Abstract
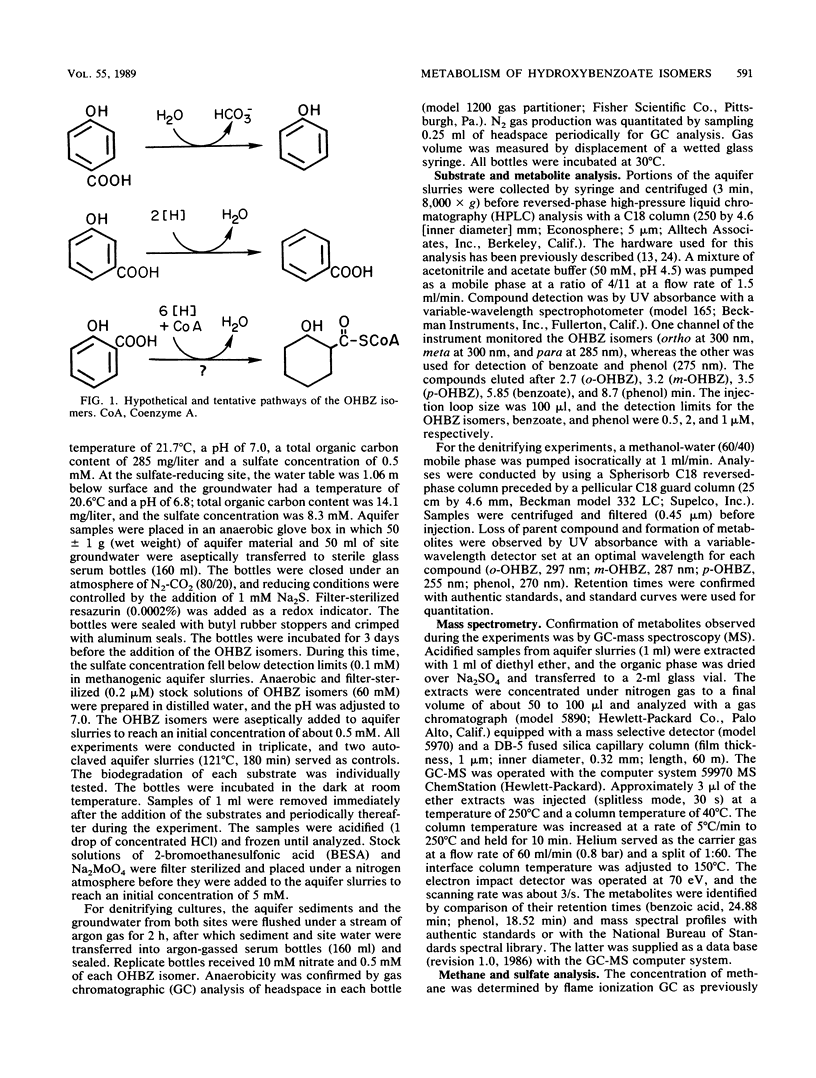
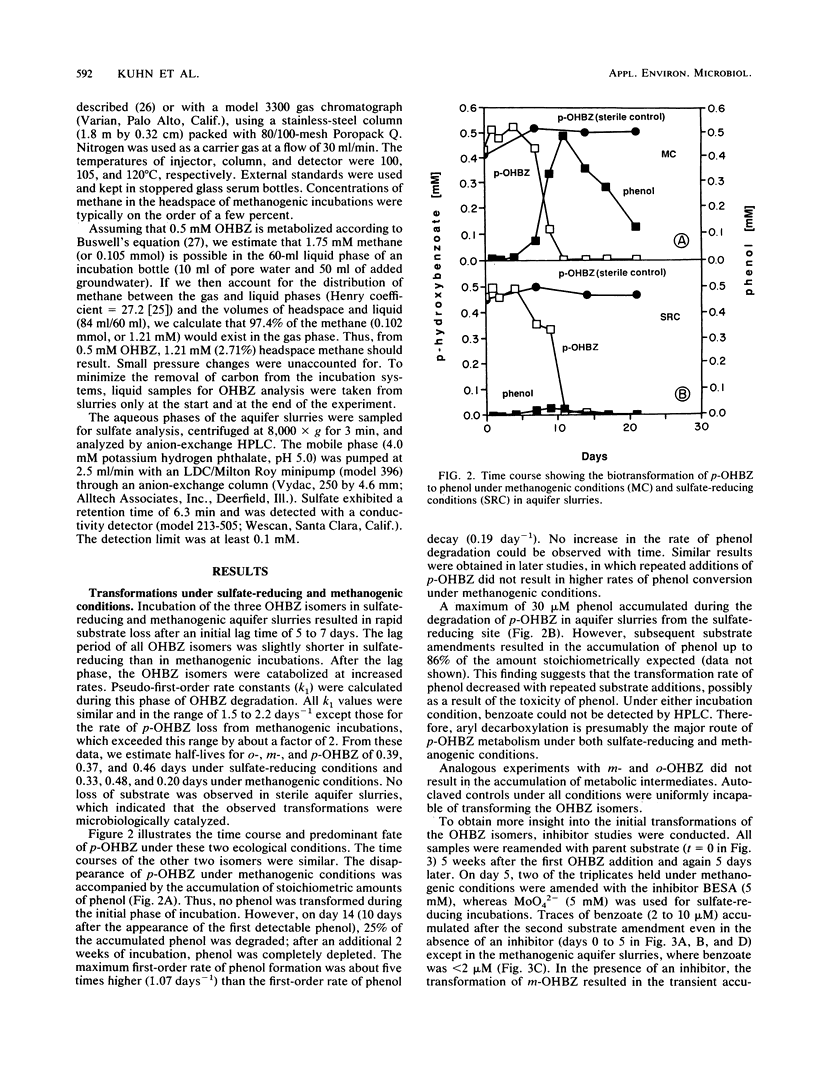
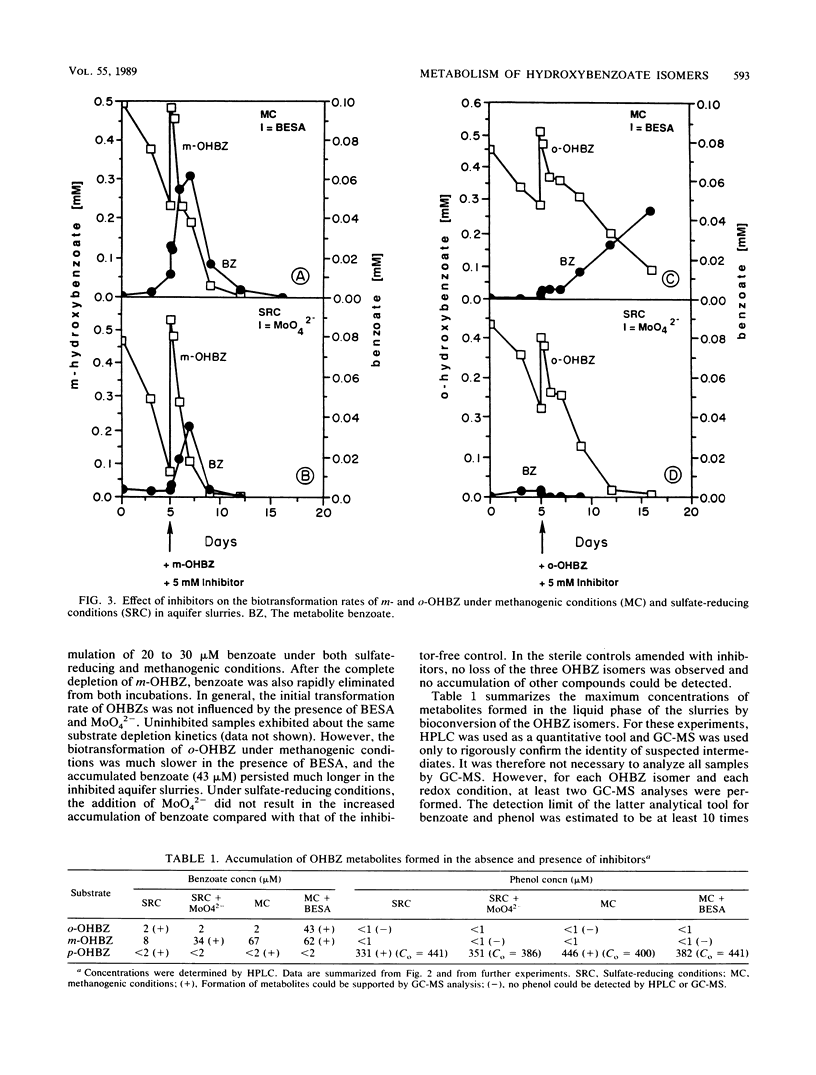
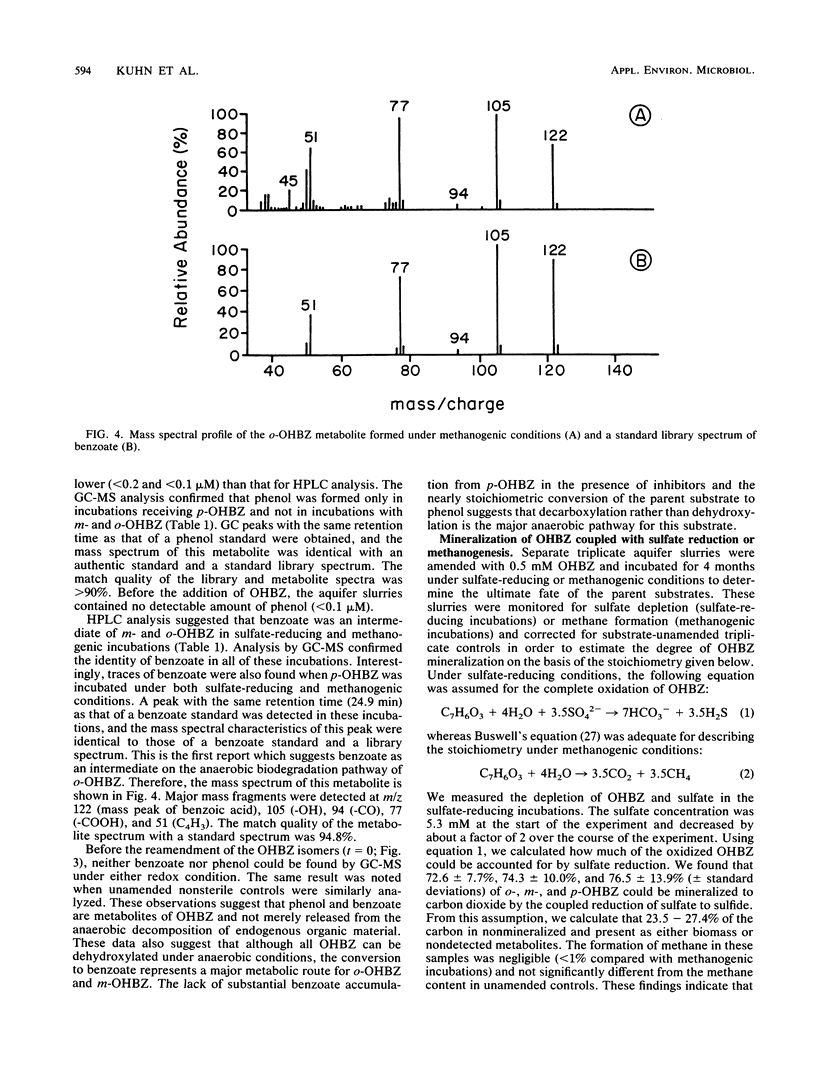
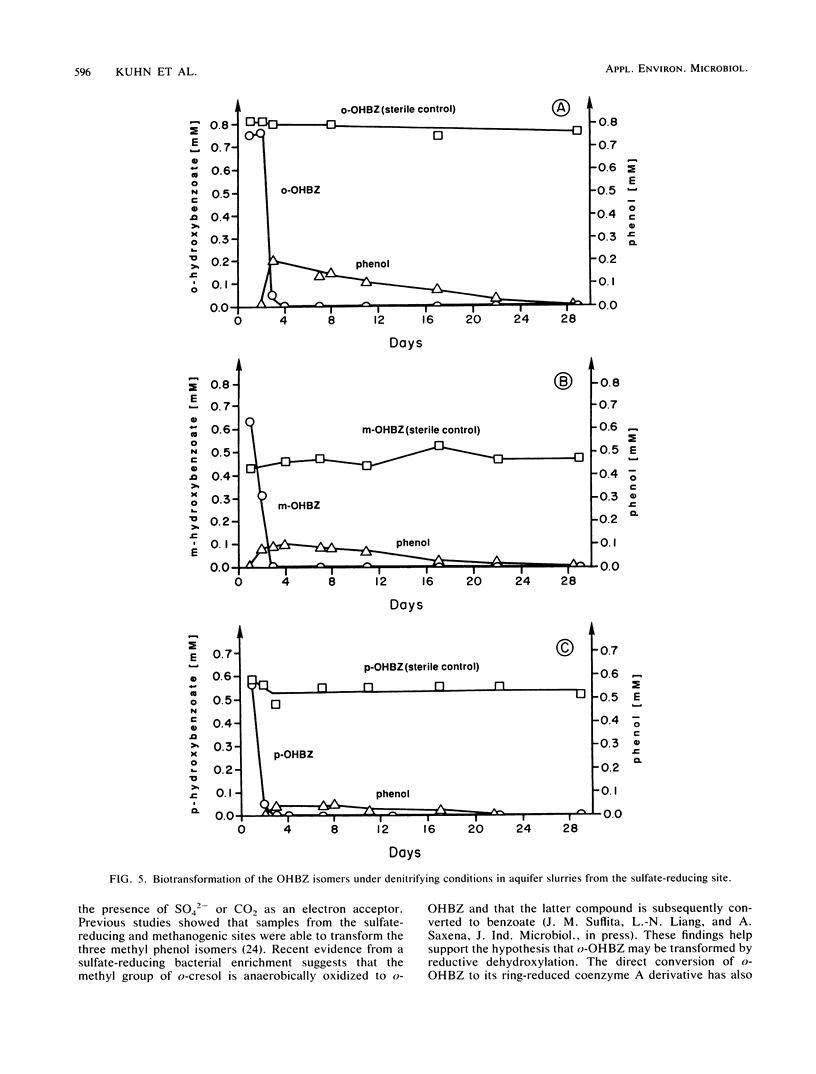
The biodegradation of hydroxybenzoate isomers was investigated with samples obtained from two sites within a shallow anoxic aquifer. The metabolic fates of the substrates were compared in denitrifying, sulfate-reducing, and methanogenic incubations. Under the latter two conditions, phenol was detected as a major intermediate of p-hydroxybenzoate, but no metabolites were initially found with m- or o-hydroxybenzoate. However, benzoate accumulation was noted when metabolic inhibitors were used with these samples. About 9 to 17 days was required for >95% removal of the parent isomers under these conditions. When aquifer slurries were amended with nitrate, the equivalent removal of the hydroxybenzoates occurred within 4 days. In the denitrifying incubations, phenol was formed from all three hydroxybenzoates and accounted for about 30% of the initial substrate amendment. No benzoate was measured in these samples. All metabolites were identified by chromatographic mobility, mass spectral profiles, or both. Autoclaved controls were uniformly incapable of transforming the parent substrates. These results suggest that the anaerobic fate of hydroxybenzoate isomers depends on the relative substitution pattern and the prevailing ecological conditions. Furthermore, since these compounds are central metabolites formed during the breakdown of many aromatic chemicals, our findings may help provide guidelines for the reliable extrapolation of metabolic fate information from diverse anaerobic environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H. A. Amino acid degradation by anaerobic bacteria. Annu Rev Biochem. 1981;50:23–40. doi: 10.1146/annurev.bi.50.070181.000323. [DOI] [PubMed] [Google Scholar]
- Berry D. F., Francis A. J., Bollag J. M. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol Rev. 1987 Mar;51(1):43–59. doi: 10.1128/mr.51.1.43-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert I. D., Young L. Y. Anaerobic oxidation of p-cresol by a denitrifying bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1117–1122. doi: 10.1128/aem.52.5.1117-1122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Gibson J. Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris. Appl Environ Microbiol. 1988 Mar;54(3):712–717. doi: 10.1128/aem.54.3.712-717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilpert W., Schink B., Dimroth P. Life by a new decarboxylation-dependent energy conservation mechanism with Na as coupling ion. EMBO J. 1984 Aug;3(8):1665–1670. doi: 10.1002/j.1460-2075.1984.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janshekar H., Fiechter A. Lignin: biosynthesis, application, and biodegradation. Adv Biochem Eng Biotechnol. 1983;27:119–178. doi: 10.1007/BFb0009107. [DOI] [PubMed] [Google Scholar]
- Jenneman G. E., McInerney M. J., Knapp R. M. Effect of nitrate on biogenic sulfide production. Appl Environ Microbiol. 1986 Jun;51(6):1205–1211. doi: 10.1128/aem.51.6.1205-1211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E. P., Zeyer J., Eicher P., Schwarzenbach R. P. Anaerobic degradation of alkylated benzenes in denitrifying laboratory aquifer columns. Appl Environ Microbiol. 1988 Feb;54(2):490–496. doi: 10.1128/aem.54.2.490-496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenski W. J., Suflita J. M. Biodegradation of cresol isomers in anoxic aquifers. Appl Environ Microbiol. 1987 Apr;53(4):710–716. doi: 10.1128/aem.53.4.710-716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Campbell W. L., Chinoy I. Anaerobic degradation of the benzene nucleus by a facultatively anaerobic microorganism. J Bacteriol. 1970 May;102(2):430–437. doi: 10.1128/jb.102.2.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschech A., Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987 Sep;148(3):213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- Vogel T. M., Grbìc-Galìc D. Incorporation of Oxygen from Water into Toluene and Benzene during Anaerobic Fermentative Transformation. Appl Environ Microbiol. 1986 Jul;52(1):200–202. doi: 10.1128/aem.52.1.200-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L. A., Johnson K. A., Robinson I. M., Yokoyama M. T. Isolation from swine feces of a bacterium which decarboxylates p-hydroxyphenylacetic acid to 4-methylphenol (p-cresol). Appl Environ Microbiol. 1987 Jan;53(1):189–192. doi: 10.1128/aem.53.1.189-192.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


