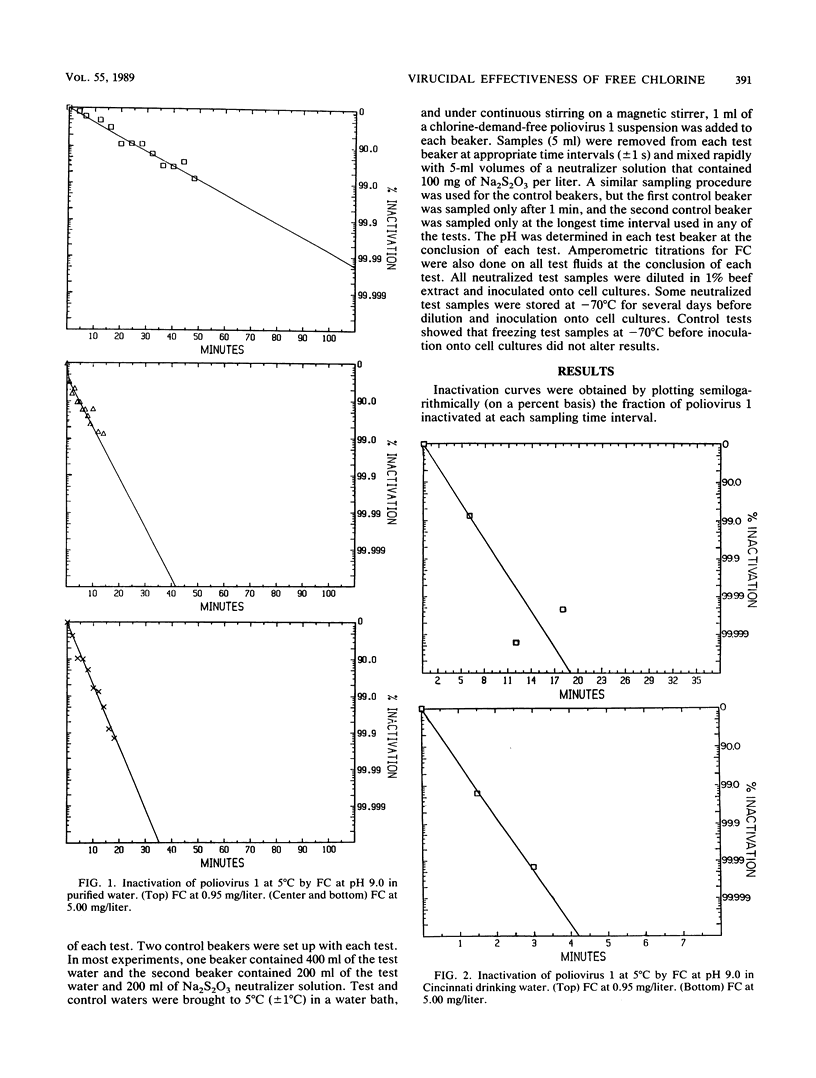
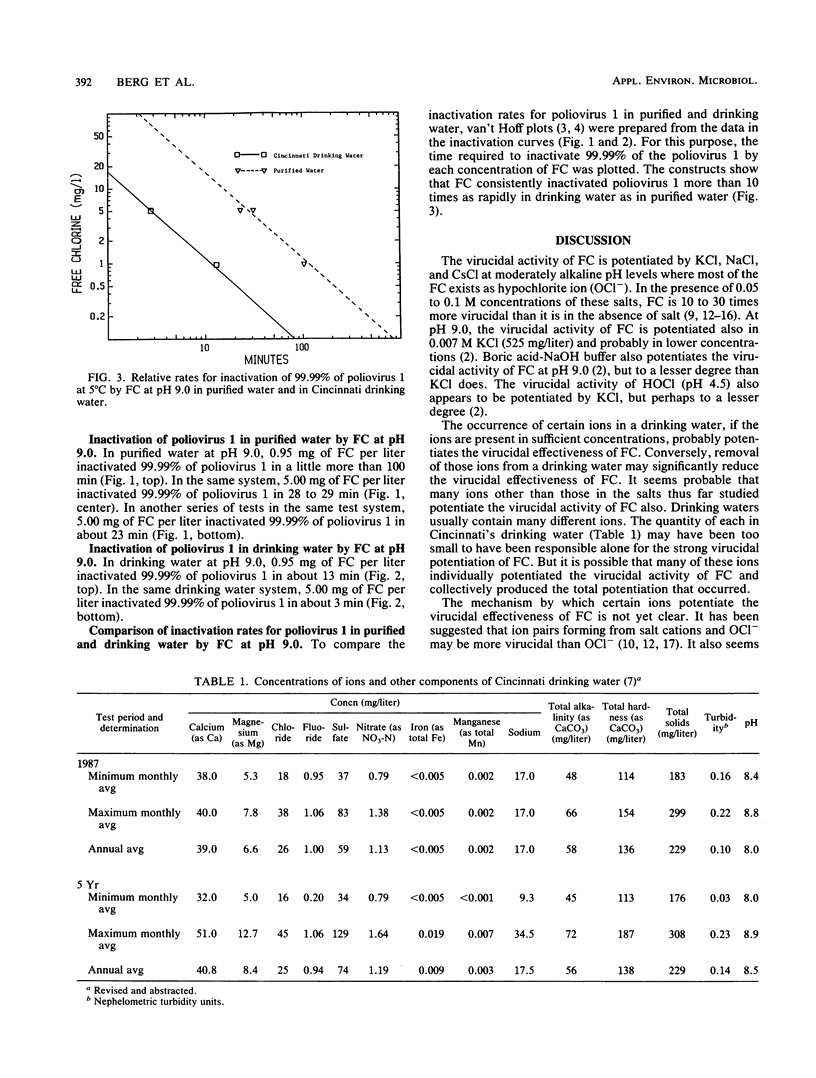
At 5 degrees C, poliovirus 1 was inactivated by free chlorine (FC) at pH 9.0 more than 10 times as rapidly in drinking water as in purified water. Because ions that comprise many salts potentiate the virucidal effectiveness of FC, we believe that ions and possible other substances in the drinking water potentiated the virucidal effectiveness of FC. Since viruses may be much more sensitive to chlorination in drinking waters than laboratory tests in purified waters have heretofore led us to believe, it may be possible to reduce the amounts of FC applied to many water supplies for disinfection and thereby perhaps reduce the quantities of halomethanes and other toxic compounds produced in these supplies by the chlorination process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERG G., CHANG S. L., HARRIS E. K. DEVITALIZATION OF MICROORGANISMS BY IODINE. I. DYNAMICS OF THE DEVITALIZATION OF ENTEROVIRUSES BY ELEMENTAL IODINE. Virology. 1964 Apr;22:461–481. doi: 10.1016/0042-6822(64)90068-6. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- Engelbrecht R. S., Weber M. J., Salter B. L., Schmidt C. A. Comparative inactivation of viruses by chlorine. Appl Environ Microbiol. 1980 Aug;40(2):249–256. doi: 10.1128/aem.40.2.249-256.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J. C., Akin E. W. Microbial resistance to disinfectants: mechanisms and significance. Environ Health Perspect. 1986 Nov;69:7–13. doi: 10.1289/ehp.86697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen H., Thomas K., Sharp D. G. Inactivation of coxsackieviruses B3 and B5 in water by chlorine. Appl Environ Microbiol. 1980 Sep;40(3):633–640. doi: 10.1128/aem.40.3.633-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Leong J. Inactivation of poliovirus I (Brunhilde) single particles by chlorine in water. Appl Environ Microbiol. 1980 Aug;40(2):381–385. doi: 10.1128/aem.40.2.381-385.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Young D. C., Floyd R., Johnson J. D. Effect of ionic environment on the inactivation of poliovirus in water by chlorine. Appl Environ Microbiol. 1980 Mar;39(3):530–534. doi: 10.1128/aem.39.3.530-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


