Abstract
Crossed antagonism between activities in neurons subserving alternating movements such as swimming or walking has been described in a number of systems. The role of reciprocal inhibition has been implicated in these activities, but involvement of rhythmic ongoing fluctuations of membrane potential, called synaptic “noise,” has not been examined. In the Mauthner (M) cells, which control the direction of escape, this activity is inhibitory. We report that in the zebrafish (Danio rerio), inhibitory synaptic noise exhibits prolonged bursts of rhythmic, inhibitory postsynaptic potentials, which attenuate the M cell’s sensibility to excitatory sensory drives. Furthermore, paired intracellular recordings have shown that inhibitory synaptic noise alternates between two distinct states, noisy and quiet, which are out of phase in the two cells. Firing of either M cell resets this pattern by reducing the inhibition in the contralateral one. This suggests that an avoidance reflex in one direction may favor initiation, by the opposite M cell, of a subsequent escape toward a more appropriate location.
Mauthner (M) cells are responsible for determining the initial left or right direction of the escape reaction in response to aversive stimuli. Only one of them fires an action potential (1, 2) and, thus, governs activity in the relevant spinal networks. M cell excitability is controlled by two sets of presynaptic inhibitory interneurons (Fig. 1A), which ensure that the cell fires only in response to relevant sensory inputs (3). The electrophysiology and anatomy of these interneurons have been characterized in the goldfish (4–6) and the zebrafish (7). They include (i) second-order vestibular commissural interneurons, which are activated by primary auditory fibers via mixed (electronic and chemical) synapses and regulate both M cells’ threshold, and (ii) ipsilateral recurrent collateral interneurons, disynaptically activated by collaterals of both M axons. Their glycinergic (8) terminal synapses generate in the M cell an intense inhibitory synaptic noise (ISN), which, as suggested by models, modifies the input–output relation of this neuron (9). In this work we demonstrate an alternating, and bilateral control, of this background activity.
Figure 1.
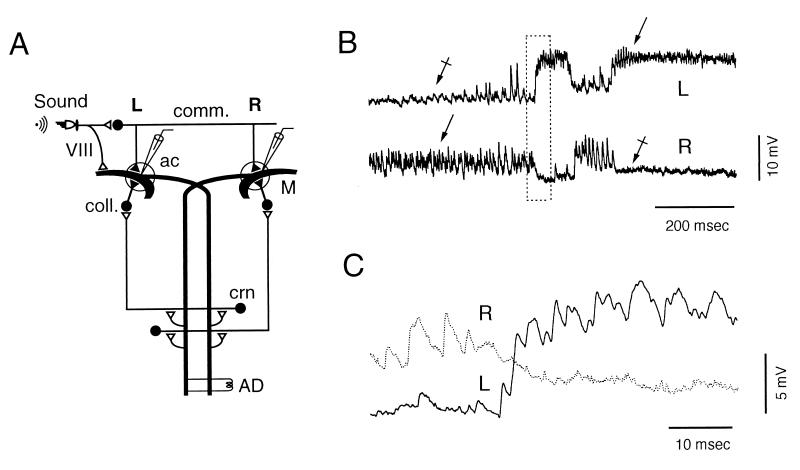
Alternate states of ISN. (A) Diagram of the experimental set-up and of M cells’ inhibitory networks. Action potentials were induced in the left (L) or the right (R) M cells by antidromic (AD) stimulation of the M axon(s) or by brief, intracellular current injections (≈1 msec) through the recording microelectrode. Both procedures activated the M cell ipsilateral recurrent pathway via the cranial relay neurons (crn), which synapse on collateral inhibitory interneurons (coll.). The commissural inhibitory interneurons (comm.), which terminate on both M cells, were activated by auditory stimuli via VIII nerve primary fibers. ac, axon cap. (▴ and ▵) Inhibitory and excitatory synapses, respectively. (B) Simultaneous intracellular recordings from both M cells. Bursts of fast IPSPs (arrows), present during the noisy state, disappear during the subsequent quiet period (crossed arrows). (C) Boxed region in B displayed at higher magnification and faster sweep speed.
Methods
Physiology.
Adult zebrafish (Danio rerio) were obtained from Bio International (Nantes, France). Animal care procedures were in accordance with local requirements. The procedures for recording from identified zebrafish M cells have been described in detail (7). Experiments were performed on animals anaesthetized with MS222 (0.2 g/liter 3-aminobenzoic acid ethyl ester/methanesulfonate salt; Sigma) and immobilized with Pavulon (pancuronium bromide, ≈1 μg/g body weight; Organon Teknika–Cappel). Intracellular recordings in the current–clamp mode were obtained from the M soma or lateral dendrite by using 2.5- to 4-MΩ electrodes filled with 3 M KCl, so that inhibitory postsynaptic potentials (IPSPs) were reversed in the depolarizing direction. All experiments were performed at room temperature (18–25°C).
Data Collection and Measurements.
Records were digitized at a sampling rate of 48 kHz and stored on tape with a digital tape recorder, DTR-1404 (Biologic Science Instruments, Claix, France). Continuous data segments were acquired by a computer (Power Macintosh 8100/100AV) at a sampling rate of 12 kHz and were analyzed by using updated versions of the program detectivent, written in labview (10).
Results and Discussion
The spontaneous unitary IPSPs comprising ISN had a rise time of 0.44 msec ± 0.04 and a decay time constant of 1.8 msec ± 0.3 (mean ± SD; n = 45 events from two cells). As in the goldfish (8), they were produced by presynaptic action potentials and mediated by glycine-activated channels because they were sensitive to tetrodotoxin (TTX) and strychnine (data not shown).
In an initial series, 32 M cells were recorded unilaterally. Despite variabilities between cells, we noticed that one of two states could exist: a “noisy” one, made of bursts of large IPSPs, and a “quiet” state. In 28 cells, switches between the two states occurred either spontaneously or after appropriate stimulation (see below), whereas in the remaining four cells, only one state prevailed. The duration of each successive phase was variable among, and within, cells. In three cells, which were studied for at least 5 min, the length of the noisy state ranged from 14 msec to 4 min. Pooled data on 102 bursts indicated that the duration of the majority of them (77%) was clustered and ranged from 14 to 302 msec, whereas that of the second group was scattered, lasting from 603 msec to 4 min before transitions occurred.
Paired recordings from both M cells (Fig. 1 B and C) showed that these distinct patterns were complementary: when one cell was in the noisy state, the other was quiet, and vice versa. Reciprocal transitions between states were simultaneous and did not require impulses in either of the cells. Again, the distribution of burst durations (not shown) was bimodal and split in two categories. The first group, comprising 74% of the bursts, ranged from 9 to 312 (mean = 146 msec ± 78, n = 64). It was accompanied by a near-complete silence in the opposite side, ranging from 5 to 304 msec (mean = 137 msec ± 77), with a nonsignificant difference between these values (one-tailed Student’s t test, 126 df, t = 0.65). The second group of bursts lasted from 314 min up to several minutes, with a near-complete silence in the opposite side.
We activated the M cell in one of three ways to determine whether it contributes to the feedback regulation of ISN. These were (i) spinal stimulations (n = 32 fish), which excite antidromically both M axons or only one of them, (ii) spontaneous spikes (n = 18 of 32) because of large Cl− loading, and (iii) transmembrane current injections (six independent experiments). The last two protocols induced spikes in the recorded cell alone. Comparison of their effects revealed certain trends.
After stimulations of the spinal cord, the results could be categorized into three types: suppressive, inductive, and variable (n = 8, 4, and 20 cells, respectively). When a cell was in the noisy state, suppressive stimulation induced a transient disappearance of spontaneous IPSPs (Fig. 2A) in 99% of trials (n = 92) during 42–500 msec (mean = 164 msec ± 110, n = 40). In contrast, the same stimulation had no effect on an initial quiet state, as in Fig. 2C (0% effect, n = 137 trials). In the inductive type and during the quiet state, spinal stimulations always induced short trains of IPSPs lasting from 44 to 560 msec (mean = 165 msec ± 120, n = 61 trials), whereas during the noisy state the stimulus was without effect. In variable cells, an average of 38% suppression and 63% induction was obtained at random (during the noisy and quiet states, respectively). At this stage, further interpretation of the data was difficult because it was not possible to determine whether one of the two M cells had been activated selectively.
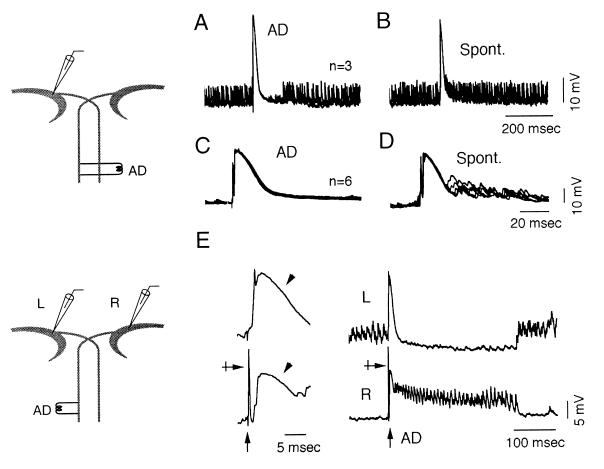
Figure 2.
Differential effects of M cell firing. (A and B) Superimposed (n = 3) intracellular recordings from a suppressive M cell. (A) A spinal stimulus (AD) produced a transient suppression of the ISN. (B) A spontaneous discharge in the zebrafish M cell increased the rate of spontaneously occurring IPSPs. (C and D) Recordings at a faster sweep speed, from the same cell as above, during a quiet period (n = 6 trials). (C) A spinal stimulation produced only an antidromic spike and a collateral IPSP. (D) A spontaneous discharge of the M cell was followed by an awakening of the ISN. (E) Simultaneous recordings at fast (Left) and slow (Right) sweep speeds from two M cells before and after a spinal stimulus (AD). The strength of the stimulation was adjusted to excite only the right cell (crossed arrow). A collateral IPSP (arrowheads) was induced in both cells but was followed by a transient burst of IPSPs in the activated neuron alone.
Two sets of observations address the issue of the opposing effects of the M cells. First, in one experiment, no spike preceded the collateral IPSPs, indicating that the latter were induced by spontaneous activation of the opposite M cell (11). Ongoing bursts were consistently suppressed for 87–432 msec (mean = 187 msec ± 96, n = 13) after these collateral IPSPs. Second, firing of the recorded M cell clearly boosted the ISN in the seven cells of the suppressive type. That is, the IPSPs were not suppressed (Fig. 2B) after a spontaneous discharge (0%, n = 62 trials) whereas when cells were in a quiet state, spontaneous firing induced bursts of IPSPs (Fig. 2D) lasting from 43 to 477 msec (mean = 133 msec ± 91, n = 32 trials).
Taken together, these data suggest that ISN is enhanced when the M cell is activated and suppressed when the contralateral cell fires, therefore, resetting an alternation that occurs in the absence of M cell firing. This conclusion was confirmed by results obtained while recording simultaneously from both M cells. First, paired recordings (n = 6 fish) showed that antidromic activation of one M cell consistently induced trains of ISN in that cell, whereas spontaneous IPSPs were suppressed in the contralateral side (Fig. 2E). Also, brief (≈1 msec) transmembrane current pulses that fired the cell induced similar bursts (145 msec ± 119, n = 110 from six cells) and an enhancement of ISN starting 11.9 ± 4 msec (n = 113 from six cells) after the peak of the evoked action potential (range, 5–19 msec). Second, in another series, recordings from both M cells showed that bursts of IPSPs in one M cell (ranging from 36 to 340 msec, mean = 150 msec ± 78) were accompanied by a suppression in the other one for an equivalent amount of time (ranging from 24 to 327 msec, mean = 147 ± 78; n = 80 trials from five fish) as in Fig. 3 A and B. Similarly, a spontaneous firing of the nonactivated cell suppressed synaptic noise in the opposite side. Thus, overall, alternating patterns in the left and right M cells remained the rule.
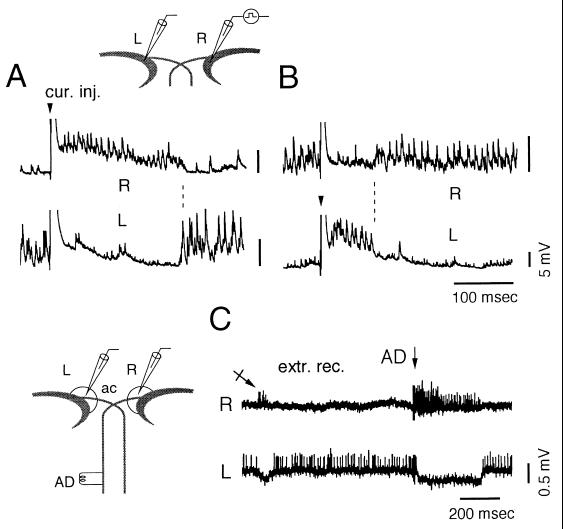
Figure 3.
Reciprocal modulation of ISN. Simultaneous recordings from the left (L) and right (R) M cells and selective activation of one of them. (A and B) A directly evoked spike (truncated, arrowhead) followed by a collateral IPSP and a burst of noise were produced in the right (A) and left (B) M cells. ISN was suppressed simultaneously in the opposite side. (C) Simultaneous extracellular recordings from the right (Upper) and left (Lower) axon caps (circled areas) from another experiment. Note the spontaneous alternation of firing in both axon caps (crossed arrow) and after a spinal stimulation (AD), which presumably activated only the right M cell (arrow).
The network that controls this switch has not been fully characterized. However, simultaneous extracellular recordings of population spikes in both axon caps that contain axons of inhibitory interneurons projecting to the M cell soma support the notion of a reciprocal control because action potentials were not present simultaneously in both sides (Fig. 3C). Furthermore, antidromic stimulation induced trains of spikes of the same duration as that of intracellularly recorded bursts (ranging from 25 to 330 msec, mean = 133 msec ± 96, n = 19 trials in three fish) accompanied by a suppression of activity in the contralateral axon cap, for periods ranging from 35 to 382 msec (mean = 161 msec ± 120).
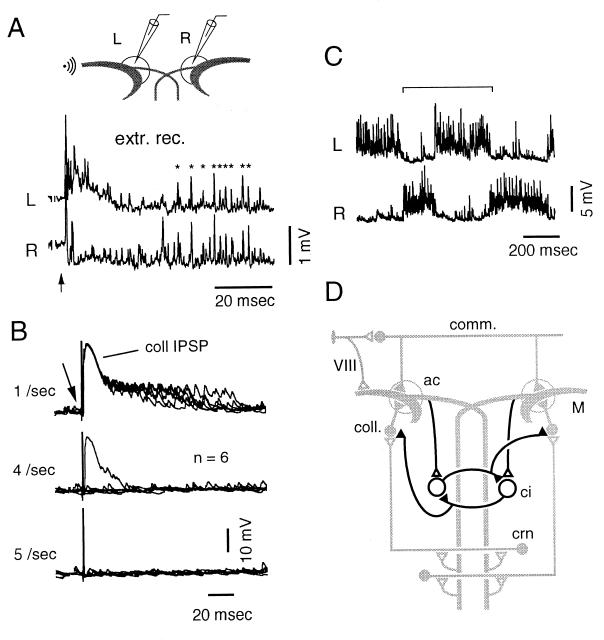
M cells’ inhibitory interneurons that have their axons in the axon cap have been named PHP neurons (4) because they exhibit a passive hyperpolarizing potential when the M cell fires. One set is the commissural cells (Fig. 1A), which respond to sound. Their involvement in the switch of ISN is unlikely because auditory stimuli evoked population spikes in both axon caps (Fig. 4A). Rather, the neurons implicated in this control share some basic properties with the collateral interneurons: as demonstrated in goldfish (11) and zebrafish (7), spinal stimulations become ineffective at rates more than four to five per sec because of a fatigue of the collateral network; induction of ISN similarly was suppressed at such frequencies (Fig. 4B).
Figure 4.
Properties of inhibitory responses and network model accounting for the alternation of ISN. (A) Spikes evoked by sound (500 Hz, 35-msec duration, 70 dB; arrow) extracellularly recorded in the left and right (L and R) axon caps (circled areas) were correlated (asterisks) in presumed commissural neurons (same experiment as in Fig. 3E). (B) Simultaneous failure to induce the collateral IPSP and ISN after direct activation (arrow) of the M cell at rates more than four to five per sec. (C) Typical rhythmic bursts (between brackets) recorded from another M cell. (D) Putative network (thick lines) accounting for the transition between states incorporated in the diagram of Fig. 1A (shaded here). Collateral interneurons (coll.) are inhibited by hypothetical, mutually connected crossed interneurons (ci), which can be activated by collaterals of the opposite M cell axon.
Occasional irregular rhythmic cycles including a noisy and a quiet state (Fig. 4C) occurred with a frequency of two to six per sec (mean = 3.6 Hz ± 1.2, n = 28 cycles from three cells). This pattern is similar to those induced by central pattern generators (CPGs), which produce left–right alternating rhythmic outputs during fictive swimming (12–15) and walking (16). Fig. 4D illustrates the supramedullary network, which could generate such a periodic process in the M cell and is consistent with schemes developed for CPGs (13, 14). It comprises sets of crossed inhibitory interneurons that reciprocally inhibit each other and provide a negative control over collateral-like inhibitory neurons. Although not demonstrated directly, this scheme is supported by the fact that crossed interneurons that terminate unilaterally have been identified at all levels of fish spinal cord (17). It implies that these interneurons belong to a set of cells that are either intrinsically active or excited from elsewhere and that fire bursts of grouped action potentials, as do some collateral interneurons in the goldfish (18).
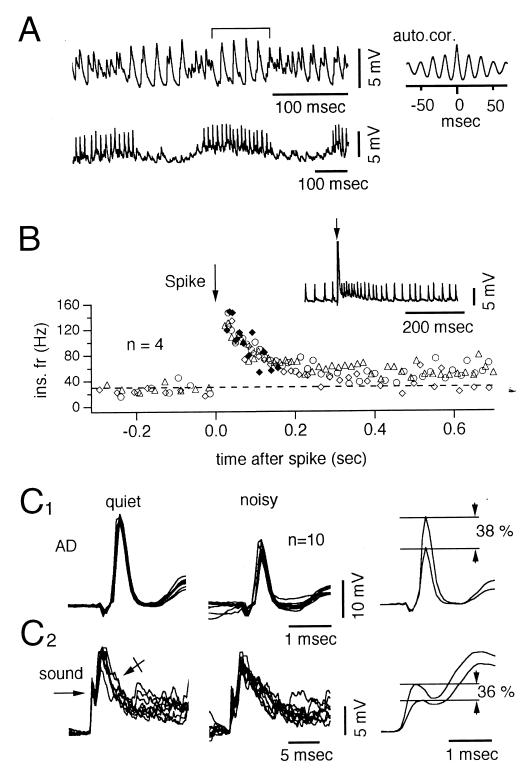
Autocorrelations of 1-sec segments of noisy state indicated that in more than half of the cells, the dominant frequency of the IPSPs was 26–100 Hz (mean = 71 ± 19, n = 26 cells), i.e., in the same range as the so-called gamma rhythm, reported in mammals including primates (19- 21) and also in the goldfish M cell (22). This ISN frequency was constant in some cells (simple cells, Fig. 5A Lower) and intermittent in others (intermittent cells, Fig. 5A Upper). In 15 cells of the intermittent group, conspicuous waves of IPSPs occurred at a mean frequency of 86 Hz ± 18 (Fig. 5A Upper). These events obviously were produced by the synchronous activation of several inhibitory cells, given the large size of the periodic IPSPs, and they were interrupted by smaller-sized periods of higher frequencies (up to 500 Hz). In the 11 simple cells (Fig. 5A Lower), IPSPs occurred at 57 Hz ± 17 and their amplitude (which averaged 20 ± 4% that of the full collateral) indicated that the rhythmicity of ISN involves the activity of several synchronized presynaptic inhibitory cells. This set of cells was particularly informative because it suggested that the same network controls both the frequency and the duration of the bursts. Specifically, the IPSP frequency was increased by an average of three times after a spike in 10 of these 11 M cells (range from 1.6 to 5.1), and it generally returned to its initial rate in 97–310 msec (mean = 161 msec ± 71; n = 30), i.e., during a period comparable to the average burst duration, or slightly longer (Fig. 5B).
Figure 5.
Temporal properties and inhibitory effects of ISN. (A Upper) Large periods of activity (between brackets) interrupted by faster waves. The autocorrelogram (Right) indicates a dominant rhythm of 60 Hz. (Lower) Bursts of IPSPs occurring at a frequency of 80 Hz in another cell. (B) Instantaneous frequencies of IPSPs in a simple ISN (Inset), plotted against time. M spikes (arrows) were evoked (n = 4 trials) by intracellular current injections that increased the frequency of the noise from 30 to 130 Hz. Control IPSPs were either continuous (n = 3, open symbols) or were induced during the quiet state (♦). (C1) Superimposed traces of antidromic M spikes (n = 10) recorded during the quiet (Left) or the noisy (Center) state and their superimposed averages (Right), indicating a 38% reduction during the noisy state (Right). (C2) Same presentation as above of excitatory postsynaptic potentials (arrow) followed by IPSPs (crossed arrow) evoked by auditory stimuli (sound, 500 Hz, 25 cycles, 50 dB). Note a 36% mean reduction during the noisy periods.
Each M cell receives excitatory inputs from a variety of sensory modalities as well as inhibitory ones and integrates them to determine which cell must fire and, consequently, the direction of the C-start (C-shaped contraction) (2). For example, if the fish is close to an obstacle, it should choose a trajectory away from it (23). But if a predator strikes suddenly, there is no time to make such a decision. In this case the supramedullary network that controls ISN may preset the preferable path for escape, thus serving as an anticipatory device. That is, once a given cell has fired it is subjected to a prolonged inhibition, whereas the contralateral one is reset to the quiet state and ready for an alternative escape. This would be consistent with the notion that the escape reaction takes priority over any other form of motor activity and overrides alternative bursts during fictive swimming but, later, resets the swimming rhythm (15). In confirmation, the C-start can occur twice, and in opposite directions, in closely spaced intervals (Robert Eaton, personal communication). This interval can be as short as 35 msec when a fish is attacked by a predator (24).
This sequence implies that the effectiveness of a given excitatory input in the M cell should be reduced during bursts of IPSPs because of their underlying shunt. Indeed, we have found that the amplitude of passively conducted (4) antidromic test spikes timed to occur during the noisy state was reduced, in comparison with controls during quieter periods (Fig. 5C1), by, on average, 24% ± 14 (n = 5 cells). This effect of ISN was confirmed by showing that the amplitude of fast-mediated excitatory postsynaptic potentials produced in the M cell lateral dendrite by the same auditory input was smaller (4, 8) during bursts of IPSPs (Fig. 5C2) than during quiet periods (mean difference = 25% ± 13, n = 3 cells).
Paired M cells and their associated networks provide a relatively simple model for investigating sensory motor integration. Thus, further studies of this system combining behavioral and genetic tools developed for zebrafish (25–27) should provide new insights on the neural basis of decision-making processes during motor control (28) as well as the origin and function of rhythms, also present in higher centers of mammals.
Acknowledgments
We thank D. S. Faber and R. Miles for their critical help during this work and N. Ankri and J. Asmanis for computer and secretarial assistance.
Abbreviations
- IPSP
inhibitory postsynaptic potential
- ISN
inhibitory synaptic noise
- M cell
Mauthner cell
References
- 1.Zottoli S J. J Exp Biol. 1977;66:243–254. doi: 10.1242/jeb.66.1.243. [DOI] [PubMed] [Google Scholar]
- 2.Eaton R C, DiDomenico R, Nissanov J. Brain Behav Evol. 1991;37:272–285. doi: 10.1159/000114365. [DOI] [PubMed] [Google Scholar]
- 3.Faber D S, Korn H, Lin J W. Brain Behav Evol. 1991;37:286–297. doi: 10.1159/000114366. [DOI] [PubMed] [Google Scholar]
- 4.Faber D S, Korn H. In: Neurobiology of the Mauthner Cell. Faber D S, Korn H, editors. New York: Raven; 1978. pp. 47–131. [Google Scholar]
- 5.Triller A, Korn H. J Comp Neurol. 1981;203:131–155. doi: 10.1002/cne.902030111. [DOI] [PubMed] [Google Scholar]
- 6.Hackett J T, Faber D S. Brain Res. 1983;264:302–306. doi: 10.1016/0006-8993(83)90829-6. [DOI] [PubMed] [Google Scholar]
- 7.Hatta K, Korn H. J Comp Neurol. 1998;395:493–509. [PubMed] [Google Scholar]
- 8.Korn H, Faber D S, Triller A. In: Handbook of Chemical Neuroanatomy. Björklund A, Hökfelt T, Wouterlood F G, van den Pol A N, editors. Vol. 8. New York: Elsevier Science; 1990. pp. 403–480. [Google Scholar]
- 9.Burnod Y, Korn H. Proc Natl Acad Sci USA. 1989;86:352–356. doi: 10.1073/pnas.86.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ankri N, Legendre P, Faber D S, Korn H. J Neurosci Methods. 1994;52:87–100. doi: 10.1016/0165-0270(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa T, Furshpan E J. J Neurophysiol. 1963;26:140–176. doi: 10.1152/jn.1963.26.1.140. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Westerfield M. J Physiol (London) 1988;403:73–89. doi: 10.1113/jphysiol.1988.sp017239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dale N, Roberts A, Soffe S R. In: Glycine Neurotransmission. Ottersen O P, Storm-Mathisen J, editors. New York: Wiley; 1990. pp. 329–353. [Google Scholar]
- 14.Grillner S, Deliagina T, Ekeberg Ö, El Manira A, Hill R H, Lansner A, Orlovsky G N, Wallén P. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- 15.Svoboda K R, Fetcho J R. J Neurosci. 1996;16:843–852. doi: 10.1523/JNEUROSCI.16-02-00843.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grillner S. In: Handbook of Physiology. Brooks V B, editor. Washington, DC: Am. Physiol. Soc.; 1981. , Sec. 1, Vol. 2, pp. 1179–1236. [Google Scholar]
- 17.Fetcho J R, Faber D S. J Neurosci. 1988;8:4192–4213. doi: 10.1523/JNEUROSCI.08-11-04192.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charpier S, Behrends J C, Chang Y-T, Sur C, Korn H. J Neurophysiol. 1994;72:531–541. doi: 10.1152/jn.1994.72.2.531. [DOI] [PubMed] [Google Scholar]
- 19.Singer W, Gray C M. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 20.Jefferys J G R, Traub R D, Whittington M A. Trends Neurosci. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- 21.MacKay W A. Trends Cognitive Sci. 1997;1:176–183. doi: 10.1016/S1364-6613(97)01059-0. [DOI] [PubMed] [Google Scholar]
- 22.Korn H, Sur C, Charpier S, Legendre P, Faber D S. In: Molecular and Cellular Mechanisms of Neurotransmitter Release. Stjärne L, Greengard P, Grillner S, Hökfelt T, Ottoson D, editors. New York: Raven; 1994. pp. 301–322. [Google Scholar]
- 23.Eaton R, Emberley D S. J Exp Biol. 1991;161:469–487. doi: 10.1242/jeb.161.1.469. [DOI] [PubMed] [Google Scholar]
- 24.Lauder G V, Liem K F. Environ Biol Fish. 1981;6:257–268. [Google Scholar]
- 25.Kimmel C B, Hatta K, Eisen J S. Development (Cambridge, UK) 1992;2,Suppl.:47–57. [Google Scholar]
- 26.Haffter P, Granato M, Brand M, Mullins M C, Hammerschmidt M, Kane D A, Odenthal J, van Eeden F J M, Jiang Y-J, Heisenberg C-P, et al. Development (Cambridge, UK) 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Driever W, Solnica-Krezel L, Schier A F, Neuhauss S C F, Malicki J, Stemple D L, Stainier D Y R, Zwartkruis F, Abdelilah S, Rangini Z, et al. Development (Cambridge, UK) 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- 28.Leon M I, Shadlen M N. Neuron. 1998;21:669–672. doi: 10.1016/s0896-6273(00)80584-x. [DOI] [PubMed] [Google Scholar]