Abstract
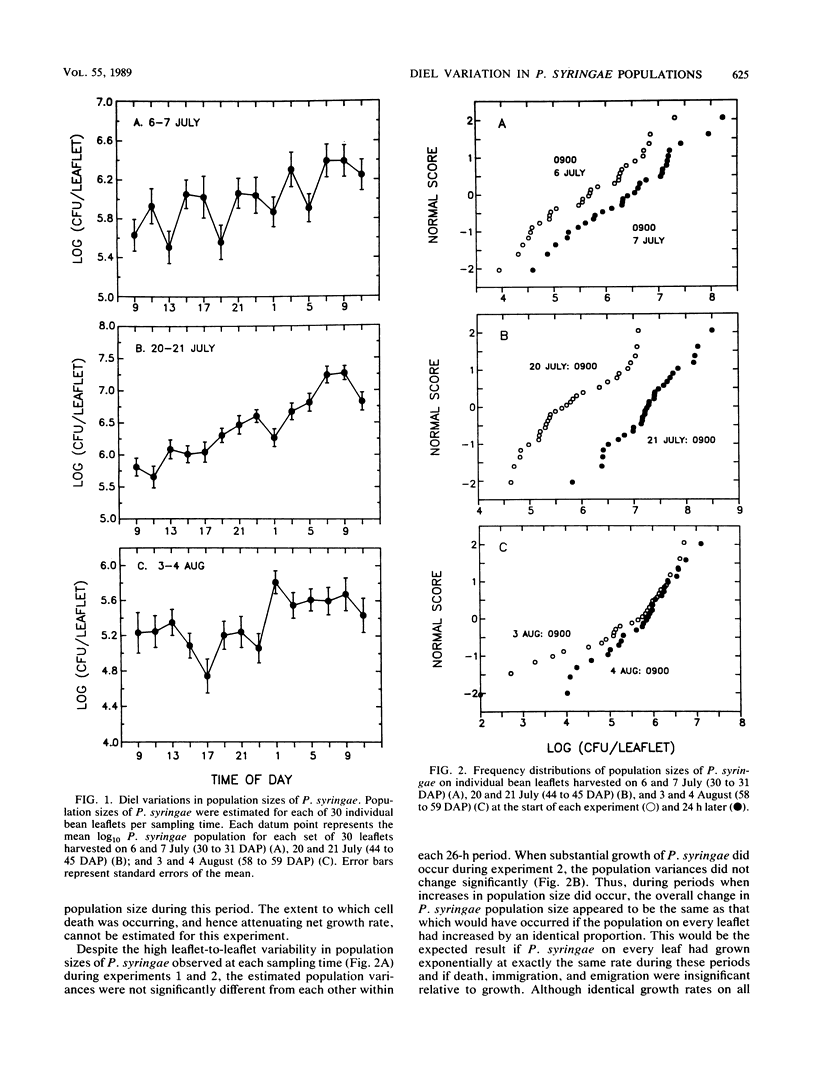
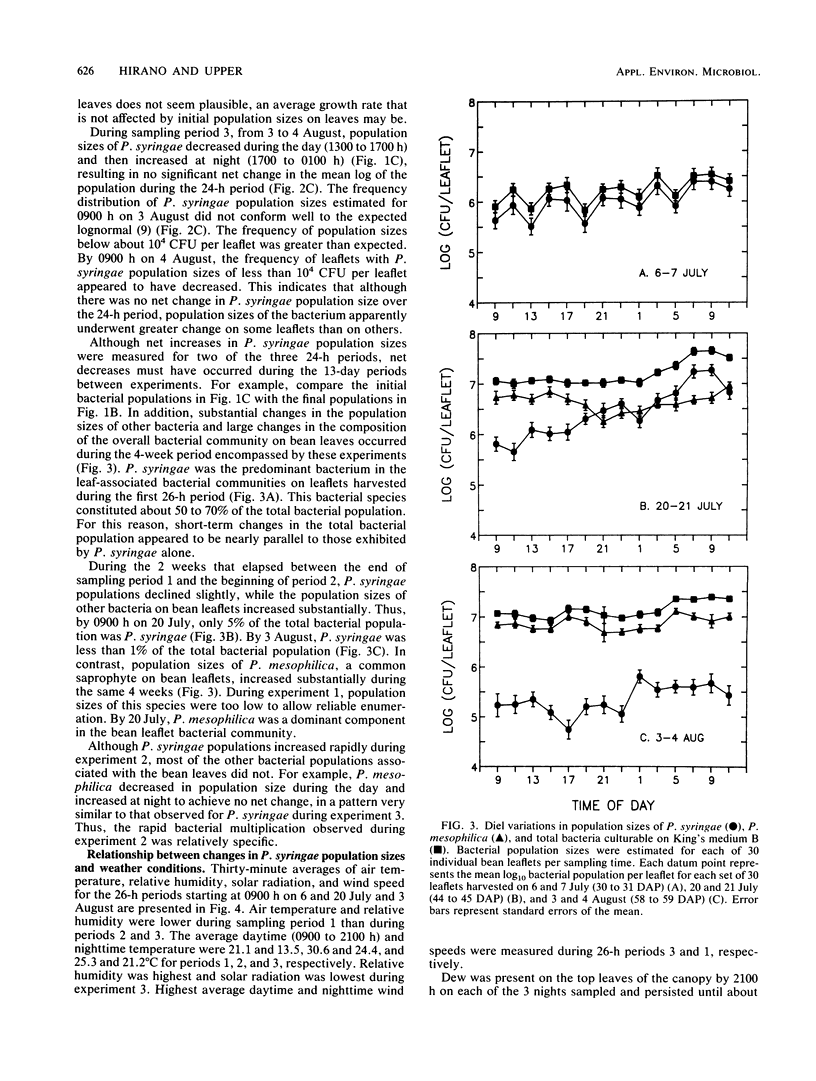
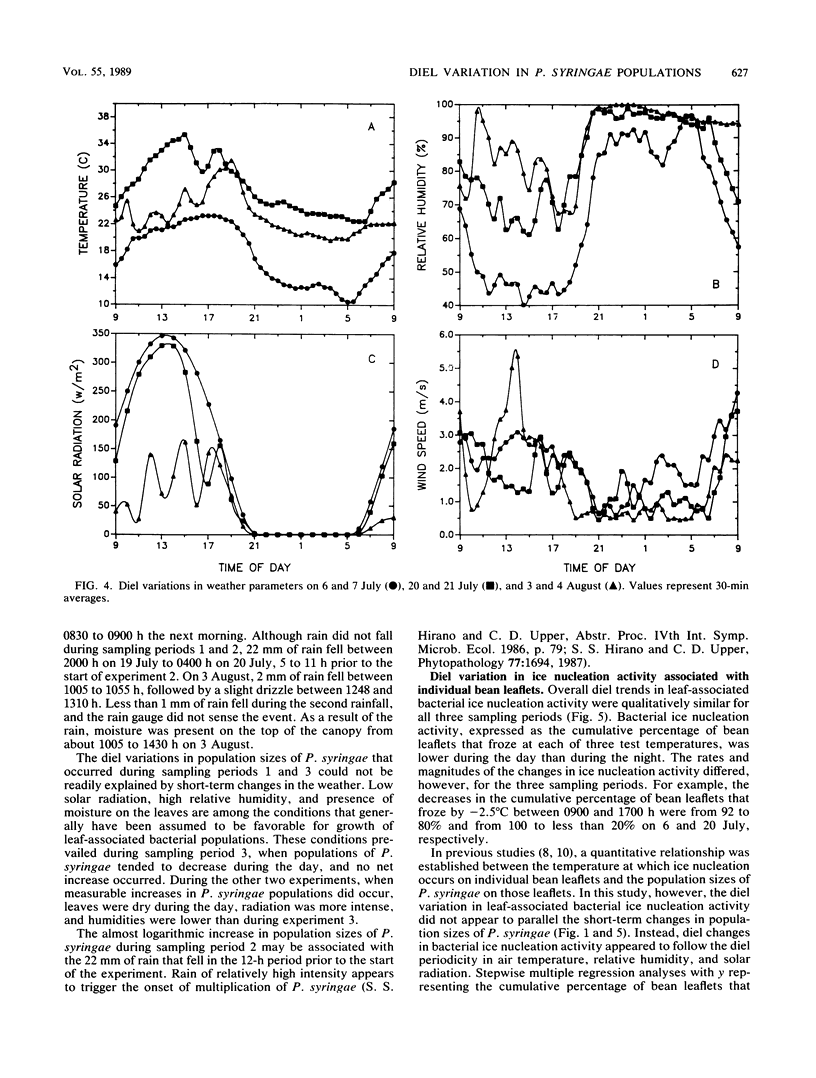
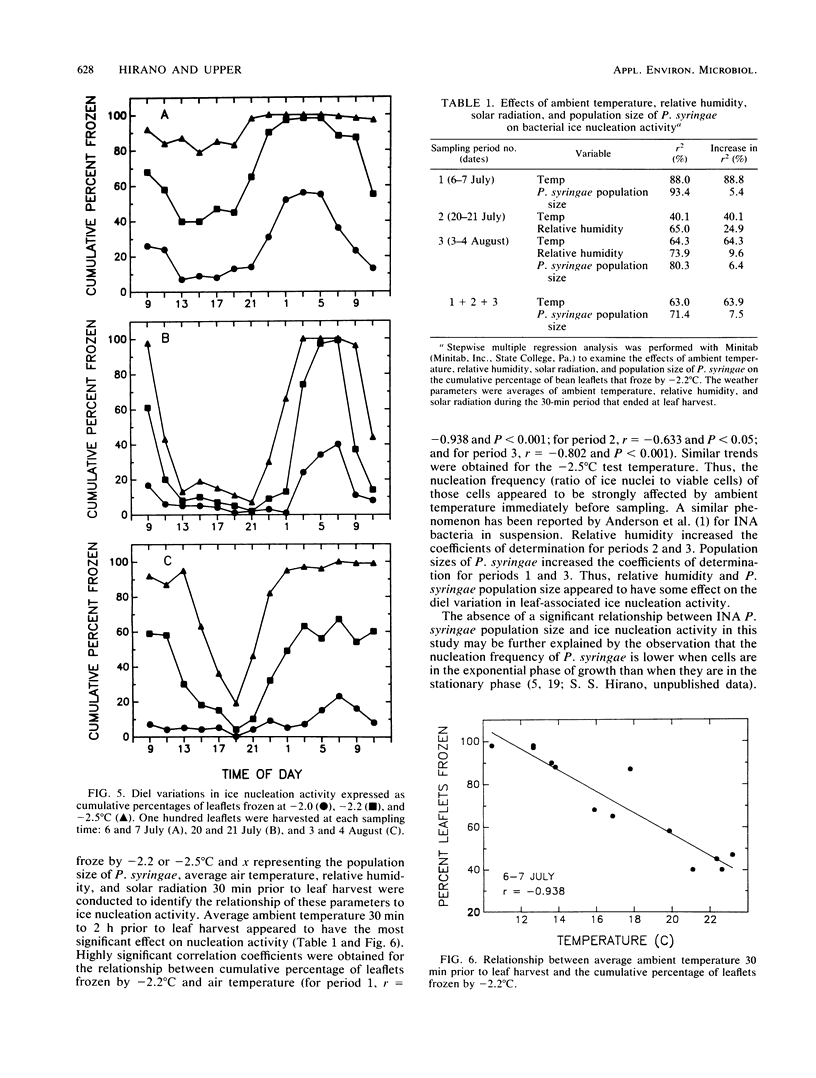
The extent to which diel changes in the physical environment affect changes in population size and ice nucleation activity of Pseudomonas syringae on snap bean leaflets was determined under field conditions. To estimate bacterial population size and ice nucleation activity, bean leaflets were harvested at 2-h intervals during each of three 26-h periods. A tube nucleation test was used to assay individual leaflets for ice nuclei. Population sizes of P. syringae were determined by dilution plating of leaflet homogenates. The overall diel changes in P. syringae population sizes differed during each of the 26-h periods. In one 26-h period, there was a continuous increase in the logarithm of P. syringae population size despite intense solar radiation, absence of free moisture on leaf surfaces, and low relative humidity during the day. A mean doubling time of approximately 4.9 h was estimated for the 28-fold increase in P. syringae population size that occurred from 0900 to 0900 h during the 26-h period. However, doubling times of 3.3 and 1.9 h occurred briefly during this period from 1700 to 2300 h and from 0100 to 0700 h, respectively. Thus, growth rates of P. syringae in association with leaves in the field were of the same order of magnitude as optimal rates measured in the laboratory. The frequency with which leaflets bore ice nuclei active at −2.0, −2.2, and −2.5°C varied greatly within each 26-h period. These large diel changes were inversely correlated primarily with the diel changes in air temperature and reflected changes in nucleation frequency rather than changes in population size of P. syringae. Thus, the response of bacterial ice nucleation activity to the physical environment was distinct from the changes in population size of ice nucleation-active P. syringae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deininger C. A., Mueller G. M., Wolber P. K. Immunological characterization of ice nucleation proteins from Pseudomonas syringae, Pseudomonas fluorescens, and Erwinia herbicola. J Bacteriol. 1988 Feb;170(2):669–675. doi: 10.1128/jb.170.2.669-675.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S. S., Baker L. S., Upper C. D. Ice nucleation temperature of individual leaves in relation to population sizes of ice nucleation active bacteria and frost injury. Plant Physiol. 1985 Feb;77(2):259–265. doi: 10.1104/pp.77.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S. S., Nordheim E. V., Arny D. C., Upper C. D. Lognormal distribution of epiphytic bacterial populations on leaf surfaces. Appl Environ Microbiol. 1982 Sep;44(3):695–700. doi: 10.1128/aem.44.3.695-700.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Lindemann J., Upper C. D. Aerial Dispersal of Epiphytic Bacteria over Bean Plants. Appl Environ Microbiol. 1985 Nov;50(5):1229–1232. doi: 10.1128/aem.50.5.1229-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Arny D. C., Upper C. D. Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiol. 1982 Oct;70(4):1084–1089. doi: 10.1104/pp.70.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Hirano S. S., Barchet W. R., Arny D. C., Upper C. D. Relationship between Ice Nucleation Frequency of Bacteria and Frost Injury. Plant Physiol. 1982 Oct;70(4):1090–1093. doi: 10.1104/pp.70.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki L. R., Galyan E. L., Chang-Chien M. M., Caldwell D. R. Ice nucleation induced by pseudomonas syringae. Appl Microbiol. 1974 Sep;28(3):456–459. doi: 10.1128/am.28.3.456-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. M., Luketina R. C., Marshall A. M. The effects on temperature on growth in vitro of Pseudomonas syringae and and Xanthomonas pruni. J Appl Bacteriol. 1977 Jun;42(3):345–354. doi: 10.1111/j.1365-2672.1977.tb00702.x. [DOI] [PubMed] [Google Scholar]



