Abstract
The ability to monitor ongoing changes in the shape of dendritic spines has important implications for the understanding of the functional correlates of the great variety of shapes and sizes of dendritic spines in central neurons. We have monitored and three-dimensionally reconstructed dendritic spines in cultured hippocampal neurons over several hours of observation in a confocal laser scanning microscope. In the absence of extrinsic stimulation, the dimensions of dendritic spines of 3-week-old cultured neurons did not change to any significant degree over 3–4 hr in the culture dish, unlike the case with younger cultures. Releasing calcium from stores with pulse application of caffeine causes a transient rise of [Ca2+]i in dendrites and spines, monitored with the calcium dye Oregon-green. Application of caffeine to a dendrite imaged with calcein caused a fast and significant increase in the size of existing dendritic spines and could lead to formation of new ones. This effect is mediated by calcium released from the ryanodine-sensitive stores, as application of caffeine in the presence of ryanodine blocked this effect on the morphology of dendritic spines. Thus, release of calcium from stores is sufficient to produce significant changes in the shape of dendritic spines of cultured hippocampal neurons.
DDendritic spines of central neurons can undergo large, rapid, and persistent morphological changes in response to variations in ambient afferent activity. These morphological changes, including elongation, shrinkage, bifurcation or expansion of spine heads, are intuitively associated with changes in the strength of synaptic efficacy underlying long-term memory (1, 2). The molecular mechanisms as well as the functional relevance of changes in spine dimensions are not known. In earlier studies, we found that dendritic spines of cultured hippocampal neurons contain independently regulated, ryanodine-sensitive calcium stores (3). These can be triggered to release calcium into the intracellular milieu with caffeine, an effect that is blocked by ryanodine. Calcium stores have been implicated in regulation of plasticity in central neurones (2, 4–6). We now examine the hypothesis that calcium released from stores can lead to changes in the morphology of dendritic spines of cultured hippocampal neurons. We developed conditions that allow us to monitor cultured neurons over hours in vitro, reconstruct them in three dimensions (3D) by using a confocal laser scanning microscope, and detect changes in their morphology on exposure to caffeine. We found that caffeine can produce ryanodine-sensitive elongation of dendritic spines in culture over several hours of observations. These changes may have important functional implications for spine/dendrite interactions.
Materials and Methods
Hippocampal cultures were prepared as described (7). In brief, dissociated hippocampi of 19-day-old embryos were plated onto poly-l-lysine-coated 12-mm glass coverslips in Eagle’s minimal essential medium (MEM) containing 5% heat-inactivated horse serum and 5% fetal calf serum. Three-week-old cultures were used for the imaging experiments.
Imaging.
A coverslip was transferred into the recording chamber in a confocal laser scanning microscope (Leica, Heidelberg, Germany), where it was perfused with MEM equilibrated with an O2/CO2 gas mixture at ≈30°C. Drugs were prepared in the recording medium from frozen stocks before use. Glutamate (0.1 mM) or caffeine (5–10 mM) was loaded in a pressure pipette with a tip diameter of 1–2 μm, which was placed near the dendrite. Ryanodine (10 μM) was applied in the perfusion medium. The confocal laser scanning microscope is equipped with an argon-ion laser for excitation at a wavelength of 488 nm. Individual cells were impaled with micropipettes containing 10 mM Oregon green 488 Bapta-1, for imaging of [Ca2+]i, or Calcein (both from Molecular Probes), which is insensitive to intracellular calcium variations, for imaging of dendritic morphology. The dye was iontophoresed into the cell for 1–2 min and was allowed to equilibrate for at least 30 min before experiments commenced to assure equal distribution of the dye in the different cellular compartments. For each experiment, a fresh spine/dendritic segment in a new dish was used. The imaged dendrite was randomly selected, with the only provision being that it is healthy looking, sharply in focus, with spines in the same plane. There was no selection on the basis of thickness of the dendrite or density of spines. In fact, because all of the analysis was post hoc, there was no a priori reason to select a given shape or size of spines or dendrites. Furthermore, the dendrites were randomized between caffeine and control treatment, and, indeed, the mean size of the spines before treatment was the same for all groups. The dendrites were imaged with a 63× water immersion objective. The dendrites were reconstructed in 3D from stacks of successive 10–15 images spaced 0.2–0.3 μm apart. For the calcium imaging experiments, a line was scanned through the center of a dendrite/spine pair (≈0.8 msec per line) to reveal fast changes in fluorescence during a response to drug application. Laser light was reduced to 1–3% of nominal intensity. Using this setting, we were able to stimulate the same spine/dendrite pair every 5–10 min for 2–3 hr with no significant loss of reactivity attributable to dye bleaching or photodynamic damage. Also, baseline fluorescence did not change across the 2- to 3-hr observation time. Further details of the calcium imaging are presented elsewhere (3).
Morphology.
For the morphological studies, the dendrite was 3D-reconstructed at least twice, 30 min apart, before drug application. In these reconstructed dendrites, each spine could be clearly visualized, in and off the plane of optical sections, regardless of the thickness of the dendrite. The dendrites did not move or turn during the drug treatment or afterward. Drugs were then applied 3–4 times, each consisting of 3–4 short pulses. 3D-reconstructed spines/dendrites were imaged immediately and 15, 30, 60, 90, 120, and 180 min after drug treatment. The cells were still alive and well at the termination of the experiment, as judged by the smooth shape of the dendrites and the somata of the recorded cell. In case of damaged cell, the dendrites blebbed, and the dye diffused out of the cell.
Analysis of spine length and shape was conducted from the 3D-reconstructed images by using a Leica confocal laser scanning microscope, adobe photoshop (Adobe Systems, Mountain View, CA) and nih-image software. All of the spines in a single image plane were measured whereas those that were at right angle to the plane of the optical section were not measured. The 2D and 3D images were used simultaneously to identify individual spines and measure them in single optical sections. The reliability of the measurements was examined by comparing results obtained by two independent observers, in a sample of five experiments comprising 23 spines. One of the observers was blind to the experimental conditions. In these experiments, the test/retest reliability score between the sets of measurements of the two observers amounted to r = 0.96 and 0.97 for measurements before and after treatment, respectively. These correlation coefficients were highly significant. The mean values of spine sizes obtained by the two observers were also nearly identical. The rest of the measurements were therefore made by one observer.
Results
Caffeine Produces a Transient Rise of [Ca2+]i in Cultured Hippocampal Neurones.
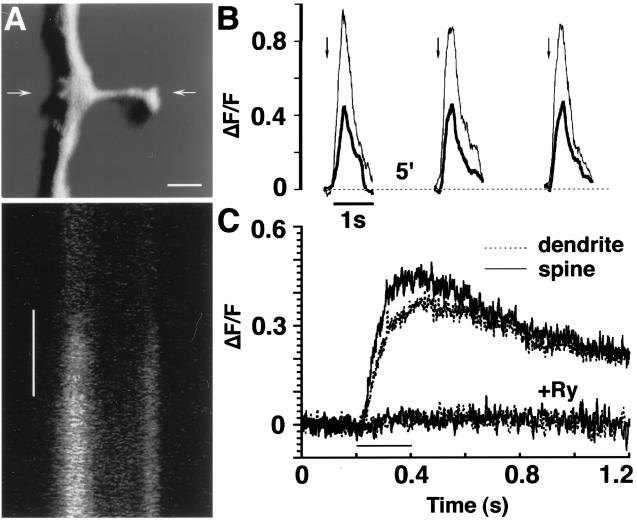
Exposure of cultured neurones to pulse application of caffeine produced a transient rise of [Ca2+]i that reached a peak within 200 msec from the onset of the response and decayed to half maximum within ≈1.2 sec (Fig. 1). This response could be evoked reliably and repetitively if spaced 5 min apart, as detailed elsewhere (3). Under these conditions, responses to caffeine were found even 3 hr after onset of the experiment. No elevation in basal fluorescence across 1 hr of treatment was obvious. The responses of spines (n = 152) and their parent dendrites to caffeine had about the same latency (Fig. 1C), but the response of the spine tended to have a larger magnitude than those of the parent dendrites (Fig. 1 B and C). Finally, the responses to caffeine were totally suppressed in the presence of ryanodine (Fig. 1C, n = 41 spines).
Figure 1.
Transient calcium responses of dendrites and spines to caffeine. (A) A sample illustration of a dendrite/spine segment loaded with Oregon green-1 and 3D-reconstructed. Arrows indicate the location of the line scanned across the spine/dendrite axis, before and during application of caffeine. (Bar = 1 μm.) (Lower) A line scan as described above. Each line is scanned for ≈0.8 msec, and the scan seen comprises ≈400 msec, from top to bottom. Caffeine (left vertical bar, 150 msec) caused a rise in calcium-sensitive fluorescence, with about the same latency in the spine and parent dendrite. (B) Example of repeated responses to caffeine in a single spine/dendrite segment comprising a spine (thin line) and dendrite (thick line). Caffeine is applied once every 5 min (arrows) with no significant reduction in magnitude of the response. (C) Summary of spine/dendrite pairs comparing responses to caffeine in control (n = 152 spines) and the presence of ryanodine (Ry, n = 41 spines). The half-time for recovery from the caffeine action is ≈1.2 sec.
Lack of Spontaneous Changes in Spine Shape in Long-Term Cultured Neurons.
Cells were stained with calcein, as detailed above, were imaged, and were 3D-reconstructed once every 15–30 min for several hours. A series of reconstructions of the dendrites and the spines taken at fixed intervals after loading of the cell with the dye showed no consistent change in spine morphology across the observation time (n = 36 spines). Furthermore, there were no apparent transient changes in spine dimensions, seen before on a minute time scale (10) and reflecting rapid movements of actin filaments in the spines. This lack of motility is in sharp contrast with the motility of filopodia seen in younger cells (refs. 7 and 8; data not shown). Thus, any significant change in shape seen below cannot be assumed to reflect spontaneous variations in spine dimensions.
Caffeine Causes Elongation of Existing Spines and Creation of New Ones.
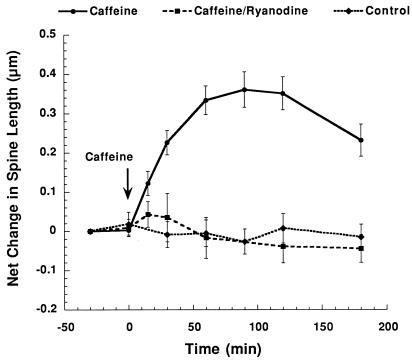
Repetitive pulse application of caffeine to calcein-filled cells caused an overall increase in length of the spines (Fig. 2 B and C). This effect could be seen in some cases already within 5 min of the first pulse of caffeine. On average, the first significant change in spine length was seen within 30 min of exposure to caffeine, and the peak change in spine length was reached within 90 min of the onset of caffeine application (Fig. 3). At this time, the change was of 0.35 ± 0.06 μm. (n = 107 spines, including all of the measured spines, in 22 experiments. The mean change of spine length of the spines that did change was 0.48 ± 0.033, n = 75 spines.)
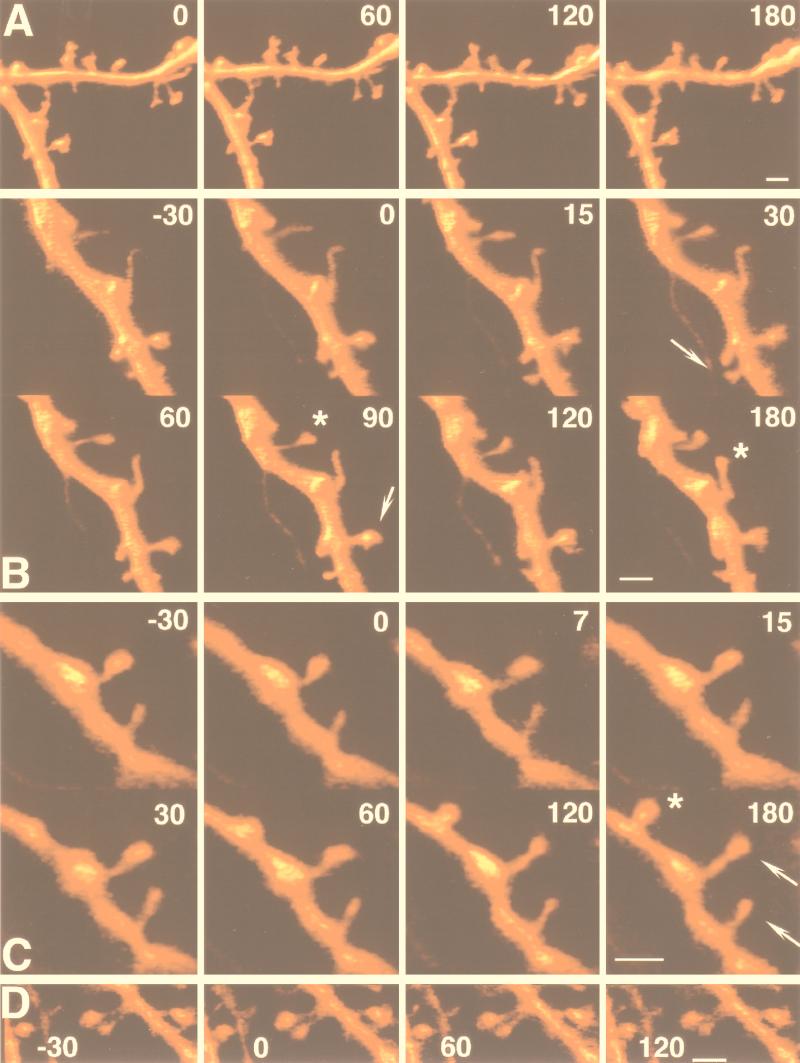
Figure 2.
Morphological changes in spines after exposure to caffeine. Cells were stained with calcein and were 3D-reconstructed in the confocal microscope at the times indicated in minutes. (A) Control dendritic segment, imaged at 1-hr intervals, showing no difference in shape or length of the dendritic spines. (B and C). Two dendritic segments, imaged before and after exposure to caffeine, applied around time 0. Note the formation of a spine head (arrows), the elongation of the spine neck of the bottom spine, and the change in position in space of the spine (asterisk). (C) Like B, a dendritic segment is exposed to caffeine at time 0, and a novel spine appears at 120 min after the onset of the caffeine treatment (asterisk). (D) Exposure to caffeine in the presence of ryanodine, under the same conditions seen in B and C. No change in spine shape or length were seen in this experiment. (Bar = 2 μm.)
Figure 3.
Time course of effects of caffeine on dendritic spines in culture. Shown is a summary diagram of changes in dendritic spines in control condition (n = 36 spines), after exposure to caffeine (n = 107 spines), and in response to caffeine in the presence of ryanodine (n = 13). A significant change in spine length is seen within 30 min of caffeine treatment whereas no change is seen in either control or in the presence of ryanodine.
Only four spines were clearly found to be formed de novo in response to caffeine in all of the 22 experiments in which dendrites were continuously monitored (e.g., Fig. 2C). In none of the control cases was there a detection of novel spines. The sample of novel spines was too small to draw conclusions concerning any possible morphological differences from existing spines.
The spine changes in response to caffeine are likely to be mediated by release of calcium from ryanodine-sensitive calcium stores because preincubation of the culture with ryanodine (10 μM) eliminated caffeine effects (two experiments, 13 spines studied; Figs. 2D and 3).
This effect of caffeine is strikingly different from the effect of glutamate applied near spiny dendrites in that glutamate caused a marked (20–40%) and rapid shrinkage of existing spines and did not cause elongation or formation of novel ones [data not shown; results are similar to those reported previously (9)]. The disparity between caffeine and glutamate actions requires further examination, but, at the present time, it is obvious that calcium influx via glutamate gated channels does not mimic the effects of caffeine.
In an attempt to dissect out factors that contribute to the caffeine-induced change in spine length, we found that the density of existing spines on the parent dendrite is a significant predictor of the response of the spines to caffeine. Thus, dendrites with a low density of spines (1–2 spines per 20-μm dendritic segment) are less likely to react to caffeine (17.8% responding, 5 of 28 spines), in comparison to dendrites with medium density (3–4 spines per 20-μm dendrite) (62.8% responding, 22 of 35 cases) and high density of spines (>4 spines per 20-μm dendrite) (80.7% of spines responding, 46 of 57 cases). This indicates that some regions of the dendrite of a given neuron have a higher propensity to react to extrinsic stimulation and will accumulate spines and that these regions will produce a better growth response to caffeine.
The thickness of the dendrites appears to be a factor that is correlated with the ability to promote growth of spines in response to caffeine; thin dendrites (1–3 μm in diameter) produced more cases of spine growth (62%) than thicker dendrites (37% of the cases). The dendrites taper off centrifugally, and, because thick dendrites have a lower spine density, this may indicate that reactive spines are more likely to be found on thinner, more remote dendrites than on thicker, proximal dendrites.
Because there is an intrinsic heterogeneity in dendritic spine dimensions, we examined the relations between the initial length of the spine and its likelihood to elongate in response to caffeine. Two independent processes were examined:
The size of the spine head.
Because elongation of the spine may reflect a change in the spine head, we measured the size of the heads, and they appear not to contribute to the change in the length of the spines in responses to caffeine: The spine head diameter in controls was 1.14 ± 0.11 μm and after caffeine was 1.11 ± 0.12 μm. This indicates that the growth of the spine is not taking place by changing the spine head dimensions.
The size of the spine neck.
The initial size of the spine seems to correlate with the magnitude of the response. In general, the shorter spines (average length, including the spine head, 0.61 ± 0.04 μm) expressed a significantly smaller change (0.43 ± 0.04 μm, n = 28) than the long ones (initial length 2.86 ± 0.17 μm, mean change 0.6 ± 0.058 μm, n = 19, t = 2.40, P < 0.02). The intermediate-sized spines (initial size 1.46 ± 0.04 μm, change of 0.45 ± 0.06 μm, n = 28) were not different from the short ones.
These experiments indicate that the type and magnitude of the response to caffeine can be predicted by the morphological characteristics of the parent dendrite and the spines undergoing the change. Further experiments are needed to analyze the intracellular factors underlying this unique response.
Discussion
Dendritic spines of 3- to 4-week old cultured hippocampal neurons are fairly stable and do not change their shape or size spontaneously in our testing conditions. This is distinctly different from the case of the less mature neurons, 1–2 weeks in culture, in which dendritic filopodia are highly motile and can expand and shrink within minutes (8). The variations in spine motility may be attributable to the fact that, in the growing neurones, axonal branches are searching for targets and may stabilize only on formation of functional synaptic connections with the postsynaptic neuron. Parenthetically, even our “mature” neurons are not so similar to the neurons in vivo that have a much higher spine density. At any rate, we found no changes in spine dimensions over several hours of observations in a resting state of the more mature cultured neurons. In this respect, our results are different from a recent report suggesting that actin undergoes spontaneous and rapid fluctuations in size that affect spine dimensions (10). This discrepancy may be attributable to different age or growth conditions of the cultures or to the fact that, in their study, Fischer et al. (10) imaged green fluorescent protein-linked actin, which may not necessarily reflect directional changes in the entire spine but local fluctuations about the same mean location of the spine, the significance of which is still not obvious. In a recent study with slice cultured neurons, in which novel dendritic spines have been formed in correlation with plasticity-producing stimuli, no spontaneous changes in spine density were reported (11).
It has been reported recently (9, 12) that topical application of glutamate can cause a rapid collapse of dendritic spines. This is diametrically opposite to the effect seen in the present study, in which the vast majority of the spines were elongated after exposure to caffeine. The difference between the two effects may result from the nature of the stimulus used to evoke a rise in [Ca2+]i, which underlies the change in spine morphology; in the glutamate case, calcium influx is the main source of the elevated [Ca2+]i whereas in the caffeine case, [Ca2+]i rises only after its release from stores. Another major difference between the effect of caffeine and glutamate is that glutamate application causes a large and prolonged rise in [Ca2+]i that can reach saturation whereas caffeine can only produce a transient and relatively small (200–400nM) change in [Ca2+]i. Taken together, these results suggest that a modest and transient rise in [Ca2+]i, such as the one produced by caffeine, will cause an elongation of dendritic spines whereas a larger and more sustained rise in [Ca2+]i will cause a collapse of dendritic spines. A similar dual role of a change in [Ca2+]i has been suggested for the regulation of growth cone motility (13). Furthermore, different biochemical pathways leading to different gene expression may be activated by different patterns of local [Ca2+]i changes (14). The assumed involvement of metabotropic receptors in synaptic processes leading to neuronal plasticity (2, 15) is consistent with this hypothesis, as the activation of metabotropic receptors leads to release of calcium from stores and is likely to cause a smaller rise in [Ca2+]i than the rise produced by influx of calcium through the ionotropic receptors. On the other hand, synaptic activation of glutamate receptors has been suggested to cause release of calcium from stores, which serve as the main source for the increased [Ca2+]i (16). Thus, the role of stores in the elevation of [Ca2+]i may even be more important than originally proposed.
A major issue in assessing the rules governing spine plasticity is the localization of the novel spine—could we predict where a new spine will be formed? That the dendrite is not a homogenous compartment has already been alluded to in previous studies; Moser et al. (17) found that the formation of novel spines is limited to certain segments on the dendritic tree, which may be more plastic or may contain higher concentrations of afferent fibers. Also, Jones et al. (18) found that the main change in experience-induced plasticity is in multiple synapses in the visual cortex. We found that dendritic segments that already have a high concentration of spines are more likely to express spine plasticity in response to stimulation than dendrites that have fewer spines. That indicates that the dendrite is indeed inhomogeneous and different dendrites can grow different densities of spines. This observation may have important implications for spine/spine interactions in control of dendrite excitability (19).
The change in spine dimension is pretty modest. An average increase of ≈33% in spine length was seen in the present study. Although this may not be a large and persistent change, it has been shown recently (19) that such a change in spine length will have an important implication for spine/dendrite interaction and for the ability to communicate calcium changes between the two compartments.
Acknowledgments
We thank Ms. V. Greenberger for the preparation of the cultures and Y. Lapid for the help with the confocal microscope. This work was supported by a grant from the U.S.–Israel Binational Foundation.
Abbreviation
- 3D
three-dimensional.
References
- 1.Harris K M, Kater S B. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 2.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Korkotian E, Segal M. Eur J Neurosci. 1998;10:2076–2084. doi: 10.1046/j.1460-9568.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 4.Meldolesi J, Villa A, Podini P, Clementi E, Zacchetti D, D’Andrea P, Lorenzon P, Grohovaz F. J Physiol (Paris) 1992;86:23–30. doi: 10.1016/s0928-4257(05)80004-x. [DOI] [PubMed] [Google Scholar]
- 5.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Physiol Rev. 1994;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 6.Schiegg A, Gerstner W, Ritz R, Van Hemmen J L. J Neurophysiol. 1995;74:1046–1055. doi: 10.1152/jn.1995.74.3.1046. [DOI] [PubMed] [Google Scholar]
- 7.Papa M, Bundman M C, Greenberger V, Segal M. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziv N E, Smith S J. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 9.Segal M. Neurosci Lett. 1995;193:73–76. doi: 10.1016/0304-3940(95)11665-j. [DOI] [PubMed] [Google Scholar]
- 10.Fischer M, Kaech S, Knutti D, Matus A. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 11.Engert F, Bonhoeffer T. Nature (London) 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 12.Halpein S, Hipolito A, Saffer L. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kater S B, Davenport R W, Guthrie P B. Prog Brain Res. 1994;102:49–60. doi: 10.1016/S0079-6123(08)60531-2. [DOI] [PubMed] [Google Scholar]
- 14.Finkbeiner S, Greenberg M E. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- 15.Weiler I J, Greenough W T. Proc Natl Acad Sci USA. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emptage N, Bliss T V, Fine A. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 17.Moser M B, Trommald M, Egeland T, Andersen P. J Comp Neurol. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Jones T A, Klintsova A Y, Kilman V L, Sirevaag A M, Greenough W T. Neurobiol Learn Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- 19.Volfovsky N, Parnas H, Segal M, Korkotian E. J Neurophysiol. 1999;82:450–462. doi: 10.1152/jn.1999.82.1.450. [DOI] [PubMed] [Google Scholar]