Abstract
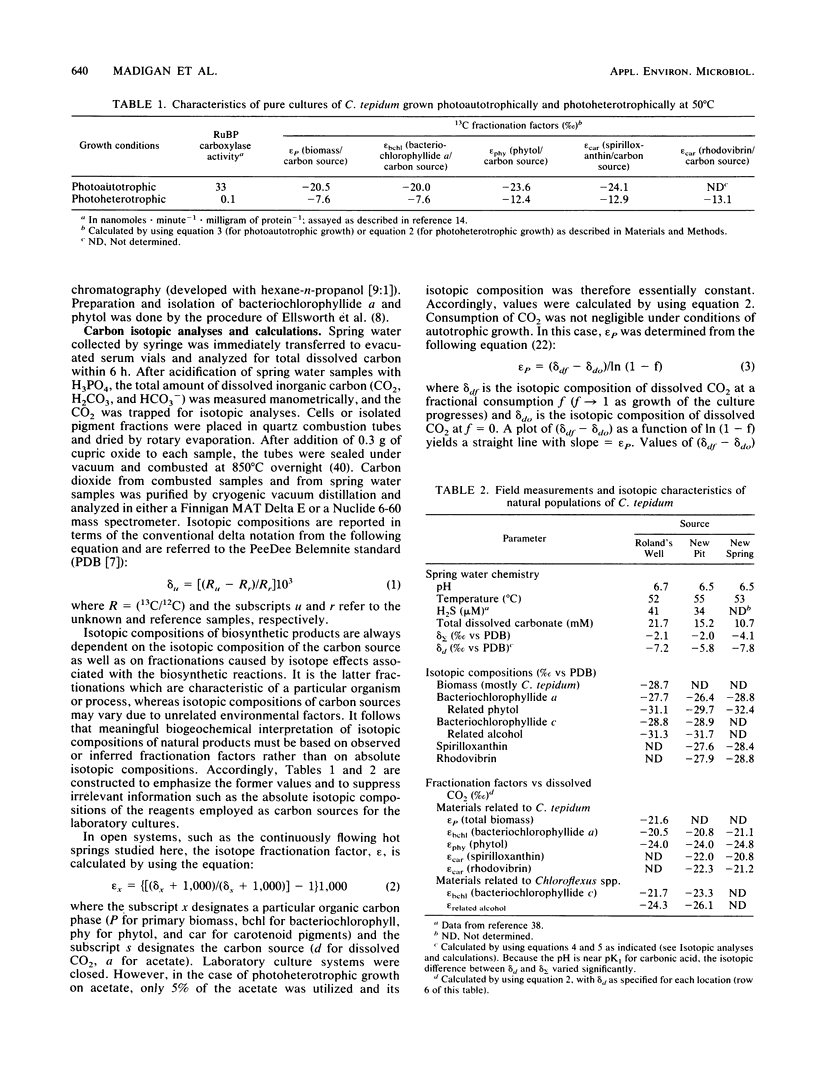
Purple phototrophic bacteria of the genus Chromatium can grow as either photoautotrophs or photoheterotrophs. To determine the growth mode of the thermophilic Chromatium species, Chromatium tepidum, under in situ conditions, we have examined the carbon isotope fractionation patterns in laboratory cultures of this organism and in mats of C. tepidum which develop in sulfide thermal springs in Yellowstone National Park. Isotopic analysis (13C/12C) of total carbon, carotenoid pigments, and bacteriochlorophyll from photoautotrophically grown cultures of C. tepidum yielded 13C fractionation factors near -20%. Cells of C. tepidum grown on excess acetate, wherein synthesis of the Calvin cycle enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase ribulose bisphosphate carboxylase) was greatly repressed, were isotopically heavier, fractionation factors of ca. -7% being observed. Fractionation factors determined by isotopic analyses of cells and pigment fractions of natural populations of C. tepidum growing in three different sulfide thermal springs in Yellowstone National Park were approximately -20%, indicating that this purple sulfur bacterium grows as a photoautotroph in nature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Tayne T. A., Ward D. M. Formation and fate of fermentation products in hot spring cyanobacterial mats. Appl Environ Microbiol. 1987 Oct;53(10):2343–2352. doi: 10.1128/aem.53.10.2343-2352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M. M., Ward D. M. Photoexcretion and fate of glycolate in a hot spring cyanobacterial mat. Appl Environ Microbiol. 1988 Jul;54(7):1738–1743. doi: 10.1128/aem.54.7.1738-1743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth R. K., Nowak C. A. A gas chromatographic-mass spectrometric analysis of the esterifying alcohols of pumpkin seed protochlorophylls. Anal Biochem. 1974 Feb;57(2):534–546. doi: 10.1016/0003-2697(74)90108-0. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. E., LASCELLES J. RIBULOSE DIPHOSPHATE CARBOXYLASE IN THIORHODACEAE. J Gen Microbiol. 1963 Dec;33:445–458. doi: 10.1099/00221287-33-3-445. [DOI] [PubMed] [Google Scholar]
- Hayes J. M., Takigiku R., Ocampo R., Callot H. J., Albrecht P. Isotopic compositions and probable origins of organic molecules in the Eocene Messel shale. Nature. 1987 Sep 3;329:48–51. doi: 10.1038/329048a0. [DOI] [PubMed] [Google Scholar]
- Madigan M. T. A novel photosynthetic purple bacterium isolated from a yellowstone hot spring. Science. 1984 Jul 20;225(4659):313–315. doi: 10.1126/science.225.4659.313. [DOI] [PubMed] [Google Scholar]
- Sadler W. R., Stanier R. Y. THE FUNCTION OF ACETATE IN PHOTOSYNTHESIS BY GREEN BACTERIA. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1328–1334. doi: 10.1073/pnas.46.10.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevåg R., Buchanan B. B., Berry J. A., Troughton J. H. Mechanisms of CO2 fixation in bacterial photosynthesis studied by the carbon isotope fractionation technique. Arch Microbiol. 1977 Feb 4;112(1):35–38. doi: 10.1007/BF00446651. [DOI] [PubMed] [Google Scholar]
- Van Gemerden H. Physiological ecology of purple and green bacteria. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):73–92. doi: 10.1016/s0769-2609(83)80098-2. [DOI] [PubMed] [Google Scholar]



